Abstract
The purpose of this study was to determine whether chronic cold exposure would increase the aerobic capacity of skeletal muscle in UCP-dta mice, a transgenic line lacking brown adipose tissue (BAT). Wild type and UCPdta mice were acclimated to either warm (23 °C), or cold (4 °C) conditions. Cold increased muscle oxidative capacity nearly equivalently in wild-type and UCP-dta mice, but did not affect the respiratory function of isolated mitochondria. Summit metabolism (V̇ O2summit) and norepinephrine-induced thermogenesis (V̇ O2NST) were significantly lower in UCP-dta mice relative to wild-type mice regardless of temperature treatment, but both were significantly higher in cold relative to warm acclimated mice. BAT mass was significantly higher in the cold relative to warm acclimated wild-type mice, but not in cold acclimated UCP-dta mice. BAT citrate synthase activity was lower in transgenic animals regardless of acclimation temperature and BAT citrate synthase activity per depot was significantly higher only in the cold acclimated wild-type mice. Muscle citrate synthase activity was increased in both genotypes. As defects in muscle oxidative function have been observed with obesity and type 2 diabetes, these results suggest that chronic cold exposure is a useful intervention to drive skeletal muscle oxidative capacity in mouse models of obesity.
Keywords: UCP-dta, Obesity, Shivering, Exercise training, Aerobic capacity
1. Introduction
Coping with environmental cold stress is an integral part of life for many animals living in temperate climates. Endothermic mammals typically respond to a cold challenge by increasing metabolic heat production in order to maintain body temperature within a narrow range (when physical mechanisms to reduce heat loss are insufficient). Shivering thermogenesis in skeletal muscle and nonshivering thermogenesis (NST) in brown adipose tissue (BAT) are the major recognized forms of thermogenesis in mammals (Heldmaier et al., 1989).
In small placental mammals, NST in BAT appears to be the primary mode of heat production during sustained cold exposure (Foster and Frydman, 1978). When activated by norepinephrine, uncoupling protein 1 (UCP1) in BAT mitochondria uncouples oxidative phosphorylation from ATP production by permitting protons to leak back into the mitochondrial matrix from the inner-membrane space. This uncoupling results in a high rate of substrate oxidation and the liberation of heat in the absence of ATP synthesis. While constituting only a small percentage of body mass, BAT can account for up to 50% of whole body oxygen consumption during maximal activation with norepinephrine (Foster and Frydman, 1978).
The importance of BAT in maintaining metabolic homeostasis was demonstrated with creation of the UCP-dta mouse, in which the insertion of a transgene partially ablated the tissue and created animals that become hyperphagic, insulin resistant, and obese (Lowell et al., 1993). Despite the reduction in BAT, the UCP-dta mouse does not appear to be intolerant of short term cold exposure (Lowell et al., 1993; Melnyk and Himms-Hagen, 1998), suggesting that skeletal muscle thermogenesis may compensate for the deficiency of BAT, similar to that described in the UCP1 null mouse, another model of BAT loss of function (Golozoubova et al., 2001). The potential importance of adaptive changes in muscle properties in response to thermogenesis is seen in placental mammals larger than 10 kg and marsupials, for whom non-shivering thermogenesis is lacking in the response to a cold challenge (see comments following Heldmaier, 1971; Hayward and Lisson, 1992). Several studies have shown that sustained cold exposure causes metabolic changes in skeletal muscle similar to those observed following endurance exercise training (Hong et al., 1987; Schaeffer et al., 2001, 2003).
The aims of this study were to determine if UCP-dta mice are capable of increasing thermogenic capacity and to determine the role of skeletal muscle in thermogenesis during chronic cold exposure. We used a multi-level approach including whole animal, tissue, and cellular metabolic measurements to address the following questions: Does shivering thermogenesis compensate for the BAT deficit and enhance muscle oxidative capacity in UCP-dta mice? Does cold exposure augment muscle mitochondrial respiration and fatty acid oxidation in wild-type or UCP-dta mice? Finally, does BAT hypertrophy result in an increase in NST capacity in cold exposed UCP-dta mice?
2. Materials and methods
2.1. Experimental animals
Wild type and UCP-dta mice (Jackson Laboratories, Bar Harbor, ME, USA), a transgenic mouse line in which the diphtheria toxin A chain is under control of the UCP-1 promoter leading to partial ablation of BAT (Lowell et al., 1993), were weaned at 21 days of age and divided into either warm (23±1 °C), or cold (4±1 °C) acclimation groups in the animal facility at Miami University. The warm acclimated (WA) group was housed in the standard animal facility at 23±1 °C until 13 weeks±1 of age. At weaning, the cold acclimated (CA) group was transferred to a controlled temperature chamber at 28 °C, and the temperature of the chamber was progressively decreased 2 °C/ day. Once the environmental chamber reached 4 °C, the animals remained at this temperature (~7 weeks) until reaching 13±1 weeks of age. All animals were housed in groups of 2–4 individuals with a 12:12-h light-dark cycle and provided with water and standard rodent chow (62% carbohydrates: 25% protein: 13% fat; PMI Nutritional International, Brentwood, MO, USA) ad libitum. The body mass of each group was measured at 13±1 weeks when experiments were performed. In all cases, we did not use animals with body weight greater than 36 g in order to prevent confounding effects of obesity and metabolic pathology (two individuals from the warm acclimated transgenic group were excluded). All animal experimentation was approved by the Institutional Animal Care and Use Committee of Miami University and complied with the “Principles of Animal Care,” publication no. 86-23, revised 1985, of the National Institutes of Health as well as the laws of the United States.
