Abstract
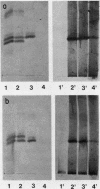
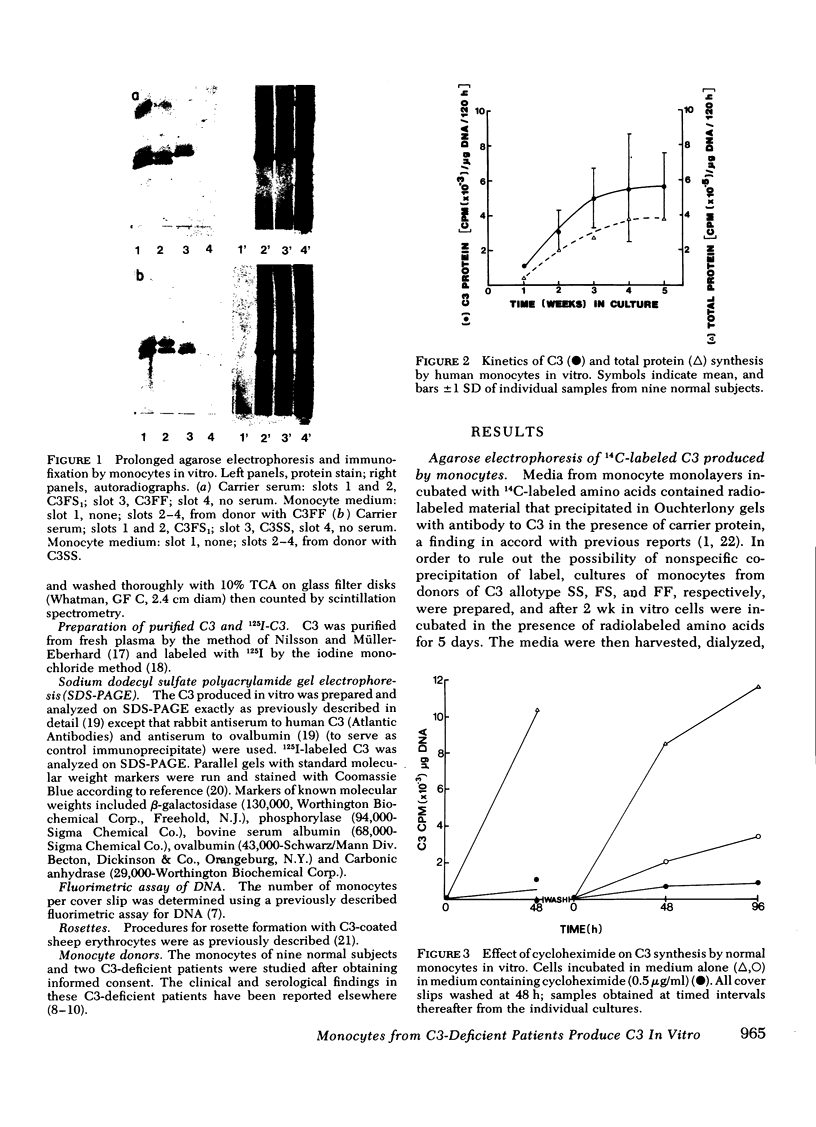
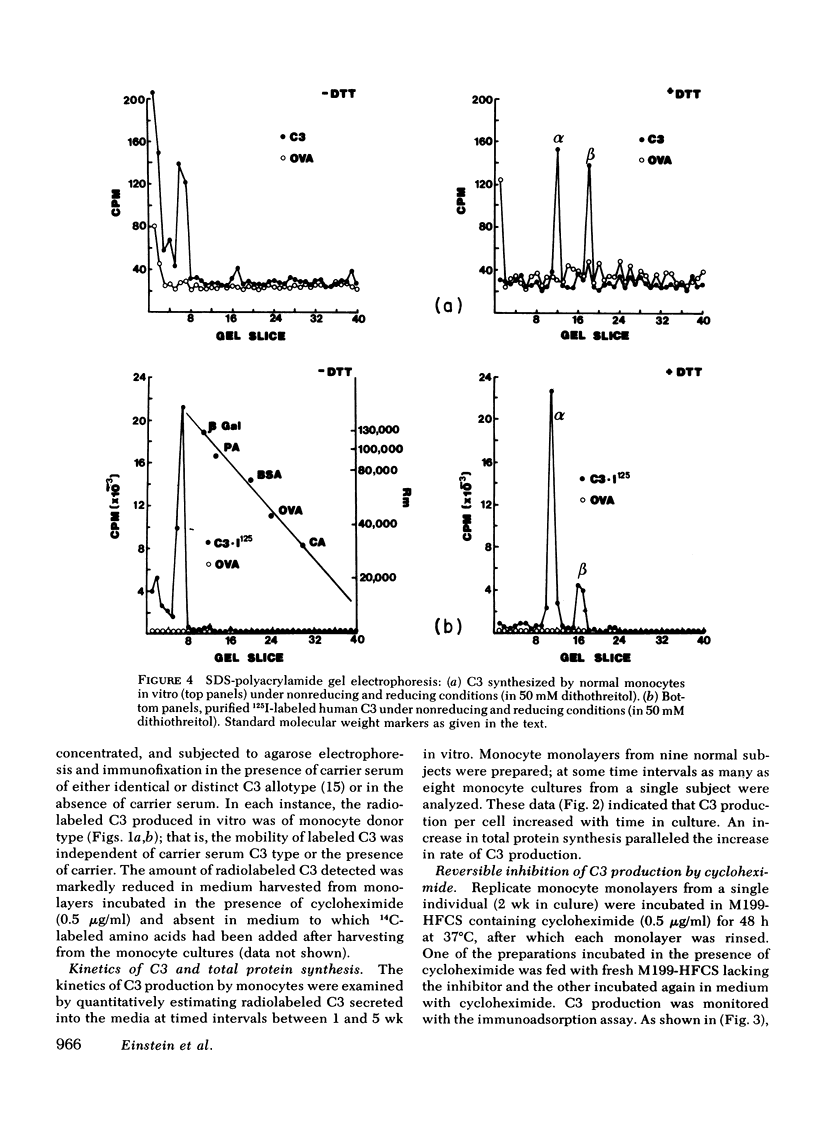
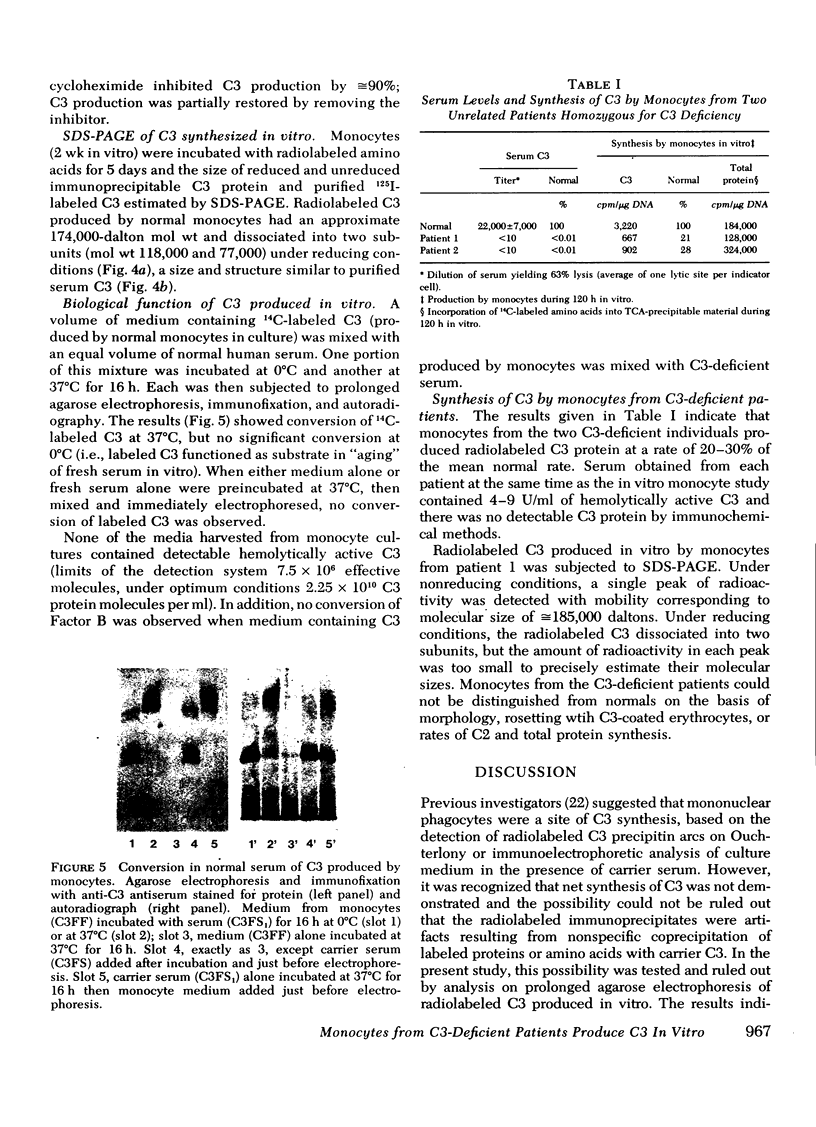
Human monocytes synthesized the third component of complement (C3) up to 5 wk in vitro. Evidence for net C3 synthesis was based on (a) incorporation of 14C-labeled amino acids into C3 protein, (b) indentity of the allotype of C3 produced in vitro with that of the doner's serum C3, even in the presence of carrier C3 protein of a different allotype; (c) correspondence of electrophoretic mobility, size, and subunit structure of C3 protein produced in vitro with serum C3; (d) inhibition of C3 production with cycloheximide. Monocytes from two unrelated C3-deficient patients were studied under conditions that supported C3 synthesis by normal monocytes. Serum from each of the patients contained less than 1% of the normal C3 concentration, buth their monocytes produced C3 at approximately equal to 25% of the normal rate when studied after 2 wk in vitro. The C3 produced in vitro by monocytes from one of the patients had the molecular weight of normal serum C3 and dissociated appropriately under reducing conditions. Monocytes from C3-deficient patients could not be distinguished from normals on the basis of morphology, rosetting with C3-coated erythrocytes, or rates of C2, and total protein synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Colten H. R., Gear J. S., Rabson A. R., Rosen F. S. Homozygous human C3 deficiency. The role of C3 in antibody production, C-1s-induced vasopermeability, and cobra venom-induced passive hemolysis. J Clin Invest. 