Abstract
Objectives
Little is known about the treatment Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans receive for chronic non-cancer pain (CNCP). We sought to describe the prevalence of prescription opioid use, types and doses of opioids received, and identify correlates of receiving prescription opioids for CNCP among OEF/OIF veterans.
Design
Retrospective review of VA administrative data.
Setting
Ambulatory clinics within a VA regional healthcare network.
Patients
OEF/OIF veterans who had at least 3 elevated pain screening scores within a 12-month period in 2008. Within this group, those prescribed opioids (n=485) over the next 12 months were compared to those not prescribed opioids (n=277). In addition, patients receiving opioids short term (<90 days, n=284) were compared to patients receiving them long-term (≥90 consecutive days, n=201).
Results
Of 762 OEF/OIF veterans with CNCP, 64% were prescribed at least one opioid medication over the 12 months following their index dates. Of those prescribed an opioid, 59% were prescribed opioids short-term and 41% were prescribed opioids long-term. The average morphine-equivalent opioid dose for short-term users was 23.7 mg (SD=20.5) compared with 40.8 mg (SD=36.1) for long-term users (p<0.001). Fifty-one percent of long-term opioid users were prescribed short-acting opioids only and one-third were also prescribed sedative-hypnotics. In adjusted analyses, diagnoses of low back pain, migraine headache, post-traumatic stress disorder, and nicotine use disorder were associated with an increased likelihood of receiving an opioid prescription.
Conclusion
Prescription opioid use is common among OEF/OIF veterans with CNCP and is associated with several pain diagnoses and medical conditions.
Keywords: Chronic pain, Opioids, Veteran, Pain/drug therapy
Introduction
Over the past 20 years, chronic non-cancer pain (CNCP) has increasingly been managed with opioid medications [1–2]. Between 1980 and 2000, the rate of opioid prescriptions at outpatient visits for chronic musculoskeletal pain approximately doubled [3]. Despite the increased use of opioids, the safety and efficacy of long-term opioid therapy for CNCP is unclear [4–6]. Some investigators have sought to identify factors associated with receiving opioid prescriptions. Psychiatric disorders, including generalized anxiety disorder, panic disorder, depression, and dysthymia have previously been found to be associated with receiving an opioid [7–9].
Pain conditions are highly prevalent among returning Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans; in ambulatory settings, 47% report at least a mild level of pain and over half report musculoskeletal pain [10–12]. There are several potential reasons for this. Compared to previous wars, injured OEF/OIF veterans have improved survival. In addition, OEF/OIF veterans have higher rates of complex injuries including polytrauma [13–14]. Polytrauma and blast injuries among returning OEF/OIF veterans have been shown to increase the prevalence of a number of pain conditions, including chronic low back pain and head pain [10, 14–16].
However, relatively little is known about the treatment OEF/OIF veterans receive for their pain. One prior report found that OEF/OIF veterans with blast injury reported higher rates of opioid analgesic use at discharge from a chronic pain rehabilitative program and reduced improvement in pain intensity following treatment compared to non-blast injured veterans [17]. In a cohort of polytrauma registry patients, approximately one-half of 133 blast-injured OEF/OIF veterans were prescribed opioid analgesics [18]. Another study reported an increase in the prevalence of chronic opioid use in young veterans within a 5-year period [19]. Further, this prior report found that primary care providers prescribed up to 80% of opioids [19]. Little information is available regarding other aspects of opioid use (e.g., types of medications, doses, duration of treatment) or the correlates of receiving opioid prescriptions in this patient population.
Given the high prevalence of pain conditions and the prevalence of associated comorbid psychiatric conditions [15, 20] among OEF/OIF veterans, we sought to describe the prevalence of prescription opioid use, as well as patterns of opioid use by OEF/OIF veterans who have at least moderate CNCP. We also evaluated demographic and clinical factors associated with receiving any opioid prescription.
Methods
This study was approved by the local VA Medical Center Institutional Review Board.