2.2. Summit V̇ O2 (V̇ O2summit)
The total thermogenic capacity (V̇ O2summit—the maximal metabolic rate measured during an acute cold challenge) was measured by exposing an animal to 4 °C in a heliox gas atmosphere in which high thermal conductance acts to facilitate heat loss (Rosenmann and Morrison, 1974). V̇ O2summit was measured via open flow respirometry (Sable Systems, Las Vegas NV, USA). Animals were placed into a 1.0 L chamber with gas flow rates of 1.3 L/min and oxygen consumption was measured. All animals were weighed prior to measuring metabolic rate. Upon placement into the chamber, the animals remained at room temperature for 10–15 min until oxygen consumption reached a steady state. Then, the chamber was placed in the refrigerator (4 °C) until the animals achieved steady state or began to exhibit hypothermia as indicated by falling levels of CO2 production (typically between 12 and 15 min). The highest rates of oxygen consumption over a 1-min interval observed during the acute cold challenge were used for our analysis.
2.3. Norepinephrine induced NST
NST capacity (V̇ O2NST) represents the contribution of BAT thermogenesis to the animal's total thermogenic capacity. The ablation of BAT in the UCP-dta mouse is not complete and some functional BAT remains. Therefore we measured V̇ O2NST via open flow respirometry at 22–24 °C (Sable Systems, Las Vegas NV) following an injection of norepinephrine in order to determine if cold acclimation alters the contribution of BAT to thermogenic capacity in UCP-dta mice. Prior to measurements, the animals were weighed and then placed into the 1.0 L respirometry chamber. Room gas flow was 0.9 L/min. Oxygen consumption was measured for 30 min to allow the animals to acclimate to the chamber. At that point, the animals were removed, given an intramuscular injection of saline, and returned to the chamber for another 30 min. At the end of the second 30-min period, the animals were given an intramuscular injection of norepinephrine and returned to the chamber for another 30 min. The dose of NE was determined from the following equation from Wunder and Gettinger (1996):
NEdose = 2.53 × Mb−0:4
where NE dose is the mg of NE administered/kg of body mass, and Mb is the mass (g) of the animal. The doses administered ranged from ~18 to 23 µg. Within 4 min of the NE injection, the animals became very inactive in the chamber. To ensure that any activity did not augment the measurement of oxygen consumption following the NE injection, NST capacity was determined by sampling the highest rates of oxygen consumption over a 1-min interval while the animals remained inactive. All oxygen consumption measurements were obtained within 10 min of the injection of NE.
2.4. Tissue enzyme activity
For tissue collection, animals were euthanized via CO2 asphyxiation followed by cervical dislocation. Immediately following euthanasia, the interscapular brown fat pad, triceps muscle and the left ventricle were removed from the animal and weighed in order to determine if cardiac and/or BAT hypertrophy occur during cold acclimation in UCP-dta mice.
To investigate whether the aerobic challenge associated with cold exposure increased the oxidative capacity of the tissues responsible for heat production, we measured citrate synthase activity of skeletal muscle and BAT in wild type and UCP-dta mice. Immediately following the weighing of BAT and triceps muscles, samples were frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis of oxidative capacity using a citrate synthase (EC 2.3.3.1) assay modified from Chi et al. (1983). The samples were diluted (1:20 volumes for triceps muscle; 1:40 volumes for BAT) in homogenization medium (50 mM Tris, 0.15 M KCl, pH=7.4), homogenized, and centrifuged at 1000g at 4 °C for 10 min. The resultant supernatant was frozen in liquid nitrogen and stored at –80 °C. Maximal citrate synthase activity was determined spectrophotometrically at 25 °C by measuring the rate of disappearance of acetyl-CoA at 232 nm in assay reagent (100 mM Tris, pH 8.1) with excess acetyl CoA (0.2 mM) and oxaloacetate (0.17 mM) over a 4-min interval.
2.5. Mitochondrial isolation and respiration
We measured oxygen consumption of mitochondria isolated from total hindlimb musculature to determine whether the aerobic demand on the muscle during cold acclimation affects the capacity of mitochondria to oxidize fuels derived from glucose or lipids. The entire musculature was dissected from the whole hind limbs of mice, trimmed of fat and connective tissue, and minced on a cold glass dish in isolation medium (0.1 M KCl, 0.05 M Tris–HCl, 2 mM EGTA, pH 7.4 at 4 °C). The minced tissue was digested for 3 min in digestion medium (0.1 M KCl, 0.05 M Tris–HCl, 2 mM EGTA, 0.5% BSA, 5 mM MgCl2, 1 mM ATP, and 245.7 units/100 mL of protease type VIII, pH 7.4 at 4 °C) and homogenized with 4–6 passes in an Eberbach glass and Teflon homogenizer (Eberbach Corp., Ann Arbor, MI, USA). The homogenate was centrifuged at 490g for 10 min. The resultant supernatant was removed and centrifuged for 10 min at 10,368g. The supernatant was separated and the mitochondrial pellet was washed and re-suspended in isolation media before another 10 min (10,368g) centrifugation. The resultant supernatant was removed and the mitochondrial pellet was re-suspended in isolation media and centrifuged for 10 min (3,841g). The supernatant was removed and the final mitochondrial pellet was re-suspended in isolation media at a concentration of 20–30 µg of protein per µL of respiration media. The concentration of the isolated mitochondrial fraction was determined using the Lowry method for protein determination (Lowry et al., 1951). All isolation steps were carried out on ice and centrifugations at 4 °C.