1976 Jan;57(1):222–229. doi: 10.1172/JCI108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Colten H. R., Rosen F. S., Rabson A. R., Macnab G. M., Gear J. S. Homozygous deficiency of C3 in a patient with repeated infections. Lancet. 1972 Dec 2;2(7788):1179–1181. doi: 10.1016/s0140-6736(72)92598-6. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Johnson A. M., Birtch A. G., Moore F. D. Human C'3: evidence for the liver as the primary site of synthesis. Science. 1969 Jan 17;163(3864):286–288. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Propp R. P. Genetic polymorphism of the third component of human complement (C'3). J Clin Invest. 1968 Sep;47(9):2181–2191. doi: 10.1172/JCI105904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Propp R. P., Klemperer M. R., Rosen F. S. Inherited deficiency of the third component of human complement (C'3). J Clin Invest. 1969 Mar;48(3):553–557. doi: 10.1172/JCI106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballow M., Shira J. E., Harden L., Yang S. Y., Day N. K. Complete absence of the third component of complement in man. J Clin Invest. 1975 Sep;56(3):703–710. doi: 10.1172/JCI108141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokisch V. A., Dierich M. P., Mūller-Eberhard H. J. Third component of complement (C3): structural properties in relation to functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1989–1993. doi: 10.1073/pnas.72.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten H. R., Frank M. M. Biosynthesis of the second (C2) and fourth (C4) components of complement in vitro by tissues isolated from guinea-pigs with genetically determined C4 deficiency. Immunology. 1972 Jun;22(6):991–999. [PMC free article] [PubMed] [Google Scholar]