Study Sample
Veterans receiving treatment within Veterans Integrated Service Network-20 (VISN-20), namely any VA facility in the Pacific Northwest (Washington, Oregon, Idaho, and Alaska) were included in the study sample. Patient data were extracted from the VISN-20 Data Warehouse, which is updated monthly and contains data from the main clinical software packages of regional VA healthcare facilities and two national VA databases [21]. VISN-20 Warehouse data were linked to OEF/OIF Roster data. The OEF/OIF Roster is developed and maintained by the VHA Service Support Center, and OEF/OIF roster files are provided by the Department of Defense, Defense Manpower Data Center (DMDC)–Contingency Tracking System Deployment file. The DMDC requires that veterans on the OEF/OIF roster were physically located within the OEF/OIF combat zones or specifically identified as directly supporting the OEF/OIF mission outside the designated combat zones in Afghanistan or Iraq.
Currently the VA healthcare system uses numeric rating scores (NRS) [22] to routinely screen for pain in clinical settings as part of its “Pain as the fifth vital sign” campaign [23]. The NRS rates pain intensity on a scale of 0–10, with 0 representing no pain and 10 representing the worst possible pain. To identify patients with CNCP for the current study, we used the VISN-20 Data Warehouse to find patients who had NRS pain scores ≥4 recorded in at least three different months during 2008. This is consistent with common definitions of chronic pain that require at least three to six month duration of moderate to severe pain [24]. A cutoff of four was used for this study due to its consistency with VA clinical practice and policy regarding indication for further evaluation of pain [25–26]. An index date was calculated for each subject, defined as the date the last pain score ≥4 occurred in calendar year 2008. Exclusion criteria included: any visits to a VA opioid substitution (methadone maintenance) program in VISN20 within 12 months following the index date, any cancer diagnosis made in the 12 months following the index date, or surgery six months prior to or 12 months following the index date. Any patient who died within 12 months following the index date was also excluded.
Included subjects were classified into two groups: CNCP patients prescribed opioid medications or CNCP patients not prescribed any opioid medications within 12 months following the index date. CNCP patients who were prescribed an opioid were classified into two subgroups: CNCP patients prescribed <90 days of opioid medications (short-term use) or CNCP patients prescribed any opioid medications ≥90 consecutive days (long-term use) during the 12 month study period. To account for brief periods of time during which a patient might be waiting for a new prescription, if a patient had been prescribed at least 90 days of opioid medications within any 104-day block, the patient was defined as being a long-term user. This definition allowed for up to a 14-day gap in opioid prescriptions. Prescription rates for short-acting and long-acting opioids were compared across age groups for short-term and long-term users.
Demographic data including age, sex, education, marital status, ethnicity, branch of service, and military component of last deployment were collected for all subjects. Inpatient and outpatient diagnoses were based on International Classification of Diseases, Clinical Modification–9th Revision (ICD-9-CM). If a diagnostic code for a disorder was documented by a provider within 12 months following the index date, the patient was coded as having the disorder. We collected information regarding substance use disorder (defined as substance abuse/dependence diagnoses for alcohol, amphetamine, cannabis, cocaine, opioids, polysubstance abuse, or other unspecified drugs of abuse), tobacco use, major depression, dysthymic disorder, panic disorder, and post-traumatic stress disorder (PTSD) diagnoses. Pharmacy data were reviewed to extract information on prescriptions of opioids, non-opioid analgesics, and adjunctive medications often used for pain. Opioid prescription dosages were translated into morphine equivalent dosages [27].
Statistical Analyses
Demographic and diagnostic data were analyzed using chi-square or Fisher’s exact tests for categorical variables and t-tests or analysis of variance (ANOVA) for continuous variables. Post-hoc tests were evaluated with Scheffe. Logistic regression was used to evaluate characteristics associated with receipt of any opioid prescription (yes/no). Regression models included variables for age, sex, pain score at index date, and diagnoses given during the study year (yes/no), including chronic low back pain, chronic neck and joint pain, migraine headache, PTSD, and nicotine use disorder. These particular diagnoses were included because we detected significant differences (p<0.05) between groups in bivariate testing; in addition, these diagnoses have previously been shown to be associated with receipt of prescription opioids in other patient populations [7–9, 28–29]. Major depressive disorder was not entered into the model due to its high correlation with PTSD (Spearman’s correlation = 0.288, p<0.001); PTSD was included instead because of its high prevalence in this population [15, 20] and due to its greater significance value in bivariate testing.