Mitochondrial respiration was measured using a fiber optic oxygen sensor (Neofox: Ocean Optics, Dunedin, FL, USA) at 37 °C by adding 0.0875 mg of mitochondrial protein to 250 µL of respiration buffer (125 mM KCl, 20 mM HEPES, 3 mM Mg-acetate, 0.4 mM EGTA, 0.3 mM DTT, 5 mM KH2PO4, 2 mg/mL BSA, 5 mM malate, pH 7.1) in the presence of either 10 mM pyruvate or 20 mM palmitoylcarnitine. Maximal substrate oxidation in the presence of ADP and substrate (i.e. state 3 respiration) was measured following the addition of 8 µL ADP (250 mM) to the incubating assay buffer. Oxygen consumption in the absence of ATP synthesis reflecting the maintenance of the proton motive force (i.e. state 4 respiration) was measured following the addition of 8 µL of oligomycin (1 µg/mL), an inhibitor of the F0-F1 ATP synthase.
2.6. Statistical analysis
Group differences in body mass, V̇ O2, citrate synthase activity, BAT mass, left ventricle mass, and mitochondrial respiration were analyzed using a two-way analysis of variance (ANOVA) with genotype and temperature as factors using JMP statistical software (version 8.0.1). Outcomes of the ANOVA for all results are presented in Table 1. Pairwise comparisons are reported in the running text of the results section. When significant differences were detected in parameters, pairwise comparisons were run using the Tukey HSD method. The level of significance was set at p<0.05. Data are presented as means ± S.E.M. (n).
Table 1.
Outcomes of analysis of variance (p-values) for all presented variables.
| Genotype | Temperature | Interaction | |
|---|---|---|---|
| Body mass | 0.18 | 0.09 | 0.63 |
| Left ventricle mass | 0.18 | <0.001 | 0.36 |
| V̇ O2summit | <0.001 | <0.001 | 0.50 |
| Muscle CS activity | 0.20 | <0.01 | 0.37 |
| Mitochondrial respiration | |||
| - State 3 (pyruvate) | 0.30 | 0.67 | 0.85 |
| - State 4 (pyruvate) | 0.90 | 0.55 | 0.27 |
| - State 3 (palmitate) | 0.88 | 0.63 | 0.99 |
| - State 4 (palmitate) | 0.47 | 0.09 | 0.86 |
| V̇ O2NST | |||
| - Rest | 0.08 | 0.18 | 0.49 |
| - Saline | 0.20 | 0.32 | 0.10 |
| - Norepinephrine | <0.001 | <0.001 | 0.87 |
| BAT mass | <0.01 | <0.001 | 0.11 |
| BAT CS activity | <0.05 | 0.75 | 0.67 |
| BAT depot CS activity | <0.001 | <0.001 | <0.05 |
3. Results
3.1. Body mass
There were no significant effects of genotype or acclimation temperature on the body mass (Table 1) of the animals used in these experiments. Body mass of the WA wild-type mice was 29.2±0.5 g (21), of the WA UCP-dta mice was 29.6±0.6 g (14), of the CA wildtype was 29.8±0.5 g (19), and of the CA UCP-dta mice was 30.9± 0.5 g (20). Therefore, we are confident that subsequent comparisons between groups are not confounded by body mass or the development of obesity.
3.2. Heart mass
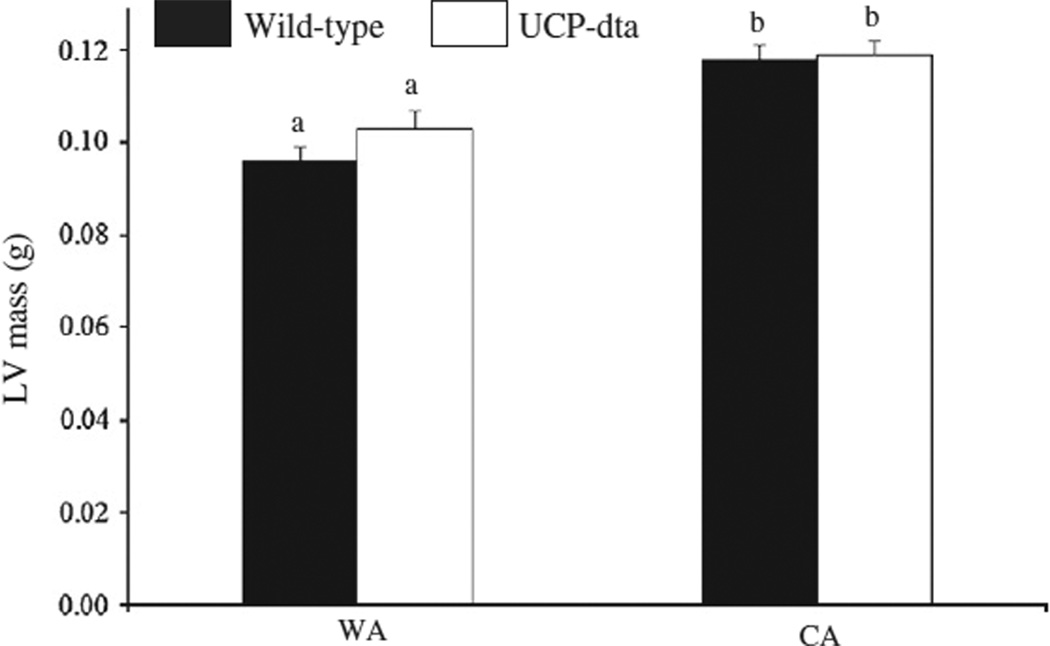
Acclimation temperature had a significant effect (Table 1) on LV mass in wild type and UCP-dta mice (Fig. 1) as LV mass was higher in CA wild type mice (p<0.001) and UCP-dta mice (p<0.05) relative to their WA counterparts. There were no significant differences in LV mass between the genotypes within each temperature acclimation group. These results suggest that cold challenge generated sufficient energetic demand to induce cardiac hypertrophy in wild type and UCP-dta mice.