- Colten H. R. Ontogeny of the human complement system: in vitro biosynthesis of individual complement components by fetal tissues. J Clin Invest. 1972 Apr;51(4):725–730. doi: 10.1172/JCI106866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Alper C. A., Bloch K. J., Herrin J. T., Rosen F. S., David J. R., Colten H. R. Biosynthetic defect in monocytes from human beings with genetic deficiency of the second component of complement. N Engl J Med. 1975 May 29;292(22):1169–1171. doi: 10.1056/NEJM197505292922207. [DOI] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. E., Colten H. R. Cell-free synthesis of the fourth component of guinea pig complement (C4): identification of a precursor of serum C4 (pro-C4). Proc Natl Acad Sci U S A. 1977 Apr;74(4):1707–1710. doi: 10.1073/pnas.74.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osofsky S. G., Thompson B. H., Lint T. F., Gewurz H. Hereditary deficiency of the third component of complement in a child with fever, skin rash, and arthralgias: response to transfusion of whole blood. J Pediatr. 1977 Feb;90(2):180–186. doi: 10.1016/s0022-3476(77)80626-4. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Colten H. R. Rheumatoid arthritis. Biosynthesis of complement proteins by synovial tissues. N Engl J Med. 1974 Jun 6;290(23):1284–1288. doi: 10.1056/NEJM197406062902304. [DOI] [PubMed] [Google Scholar]
- Stecher V. J., Thorbecke G. J. Sites of synthesis of serum proteins. OI. Serum proteins produced by macrophages in vitro. J Immunol. 1967 Oct;99(4):643–652. [PubMed] [Google Scholar]
- Strunk R. C., Tashjian A. H., Jr, Colten H. R. Complement biosynthesis in vitro by rat hepatoma cell strains. J Immunol. 1975 Jan;114(1 Pt 2):331–335. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]