Results
Patient Characteristics
Table 1 compares demographic differences between CNCP patients not prescribed opioids and those prescribed opioids over the 12-month study period. The majority of the sample was male (85%), white (84%), and the average age was 34 (SD=8.8) years. Just over half of the cohort was married (51%) and almost all had VA service-connected disabilities (95%) (Table 1). There was a greater proportion of males in the group prescribed opioids long-term (92.5%) compared to the group prescribed opioids short-term (82.4%) or patients not prescribed opioids (81.6%), (p=0.002). Otherwise, no significant demographic differences were detected when comparing CNCP patients prescribed short-term or long-term opioids and patients not prescribed opioids.
Table 1.
Demographic Characteristics and Patterns of Opioid Use.
| Opioid Patterns of Use | No opioids | Short-term | Long-term | ||
|---|---|---|---|---|---|
|
| |||||
| 0 days (n=277) | <90 days (n=284) | ≥90 days (n=201) | Test (df) | p-value | |
| Age | 34.3 (9.0) | 34.0 (9.0) | 33.1 (8.1) | F (2, 759) = 1.065 | 0.345 |
| Male Gender | 226 (81.6%) | 234 (82.4%) | 186 (92.5%) | χ2 (2) = 12.811 | 0.002 |
| Marital Status | χ2 (6) = 6.950 | 0.325 | |||
| Single/Never Married | 68 (24.5%) | 133 (46.8%) | 114 (56.7%) | ||
| Married | 141 (50.9%) | 78 (27.5%) | 37 (18.4%) | ||
| Separated/Divorced/Widowed | 62 (22.4%) | 67 (23.6%) | 44 (21.9%) | ||
| Unknown | 6 (2.2%) | 6 (2.1%) | 6 (3.0%) | ||
| Race | χ2 (6) = 9.679 | 0.139 | |||
| Caucasian | 169 (61.0%) | 198 (69.7%) | 137 (68.2%) | ||
| Black | 27 (9.7%) | 19 (6.7%) | 21 (10.4%) | ||
| Other | 13 (4.7%) | 14 (4.9%) | 4 (2.0%) | ||
| Unknown or Declined to answer | 68 (24.5%) | 53 (18.7%) | 39 (19.4%) | ||
| VA Service-Connected Disability | 262 (94.6%) | 268 (94.4%) | 195 (97.0%) | χ2 (2) = 2.082 | 0.353 |
| Active Duty (years) | 4.5 (5.2) | 4.7 (5.0) | 4.7 (4.5) | F (2, 759) = 0.138 | 0.871 |
| Military Component | χ2 (4) = 6.066 | 0.194 | |||
| Active | 148 (53.4%) | 161 (56.7%) | 118 (58.7%) | ||
| Reserve | 31 (11.2%) | 23 (8.1%) | 10 (5.0%) | ||
| Guard | 98 (35.4%) | 100 (35.2%) | 73 (36.3%) | ||
| Branch of Service | χ2 (6) = 6.018 | 0.421 | |||
| Army | 205 (74.0%) | 209 (73.6%) | 155 (77.1%) | ||
| Air Force | 32 (11.6%) | 22 (7.7%) | 17 (8.5%) | ||
| Marines | 15 (5.4%) | 25 (8.8%) | 15 (7.5%) | ||
| Navy | 25 (9.0%) | 28 (9.9%) | 14 (7.0%) | ||
Table 2 compares clinical diagnoses among patients prescribed opioids and those not prescribed opioids. There were greater proportions of patients prescribed opioids (both long-term and short-term) with documented diagnoses of chronic low back pain (66%) and migraine headache (24%) compared to patients not prescribed opioids (48% and 14%, respectively) (p<0.001 and p=0.002, respectively). Patients prescribed opioids long-term had a greater prevalence of chronic neck or joint pain (70%) compared to short-term opioid users (60%) and patients not prescribed opioids (59%) (p=0.04). There were also greater proportions of diagnoses for major depressive disorder, PTSD, and nicotine use disorder in the group prescribed opioids compared to the group not prescribed opioids (all p-values < 0.05). There was no significant difference between groups in the rates of substance use disorders. In the overall sample, the most commonly diagnosed substance use disorders were alcohol (11.8%), cannabis (3.7%), or other substance use disorder (8.8%).