Fig. 1.
Left ventricle (LV) mass was higher in cold acclimated wild type and UCP-dta mice. Within each temperature treatment, LV mass was not significantly different between genotypes; however the chronic demand of cold exposure caused significantly higher LV mass in both wild-type and UCP-dta mice. Values are means ± S.E.M. (WA wild type, n = 8; WA UCP-dta, n = 7; CA wild type, n = 9; CA UCP-dta, n = 10) and different superscripted letters indicate significant pairwise differences between groups.
3.3. Summit V̇ O2
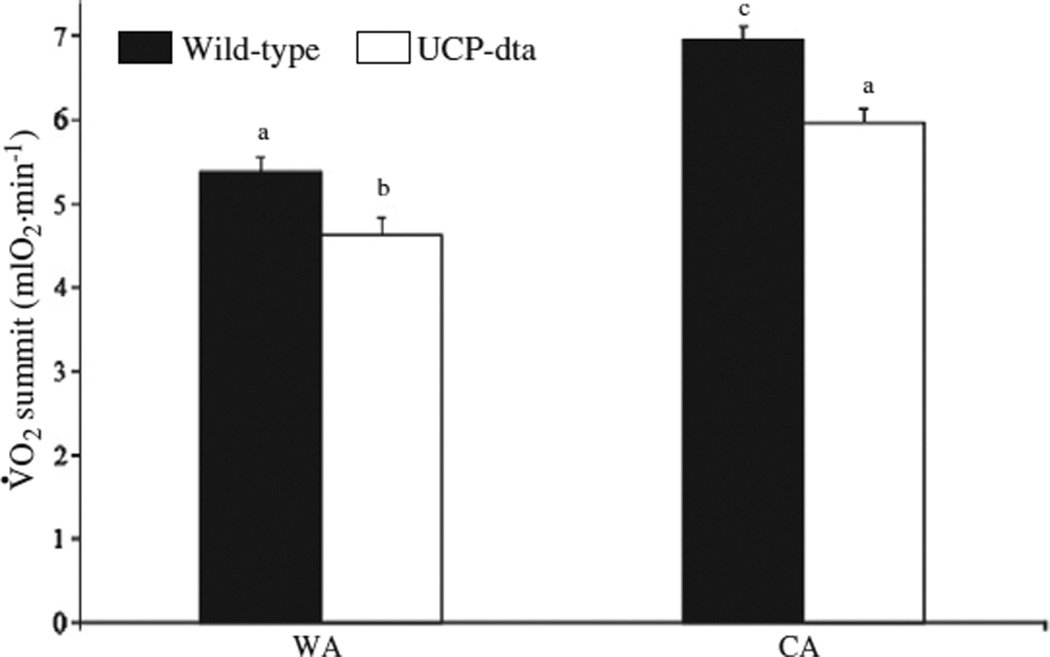
Genotype and acclimation temperature both significantly (Table 1) affected V̇ O2summit (Fig. 2). Within temperature acclimation groups, V̇ O2summit was lower in UCP-dta mice relative to wild type mice (WA p<0.05, CA p<0.01). In addition, V̇ O2summit was higher in CA wild type and UCP-dta mice relative to their WA counterparts (wild type p<0.001, UCP-dta p<0.001). These results suggest that, although the extent to which UCP-dta mice increase their V̇ O2summit (28%) is similar to wild-type mice (29%), thermogenic capacity is still compromised in CA UCP-dta mice.
Fig. 2.
Thermogenic capacity was higher in cold acclimated wild type and UCP-dta mice. Summit metabolism was measured in 12–13 week old warm acclimated and cold acclimated wild type and UCP-dta mice. V̇ O2summit of wild type mice was significantly higher than UCP-dta mice within both temperature regimes. V̇ O2summit was also significantly higher in cold acclimated wild type and UCP-dta mice relative to their warm acclimated counterparts. Values are means ± S.E.M. (WA wild type, n = 9; WA UCP-dta, n = 6; CA wild type, n = 10; CA UCP-dta, n = 9) and different superscripted letters indicate significant pairwise differences between groups.