Table 2.
Clinical characteristics of non-opioid and opioid users.
| Opioid Patterns of Use | No opioids | Short-term | Long-term | ||
|---|---|---|---|---|---|
|
| |||||
| 0 days (n=277) | <90 days (n=284) | ≥90 days (n=201) | Test (df) | p-value | |
|
Pain Diagnoses
| |||||
| Fibromyalgia | 18 (6.5%) | 15 (5.3%) | 18 (9.0%) | χ2 (2) = 2.570 | 0.277 |
| IBD* | 6 (2.2%) | 11 (3.9%) | 9 (4.5%) | χ2 (2) = 2.181 | 0.336 |
| Low Back Pain | 133 (48.0%) | 168 (59.2%) | 150 (74.6%) | χ2 (2) = 34.150 | < 0.001 |
| Neck or Joint Pain | 164 (59.2%) | 170 (59.9%) | 140 (69.7%) | χ2 (2) = 6.466 | 0.039 |
| Migraine Headache | 40 (14.4%) | 65 (22.9%) | 51 (25.4%) | χ2 (2) = 10.173 | 0.006 |
| Rheumatism/Arthritis | 74 (26.7%) | 82 (28.9%) | 63 (31.3%) | χ2 (2) = 1.222 | 0.543 |
|
| |||||
|
Psychiatric Diagnoses
| |||||
| MDD** | 101 (36.5%) | 132 (46.5%) | 111 (55.2%) | χ2 (2) = 16.882 | < 0.001 |
| Dysthymic Disorder | 7 (2.5%) | 15 (5.3%) | 9 (4.5%) | χ2 (2) = 2.844 | 0.241 |
| Panic Disorder | 7 (2.5%) | 14 (4.9%) | 7 (3.5%) | χ2 (2) = 2.315 | 0.314 |
| PTSD*** | 135 (48.7%) | 168 (59.2%) | 138 (68.7%) | χ2 (2) = 19.263 | < 0.001 |
| Any SUD**** | 33 (11.9%) | 52 (18.3%) | 31 (15.4%) | χ2 (2) = 4.454 | 0.108 |
| Nicotine Use Disorder | 35 (12.6%) | 76 (26.8%) | 55 (27.4%) | χ2 (2) = 21.406 | < 0.001 |
Note. IBD = inflammatory bowel disease; MDD = major depressive disorder; PTSD = post traumatic stress disorder;
SUD = substance use disorder.
Opioids were prescribed to 64% (n=485) of all patients. CNCP patients prescribed opioids had reported greater pain NRS intensity at their index dates (6.5, SD=1.6) compared to CNCP patients not prescribed opioids (6.0, SD=1.6, t (760) = −3.80, p<0.001). The mean duration of opioid prescription was 61 days for patients in the short-term group and 285 days for patients in the long term group. Opioids were prescribed in primary care settings with the highest frequency (67.9%) compared to emergency department/urgent care settings (13.8%), outpatient surgical settings (5.3%), dental services (1.8%), podiatry (0.7%), or other settings (10.4%).
Of veterans prescribed any opioid, 59% were prescribed opioids short-term compared to 41% prescribed opioids long-term. CNCP patients who were prescribed opioids short-term or long-term received an average daily dose of 23.7 mg and 36.1 mg morphine equivalent, respectively (p<0.001). The range of average morphine equivalent dose for short-term users was 1.7 to 180 mg per day compared to 5 to 322 mg per day for long-term users. Patients prescribed opioids long-term were more likely to be administered one or more urine drug screens (31%) compared to patients prescribed opioids short-term (21%) or patients not prescribed opioids (13%) (χ2 (2) = 21.59; p<0.001).
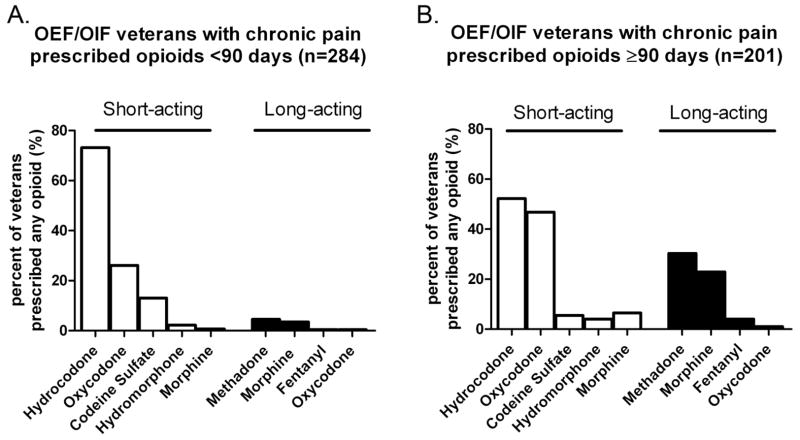
The proportions of CNCP patients receiving particular opioids are shown in Figure 1. Short-term opioid users had greater prescription rates for hydrocodone and codeine compared to long-term opioid users. Long-term opioid users had increased rates of short-acting oxycodone and short-acting morphine and prescriptions for long-acting morphine and methadone compared to short-term users.
Figure 1.
Frequency of short-acting and long-acting opioid medications prescriptions during 12 month study period for A) patients prescribed opioids short term (≤90 days) (n=284) and B) patients prescribed opioids long-term (>90 days) (n=201).
The majority of short-term users were prescribed short-acting opioids (97%); few (8.5%) short-term users were prescribed long-acting opioids (Figure 2). Ninety-two percent of long-term users were prescribed at least one short-acting opioid and 49% of long-term users were prescribed at least one long-acting opioid. Forty-one percent of long-term users were prescribed both short-and long-acting opioids. A short-acting opioid only was prescribed to 51% of long-term opioid users.
Figure 2.
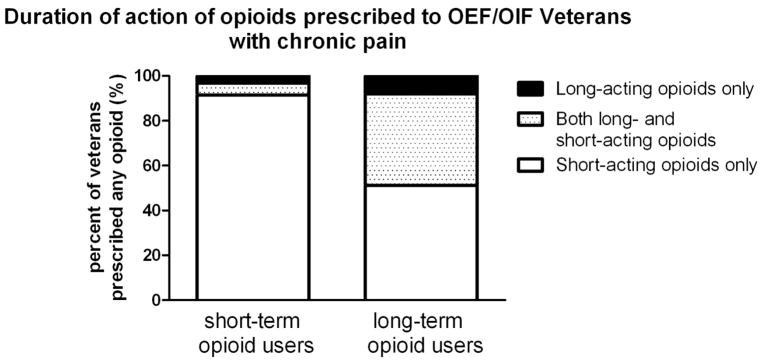
Frequencies of Veterans (% of total number of patients within each pattern) prescribed short-acting opioids only, both short-acting and long-acting opioids, or only long-acting opioids according to short-term (≤90 days) (n=284) and long term (>90 days) (n=201) use.
Opioid users (short-term and long-term, respectively) were prescribed non-steroidal anti-inflammatory medications (NSAIDs) or acetaminophen more frequently than non-opioid users (69% and 76% vs. 59%, χ2 (2) = 18.34, p< 0.001). Capsaicin prescriptions were also more frequently prescribed to opioid users (short-term and long-term, respectively) compared to non-opioid users (12% and 8% vs. 5%, χ2 (2) = 8.79, p< 0.05) as were prescriptions of anticonvulsants (29% and 44% vs. 24%, χ2 (2) = 24.53, p< 0.001), benzodiazepines (26% and 33% vs. 14%, χ2 (2) = 23.33, p< 0.001), and antidepressants (73% and 84% vs. 57%, χ2 (2) = 41.29, p< 0.001).