3.4. Muscle citrate synthase activity
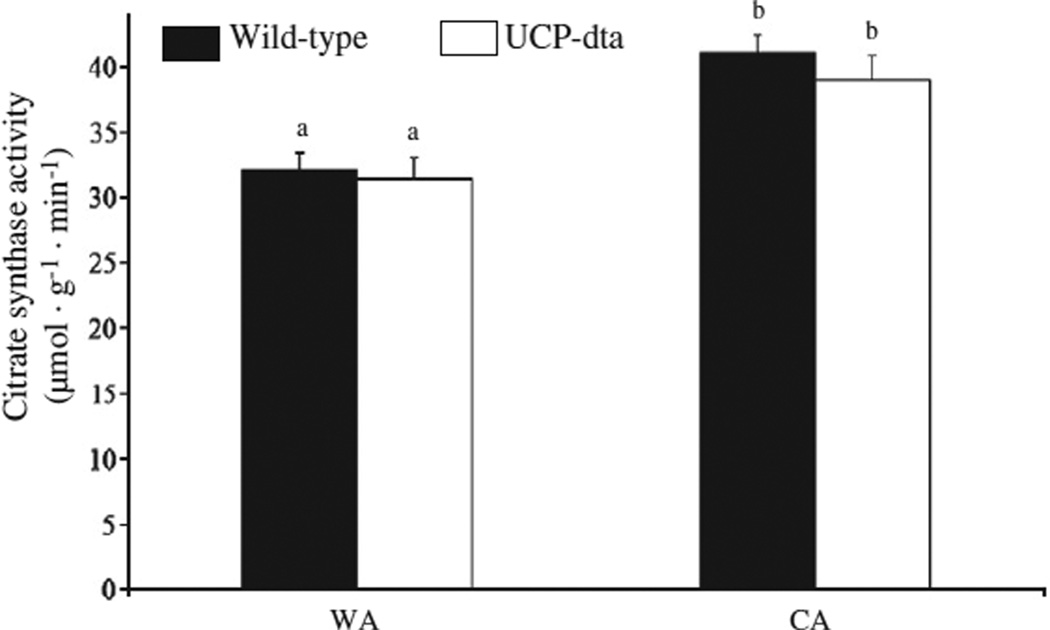
Cold exposure significantly (Table 1) increased citrate synthase (CS) activity per gram wet mass in the triceps muscle of both wildtype and UCP-dta mice (Fig. 3), as CS activity per gram wet mass was higher in CA wild type mice (p<0.01) and CA UCP-dta mice (p<0.05) relative to their WA counterparts. Cold exposure increased citrate synthase activity per gram wet mass to a similar extent in UCP-dta and in wild type mice.
Fig. 3.
Muscle citrate synthase activity was higher in cold acclimated wild-type and UCP-dta mice. Citrate synthase activity in triceps muscle from 12 to 13 week old mice was higher following cold acclimation in both genotypes, but there were no significant differences due to genotype within either warm or cold acclimation groups. Values are means ± S.E.M. (WA wild type, n = 8; WA UCP-dta, n = 8; CA wild type, n = 8; CA UCP-dta, n = 6) and different superscripted letters indicate significant pairwise differences between groups.
3.5. Muscle mitochondrial respiration
There were no significant effects (Table 1) of temperature or genotype on state 3 or state 4 respiration of isolated skeletal muscle mitochondria when respiring with either pyruvate or palmitoyl-carnitine (Table 2). Therefore, we conclude that neither genotype nor cold acclimation altered the respiratory capacity of isolated mitochondria, whether oxidizing glucose or lipid derived fuels.
Table 2.
There was no effect of either BAT ablation or cold acclimation on State 3 or State 4 respiration in mitochondria isolated from hindlimb muscle of 12–13 week old mice whether the respiratory substrate was pyruvate + malate or palmitoyl-carnitine + malate. Values are means ± S.E.M. (n).
| Pyruvate |
Palmitate |
|||
|---|---|---|---|---|
| State 3 (nmol O2 min−1 mg protein−1) |
State 4 (nmol O2 min−1 mg protein−1) |
State 3 (nmol O2 min−1 mg protein−1) |
State 4 (nmol O2 min−1 mg protein−1) |
|
| Warm | acclimated | |||
| Wild-type | 395±25 (6) | 35±7 | 154±8 (5) | 31±4 |
| UCP-dta | 424±15 (5) | 40±6 | 152±7 (5) | 28±3 |
| Cold | acclimated | |||
| Wild-type | 390±25 (9) | 43±4 | 159±9 (5) | 38±5 |
| UCP-dta | 410±18 (8) | 37±3 | 157±13 (5) | 36±4 |
3.6. NST capacity
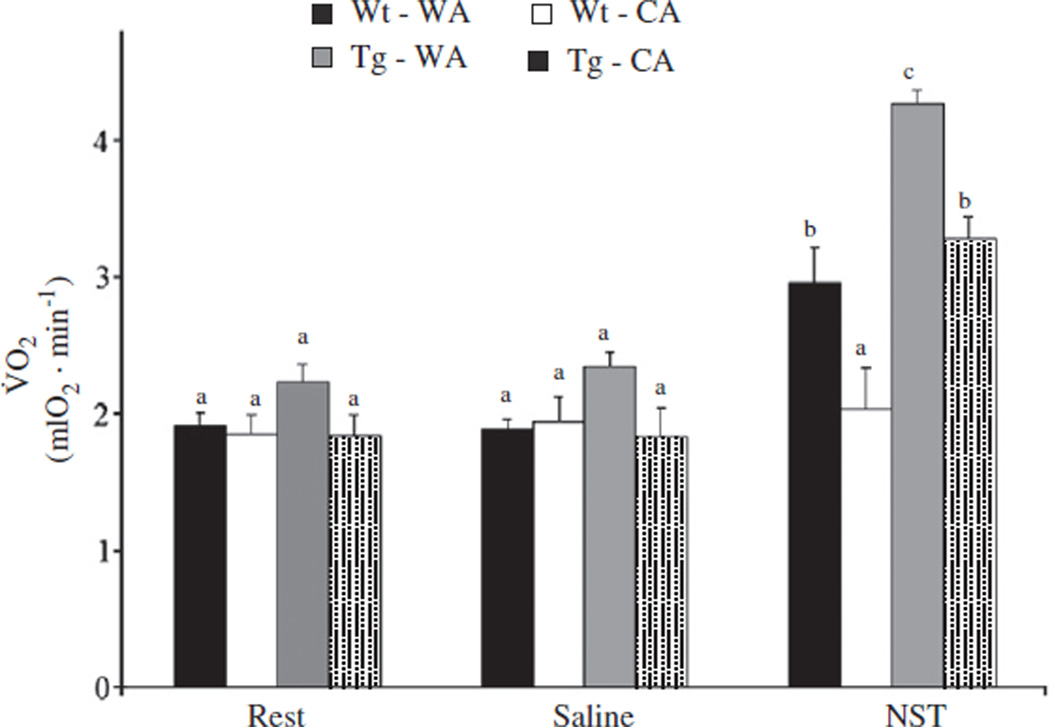
Cold exposure and genotype significantly (Table 1) affected V̇ O2NST (Fig. 4; see Supplemental Fig. 1 for an example trace). V̇ O2 at rest or following saline injection did not differ between groups (Table 1), suggesting that the majority of the group differences in V̇ O2NST are due to the response to NE injection. Within temperature acclimation groups, V̇ O2NST was significantly lower in UCP-dta mice relative to wild type mice (WA p<0.01, CA p<0.01). However, V̇ O2NST was higher in CA UCP-dta mice (p<0.001) and wild type mice (p<0.001) relative to their warm acclimated counterparts, suggesting that BAT hypertrophy increased NST capacity in CA UCP-dta mice despite partial BAT ablation. While cold acclimation is accompanied by an increase in the functional capacity of BAT, like V̇ O2summit, NST capacity is still partially compromised following cold exposure in these mice.
Fig. 4.
Oxygen consumption is measured at rest, following a saline injection and after norepinephrine injection. V̇ O2NST was not different between groups at rest or after saline, or between rest and saline injection. However, V̇ O2NST was significantly lower in 12–13 week old UCP-dta mice than wild-type mice within both temperature regimes. Norepinephrine injection caused oxygen consumption to increase over rest or saline in all groups except the warm acclimated UCP-dta mice. V̇ O2NST was significantly higher in CA wild type mice than WA wild type mice and in CA UCP-dta mice compared to WA UCP-dta mice. Values are means ± S.E.M. (WA wild type, n = 5; WA UCP-dta, n = 5; CA wild type, n = 5; CA UCP-dta, n = 6) and different superscripted letters indicate significant pairwise differences between groups.
3.7. BAT citrate synthase activity
Acclimation temperature and genotype significantly (Table 1) affected BAT mass (Table 3). While there was no significant pairwise difference between the BAT mass of wild type and UCP-dta mice within the WA group, BAT mass was significantly higher in CA wild type mice relative to the CA UCP-dta mice (p<0.05). Furthermore, BAT mass was higher in both CA wild type (p<0.001) and UCP-dta mice (p<0.05) relative to their WA counterparts, suggesting that cold exposure does cause BAT hypertrophy in UCP-dta mice despite the partial ablation of the tissue. Genotype significantly (Table 1) affected the BAT citrate synthase activity per gram of tissue (Table 3) such that BAT in the UCP-dta mice had lower oxidative capacity, although pairwise comparisons did not reveal significant differences between groups. When CS activity is expressed as total activity of the entire interscapular BAT depot (representing the whole tissue NST capacity), there was a significant effect (Table 1) of genotype and acclimation temperature on CS activity/depot, and a significant interaction between genotype and temperature (Table 3). Pairwise comparisons revealed that BAT CS activity/depot in the CA wild type mice was significantly higher than the other groups (p<0.001). Thus, cold acclimation increases the CS activity of BAT via tissue hypertrophy in the wild type mice, and while similar results were observed in UCP-dta mice, these did not reach statistical significance.
Table 3.
Main effects indicate that BAT mass is higher in cold acclimated mice while BAT citrate synthase activity is lower in UCP-dta mice. When expressed as activity of the whole interscapular BAT depot, cold acclimated wild type mice had higher tissue oxidative capacity.
| Warm acclimated | Cold acclimated | |||
|---|---|---|---|---|
| Wild type (8) | UCP-dta (5) | Wild type (8) | UCP-dta (7) | |
| BAT mass * † (g) | 0.098±0.012a,c | 0.099±0.013a | 0.177±0.012b | 0.1412±0.012c |
| CS activity * (µmol · g−1 · min−1) | 337.3±19.1 | 266.3±26.1 | 340.0±28.0 | 252.2±20.0 |
| CS activity/depot * † (µmol · depot−1 · min−1) | 32.3±1.4a | 25.3±2.9a | 59.3±5.2b | 34.0±2.3a |
Values are means ± S.E.M. (n).
* and † indicate a significant main effect of genotype and temperature acclimation, respectively. Different superscripted letters indicate significant pairwise differences between groups.
4. Discussion
This is the first report describing long-term cold exposure of UCPdta mice. It has previously been demonstrated that despite the partial ablation of BAT and a reduction in NST capacity, UCP-dta mice can tolerate a short-term cold exposure (Lowell et al., 1993; Melnyk and Himms-Hagen, 1998). Our study extends these previous observations by demonstrating that UCP-dta mice can tolerate a prolonged cold challenge (~7 weeks at 4 °C).