The results of the logistic regression analysis examining factors associated with receipt of any opioid prescription are displayed in Table 3. When adjusting for age, sex, and pain scores at index dates, diagnoses of chronic low back pain (OR = 1.83, 95% CI = 1.33–2.51) and migraine headache (OR = 1.66, 95% CI = 1.10–2.51) were associated with increased likelihood of being prescribed any opioid. Diagnoses of PTSD (OR = 1.42, 95% CI = 1.04–1.96) and nicotine use disorder (OR = 2.14, 95% CI = 1.41–3.27) were also associated with an increased likelihood of being prescribed opioids.
Table 3.
Factors associated with receiving an opioid prescription among OEF/OIF veterans with CNCP (n=762).
| Beta (Standard Error) | Wald | p-value | Odds Ratio (95% Confidence Interval) | |
|---|---|---|---|---|
| Age | −0.007 (0.01) | 0.629 | 0.428 | 0.993 (0.975–1.011) |
| Male Gender | 0.189 (0.22) | 0.775 | 0.379 | 1.208 (0.793–1.840) |
| Pain Score at Index Date | 0.150 (0.05) | 9.159 | 0.002 | 1.162 (1.054–1.280) |
| Chronic Low Back Pain | 0.602 (0.16) | 13.89 | <0.001 | 1.826 (1.330–2.506) |
| Chronic Neck and Joint Pain | 0.022 (0.17) | 0.017 | 0.896 | 1.022 (0.738–1.415) |
| Migraine Headache | 0.505 (0.21) | 5.681 | 0.017 | 1.657 (1.094–2.510) |
| Posttraumatic Stress Disorder | 0.355 (0.16) | 4.794 | 0.029 | 1.426 (1.038–1.960) |
| Nicotine Use Disorder | 0.762 (0.22) | 12.56 | <0.001 | 2.142 (1.406–3.265) |
Note. All variables listed above were simultaneously forced into the model.
Discussion
In this study, we found that approximately two-thirds of OEF/OIF veterans with CNCP were prescribed opioids over a one-year timeframe, and that over one-third were prescribed opioids on a long-term basis. This study extends prior literature documenting high rates of opioid use among OEF/OIF veterans suffering from war-related injuries [17–19]. Almost half of returning OEF/OIF veterans experience pain, compared to 10–20% of the general population [10, 30] and higher rates have been documented to occur among those who have experienced polytrauma [15]. Based on these study findings, it appears that opioid medications are frequently used to treat CNCP in OEF/OIF veterans.
Our findings suggest that in several ways prescribers are adhering to guidelines for the treatment of chronic pain [31–32]. Patients who receive opioids are more likely to receive adjunctive pain medications, such as NSAIDs/acetaminophen or antidepressants, and are more likely to be administered urine drug screening. However, our findings also suggest that in other ways, OEF/OIF veterans may not be receiving care consistent with pain and opioid treatment guidelines. Almost half of long-term opioid users were prescribed short-acting opioids only. Guidelines for the treatment of chronic pain often recommend that patients transition to long-acting opioids to improve adherence and provide more consistent pain control, though empirical evidence supporting the effectiveness or safety of long-acting over short-acting opioids is limited [4–5]. In addition, the high rate of benzodiazepine prescriptions among opioid users is concerning. In our study, 33% of long-term opioid users were prescribed sedative-hypnotic medications, a finding similar to those found in two prior studies of the general population which found that 30% and 44% of long-term opioid users were regular users of sedative-hypnotics [29, 33]. The use of opioids and sedative-hypnotic therapy should be carefully monitored by prescribing physicians to prevent possible overdose or death [34]. Finally, although the use of urine drug screening was greater in the opioid users, the rates were still low in the long-term treatment group (31%), similar to a previous report of young veterans prescribed chronic opioid therapy [19]. Pain treatment guidelines recommend the use of regular urine drug screening for all patients, and more intensive monitoring for patients who are at high risk of aberrant behaviors [5]. The high rate of comorbid diagnosed substance use disorder in this group (15%) suggests that more frequent or more systematic drug screening may be indicated.
In this sample of OEF/OIF patients with CNCP, we found a high prevalence of comorbid medical diagnoses, and patients who were prescribed opioids had higher rates of psychiatric diagnoses than patients who were not prescribed opioids. Specifically, our results indicate that chronic low back pain, migraine headache, PTSD, and nicotine use disorder are associated with an increased likelihood of receiving a prescription for an opioid medication. The strongest predictor of opioid use in our study of veterans with CNCP was the diagnosis of nicotine use disorder. This association between opioid prescription and tobacco use is also consistent with previous research. Nicotine dependence predicted more frequent use of opioids in a population-based cohort study of CNCP patients [28] and in patients with painful spine conditions [35]. It has been suggested that increased opioid use by smokers may be caused by an altered perception of pain by exposure to chronic cigarette smoke and nicotine’s analgesic properties resulting in cross-tolerance [36].
Several limitations should be considered when interpreting the results of this study. This was a retrospective study using data that were obtained from administrative data sets. NRS scores were obtained as part of routine clinical care, and as such, may not have been administered systematically. Participants were predominantly male, white OEF/OIF veterans receiving VA healthcare in the Pacific Northwest portion of the U.S. who had a high rate of service-connected disabilities; generalizability to other groups is therefore limited. Because prescription medication information was obtained from administrative data, patient adherence to prescribed medications cannot be confirmed.
This study showed that the majority of OEF/OIF veterans who have chronic non-cancer pain and are treated in VA outpatient clinics have high rates of service-connected disabilities and are prescribed opioids. Several of our findings suggest a need for improvement in implementing guideline-level pain care for these veterans, in particular with regard to avoiding use of sedative-hypnotics, and perhaps greater use of long-acting opioid formulations and more frequent use of urine drug screening. Additional provider education and system supports are indicated to improve the quality and safety of opioid prescribing for this population.
Acknowledgments
We appreciate comments from Teresa Hudson, Pharm D. on a prior draft of this manuscript. This material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center. This study was supported in part by award K23DA023467 from the National Institute on Drug Abuse to Dr. Morasco. Jonathan Duckart, MPS, was supported by a Research Enhancement Award Program grant (REA 06-174) from the VA Health Services Research and Development service. The authors appreciate the statistical support provided from the Oregon Clinical and Translational Research Institute, grant number Ul1RR024140 from the National Institutes of Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Footnotes
No author reports having any potential conflict of interest with this study.
References
- 1.Edlund MJ, Martin BC, DeVries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–9. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109:514–9. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Ballantyne JC, Fanciullo GJ, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–59. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207–9. doi: 10.1016/j.pain.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–93. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–50. doi: 10.1016/s1526-5900(03)00638-2. [DOI] [PubMed] [Google Scholar]
- 10.Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7:339–43. doi: 10.1111/j.1526-4637.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 11.Helmer DA, Chandler HK, Quigley KS, Blatt M, Teichman R, Lange G. Chronic widespread pain, mental health, and physical role function in OEF/OIF veterans. Pain Med. 2009;10:1174–82. doi: 10.1111/j.1526-4637.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 12.Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49:475–80. doi: 10.1097/JOM.0b013e318042d682. [DOI] [PubMed] [Google Scholar]
- 13.Lew HL, Poole JH, Vanderploeg RD, Goodrich GL, Dekelboum S, Guillory SB, et al. Program development and defining characteristics of returning military in a VA Polytrauma Network Site. J Rehabil Res Dev. 2007;44:1027–34. [PubMed] [Google Scholar]