To tolerate such a prolonged cold challenge, elevation of total thermogenic capacity can occur through responses of several components. Total thermogenic capacity (V̇ O2summit) is calculated as themetabolic sum of basal metabolic rate (BMR), NST, and shivering thermogenesis (ST) such that thermogenic capacity = BMR + NST + ST (Wunder and Gettinger, 1996; Van Sant and Hammond, 2008). The role of BAT in NST has beenwell established (Foster and Frydman, 1978) and not surprisingly, we observed a significant reduction in NST capacity in theWA UCP-dtamouse similar to that reported by Lowell et al. (1993). However, BAT ablation is incomplete in the UCP-dta mouse and we observed BAT hypertrophy following cold acclimation. Our results demonstrate that cold induced BAT hypertrophy in UCP-dta mice is incomplete, and cold acclimated UCP-dta mice show impairment of both V̇ O2summit and V̇ O2NST. Thus, they are not able to attain the level of thermogenic activity observed in the cold exposed wild type mice. Given the lack of difference of resting metabolic rate during this experiment, it is unlikely that changes in BMR had a large effect on oxygen consumption following NE injection; however its contribution to our observed effect is unknown.
Given the impairment of BAT function in these animals, we anticipated that increased muscle metabolic capacity would compensate via enhanced capacity for shivering thermogenesis. The effects of cold exposure on skeletal muscle aerobic capacity in larger animals that rely primarily on shivering are similar to those observed following endurance exercise training, profoundly increasing whole animal aerobic capacity, muscle oxidative capacity, and causing a shift towards fatty acids as the preferred metabolic substrate of skeletal muscle (Hong et al., 1987, Schaeffer et al., 2001, 2003). Golozoubova et al. (2001) demonstrated that the UCP1 knockout mice (a mouse with complete elimination of NST capacity in BAT) shiver when exposed to cold regardless of whether or not they are cold acclimated. In contrast, they found that shivering disappeared in wild type mice that were previously exposed to the cold indicating that NST replaces shivering in cold acclimated wild type mice. Meyer et al. (2010) demonstrated evidence of a myosin heavy chain shift towards a slower, more oxidative muscle fiber type in both cold acclimated wild type and UCP 1 knockout mice, indicating a role for skeletal muscle shivering thermogenesis in mice. Similarly, in our study, cold exposure increased citrate synthase activity in both wild type and UCP-dta mice. The UCP-dta mice thus resemble the UCP1-knockout mice, further supporting that cold acclimation increases skeletal muscle aerobic capacity. Of further interest, in both studies, wild-type mice responded similarly to the genetically manipulated mice. Because the wild type mice are expected to generate heat primarily through NST in BAT, higher muscle citrate synthase activity in the cold exposed wild type mice is puzzling. However, increases in ST and NST capacity have been demonstrated in some species of mice (Nespolo et al., 1999), and thus it is possible that these animals require both ST and NST for heat production at very low temperatures, although Golozoubova et al. (2001) described a lack of shivering in wild-type mice acclimated to 4 °C. Because the increase in citrate synthase activity was similar between the two genotypes, and thermogenic capacity was still compromised due to the lower NST capacity of cold exposed UCP-dta mice, it appears that muscle cannot fully compensate for the BAT deficit. Alternatively, muscle detraining in the wildtype mice may have not yet occurred at the time point when we examined the muscle phenotype.
Engagement of skeletal muscle in thermogenesis may reflect the greater demands of an animal with small body size. Investigations into the extent of muscle training during cold exposure in other animals with BAT have produced equivocal results. For instance, some studies demonstrate no effect of cold exposure on muscle oxidative capacity, while others demonstrate a training effect (Marsh, 1989). Despite the increase in muscle citrate synthase activity, cold acclimation did not affect mitochondrial oxidative capacity, similar to the observations in UCP1 knockout mice (Meyer et al., 2010). Skeletal muscle mitochondrial volume density is generally considered to be quantitatively matched to demand (Hoppeler and Lindstedt, 1985); however, see Lumini-Oliveira et al. (2009) who report that endurance training also increases the maximal (state 3) respiration rate of isolated muscle mitochondria. Our data suggest that shivering activity increases muscle oxidative capacity by increasing mitochondrial content of the tissue rather than the respiratory capacity of the individual mitochondria.
The UCP-dta mouse was developed as a model of obesity and type 2 diabetes (Lowell et al., 1993). Consequences of both diseases are reported to include impairment of muscle oxidative capacity (Kelley et al., 2002; Shen et al., 2004). We saw no evidence of a deficit in muscle oxidative capacity in this study; however the warm acclimated UCP-dta used in this study were not obese and may not have yet developed muscle metabolic pathology. As exercise is not a viable intervention for obese organisms, we wanted to investigate the role of muscle thermogenesis in cold acclimated UCP-dta mice in order to determine if cold exposure can be used as a stimulus of muscle activity in an obese mouse model. The full extent of the response of skeletal muscle to cold acclimation remains to be elucidated; however these data suggest that shivering thermogenesis is an important component of the response.