- 14.Dobscha SK, Clark ME, Morasco BJ, Freeman M, Campbell R, Helfand M. Systematic review of the literature on pain in patients with polytrauma including traumatic brain injury. Pain Med. 2009;10:1200–17. doi: 10.1111/j.1526-4637.2009.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46:697–702. doi: 10.1682/jrrd.2009.01.0006. [DOI] [PubMed] [Google Scholar]
- 16.Clark ME, Bair MJ, Buckenmaier CC, Gironda RJ, Walker RL. Pain and combat injuries from soldiers returning from Operations Enduring Freedom and Iraqi Freedom: implications for research and practice. J Rehabil Res Dev. 2007;44:179–193. doi: 10.1682/jrrd.2006.05.0057. [DOI] [PubMed] [Google Scholar]
- 17.Clark ME, Walker RL, Gironda RJ, Scholten JD. Comparison of pain and emotional symptoms in soldiers with polytrauma: unique aspects of blast exposure. Pain Med. 2009;10:447–55. doi: 10.1111/j.1526-4637.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 18.French DD, Bair MJ, Bass E, Campbell RR, Siddharthan K. Central nervous system and musculoskeletal medication profile of a veteran cohort with blast-related injuries. J Rehabil Res Dev. 2009;46:463–8. doi: 10.1682/jrrd.2008.09.0017. [DOI] [PubMed] [Google Scholar]
- 19.Wu PC, Lang C, Hasson NK, Linder SH, Clark DJ. Opioid use in young veterans. J Opioid Manag. 2010;6:133–9. doi: 10.5055/jom.2010.0013. [DOI] [PubMed] [Google Scholar]
- 20.Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167:476–82. doi: 10.1001/archinte.167.5.476. [DOI] [PubMed] [Google Scholar]
- 21.Veterans Integrated Service. Network-20 Data Warehouse. Available from: http://moss.v20.med.va.gov/v20dw/default.aspx.
- 22.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 23.Veterans Health Administration. Pain as the 5th Vital Sign Toolkit, revised. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee; 2000. [Accessed on August 1, 2010]. http://www1.va.gov/pain_management/docs/TOOLKIT.pdf. [Google Scholar]
- 24.International Association for the Study of Pain. Classification of chronic pain. Pain. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 25.Cleeland CS, Reyes-Gibby CC, Schall M, Nolan K, Paice J, Rosenberg JM, Tollett JH, Kerns RD. Rapid improvement in pain management: the Veterans Health Administration and the institute for healthcare improvement collaborative. Clin J Pain. 2003;19:298–305. doi: 10.1097/00002508-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Veterans Administration-Department of Defense Clinical Practice Guideline Working Group, Veterans Health Administration, Department of Veterans Affairs and Health Affairs, Department of Defense (DoD) Management of Opioid Therapy for Chronic Pain. Washington, DC: Office of Quality and Performance; 2003. publication 10Q-CPG/OT-03 (Version 2.0) [Google Scholar]
- 27.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151:625–32. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skurtveit S, Furu K, Selmer R, Handal M, Tverdal A. Nicotine dependence predicts repeated use of prescribed opioids. Prospective population-based cohort study. Ann Epidemiol. 2010;20:890–7. doi: 10.1016/j.annepidem.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Deyo R, Smith D, Johnson ES, Donovan M, Tillotson CJ, Yang X, Petrik A, Dobscha SK. Opioids for patients with back pain in primary care: prescribing patterns and use of services. J Am Board Family Medicine. 2011 doi: 10.3122/jabfm.2011.06.100232. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–9. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 31.American Pain Society. The use of opioids for the treatment of chronic pain: A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6–8. [PubMed] [Google Scholar]
- 32.American Pain Society. Clinical Practice Guideline No 2. Glenview, IL: American Pain Society; 2002. Guideline for the management of pain in osteoarthritis, rheumatoid arthritis and juvenile onset arthritis. [Google Scholar]
- 33.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–75. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. NCHS Data Brief. 2009:1–8. [PubMed] [Google Scholar]
- 35.Krebs EE, Lurie JD, Fanciullo G, Tosteson TD, Blood EA, Carey TS, Weinstein JN. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain. 2010;11:44–52. doi: 10.1016/j.jpain.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Weingarten TN, Mantialla CB, Hooten WM, Warner DO. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113:977–92. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]