5. Conclusion
Despite their compromised thermogenic capacity due to the partial ablation of BAT, increases in muscle oxidative capacity and BAT hypertrophy are sufficient to allow UCP-dta mice to survive a prolonged cold challenge. Furthermore, the energetic challenge associated with cold exposure caused an increase in left ventricular mass suggestive of exercise-induced cardiac hypertrophy, demonstrating the magnitude of the energetic demand of chronic cold exposure. The energetic challenge of chronic cold exposure thus represents a powerful model for the study of the role of metabolic activity in obesity and metabolic syndrome as obese mice that may be exercise intolerant will nonetheless defend body temperature in response to a cold challenge. Furthermore, as also observed by Meyer et al. (2010) these data also suggest that, even in wild type mice, muscle thermogenesis may be a component of acclimation to cold temperatures.
Supplementary Material
Acknowledgements
We thank Drs. Richard E. Lee Jr. and Jon P. Costanzo as well as several anonymous reviewers for critical reading of the manuscript. P.J.S. received support from a Madelene and George Shetler Diabetes Research Award and award number R15DK085497 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Abbreviations
- WA
warm acclimated
- CA
cold acclimated
- NST
nonshivering thermogenesis
Footnotes
Supplementary materials related to this article can be found online at doi: 10.1016/j.cbpa.2011.12.012.
References
- Chi MMY, Hintz CS, Coyle EF, Martin WH, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am. J. Physiol. 1983;244:C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- Foster DO, Frydman ML. Nonshivering thermogenesis in the rat 2. Measurements of blood-flow with microspheres point to brown adipose tissue as dominant site of calorigenesis induced by noradrenaline. Can. J. Physiol. Pharmacol. 1978;56:110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- Hayward JS, Lisson PA. Evolution of brown fat: its absence in marsupials and monotremes. Can. J. Zool. 1992;70:171–179. [Google Scholar]
- Heldmaier G. Relationship between nonshivering thermogenesis and body size. In: Jansky L, editor. Non-shivering Thermogenesis. Prague: Academia; 1971. pp. 73–81. [Google Scholar]
- Heldmaier G, Klaus S, Wiesinger H, Friedrichs U, Wenzel M. Cold acclimation and thermogenesis. In: Malan A, Canguilhem B, editors. Living in the Cold: 2nd International Symposium. London: John Libbey; 1989. pp. 347–358. [Google Scholar]
- Hong SK, Rennie DW, Park YS. Humans can acclimatize to cold: a lesson from Korean women divers. News Physiol. Sci. 1987;2:79–82. [Google Scholar]
- Hoppeler H, Lindstedt SL. Malleability of skeletal muscle in overcoming limitations: structural elements. J. Exp. Biol. 1985;115:355–364. doi: 10.1242/jeb.115.1.355. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himmshagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lumini-Oliveira J, Magalhaes J, Pereira CV, Aleixo I, Oliveira PJ, Ascensao A. Endurance training improves gastrocnemius mitochondrial function despite increased susceptibility to permeability transition. Mitochondrion. 2009;9:454–462. doi: 10.1016/j.mito.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Marsh R. Skeletal muscle in cold adaptation of endotherms. In: Malan A, Canguilhem B, editors. Living in the Cold: 2nd International Symposium. London: John Libbey; 1989. pp. 205–215. [Google Scholar]
- Melnyk A, Himms-Hagen J. Temperature-dependent feeding: lack of role for leptin and defect in brown adipose tissue ablated obese mice. Am. J. Physiol. 1998;274:R1131–R1135. doi: 10.1152/ajpregu.1998.274.4.R1131. [DOI] [PubMed] [Google Scholar]
- Meyer CW, Willershauser M, Jastroch M, Rourke BC, Fromme T, Oelkrug R, Heldmaier G, Klingenspor M. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am. J. Physiol. 2010;299:R1396–R1406. doi: 10.1152/ajpregu.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespolo RF, Opazo JC, Rosenmann M, Bozinovic F. Thermal acclimation, maximum metabolic rate, and nonshivering thermogenesis of Phyllotis xanthopygus (Rodentia) in the Andes mountains. J. Mammal. 1999;80:742–748. [Google Scholar]
- Rosenmann M, Morrison P. Maximum oxygen consumption and heat loss facilitation in small homeotherms by He–O2. Am. J. Physiol. 1974;226:490–495. doi: 10.1152/ajplegacy.1974.226.3.490. [DOI] [PubMed] [Google Scholar]
- Schaeffer PJ, Hokanson JF, Wells DJ, Lindstedt SL. Cold exposure increases running VO2max and cost of transport in goats. Am. J. Physiol. 2001;280:R42–R47. doi: 10.1152/ajpregu.2001.280.1.R42. [DOI] [PubMed] [Google Scholar]
- Schaeffer PJ, Villarin JJ, Lindstedt SL. Chronic cold exposure increases skeletal muscle oxidative structure and function in Monodelphis domestica, a marsupial lacking brown adipose tissue. Physiol. Biochem. Zool. 2003;76:877–887. doi: 10.1086/378916. [DOI] [PubMed] [Google Scholar]
- Shen X, Zheng SR, Thongboonkerd V, Xu M, Pierce WM, Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am. J. Physiol. 2004;287:E896–E905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- Van Sant MJ, Hammond KA. Contribution of shivering and nonshivering thermogenesis to thermogenic capacity for the deer mouse (Peromyscus maniculatus) Physiol. Biochem. Zool. 2008;81:605–611. doi: 10.1086/588175. [DOI] [PubMed] [Google Scholar]
- Wunder B, Gettinger R. Effects of body mass and temperature acclimation on the nonshivering thermogenic response of small mammals. In: Geiser F, Hulbert A, Nicol S, editors. Adaptations to the Cold: Tenth International Hibernation Symposium. Armidale: University of New England Press; 1996. pp. 131–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.