Abstract
Background
There is limited high quality evidence regarding the impact of patient navigation (PN) on outcomes for patients with diagnosed cancer.
Methods
We pooled data from two sites from the national Patient Navigation Research Program (PNRP). Patients (n=438) with newly diagnosed breast (n=353) or colorectal cancer (n=85) were randomized to PN or usual care. Trained lay navigators met with patients randomized to PN to help them assess treatment barriers and identify resources to overcome barriers. We used intent-to-treat analysis to assess time to completion of primary treatment, psychological distress (Impact of Events Scale) and satisfaction (Patient Satisfaction with Cancer-Related Care) within three months after initiation of cancer treatment.
Results
The sample was predominantly middle-aged (mean age=57) and female (90%); 44% were race-ethnic minorities (44%), 46% reported lower education levels, 18% were uninsured and 9% reported a non-English primary language. The randomized groups were comparable in baseline characteristics. Primary analysis showed no statistically significant group differences in time to completion of primary cancer treatment, satisfaction with cancer-related care, or psychological distress. Subgroup analysis showed that socially disadvantaged patients (i.e. uninsured, low English proficiency and non-English primary language) who received PN reported higher satisfaction than those receiving usual care (all ps < 0.05). Navigated patients living alone reported greater distress than those receiving usual care.
Conclusions
Although the primary analysis showed no overall benefit, the subgroup analysis suggests that PN may improve satisfaction with care for certain disadvantaged individuals.
Impact
PN for cancer patients may not necessarily reduce treatment time nor distress.
Keywords: Community Health Workers, Breast Cancer, Colorectal Cancer, Healthcare Disparities, Medical Oncology
INTRODUCTION
Patient navigation (PN), defined as instrumental and emotional support for patients during diagnosis and treatment for cancer, has been widely promoted as a means for reducing cancer health disparities (1). Patient navigators identify and address barriers to accessing timely and effective cancer treatment (2). Findings from randomized controlled trials show that PN improves rates of cancer screening (3–9). Most randomized trials of PN for follow-up of abnormal cancer screening show benefit in terms of diagnostic resolution (10–13). In contrast, there are limited data regarding the effectiveness of PN to optimize treatment of patients with diagnosed cancer. In the only published randomized trial of post-cancer diagnosis navigation, Ell et al reported no difference in adjuvant treatment adherence and follow-up from telephone navigation compared to written informational materials for low-income women undergoing treatment for breast or gynecologic cancer (14).
As part of the National Cancer Institute (NCI)-sponsored Patient Navigation Research Program (PNRP), two of the 10 sites conducted patient-level randomized trials of PN among patients with recently diagnosed breast or colorectal cancer. We pooled data from these two sites (Denver, CO and Rochester, NY) to examine the hypothesized effect of PN: 1) shorter time to treatment completion; 2) less psychological distress; and 3) greater satisfaction with cancer care. We also expected that patients with inadequate or no insurance, language barriers, lower education, and lower income, would show greater benefit from PN than those with fewer needs.
MATERIALS AND METHODS
Details regarding the Rochester protocol have been previously reported (15). We briefly summarize them along with those from the Denver site.
Setting and study participants
Participants with a definitive diagnosis of breast or colorectal cancer were enrolled in a randomized controlled trial for PN from September 2006 to June 2010 at the two study sites. In Rochester, participants were primarily recruited from participating oncology practices (n=13), both hospital and community-based. In Denver, participants were recruited from a single oncology practice within the Denver Health System, an integrated public safety net that includes a hospital and multiple health center sites. Because both sites employed similar models for navigation and used the same 3-month outcome measures, we pooled the follow-up data from these two sites to increase power.
Participants were eligible to be referred for the trials if they had newly-diagnosed breast (female) or colorectal (female or male) cancer and received care from any participating practice. “Newly-diagnosed” was defined as within three months of diagnosis and not beyond the second cycle of chemotherapy, where relevant. Among patients enrolled after surgery, only patients scheduled for oncology consultation for further treatment, such as chemotherapy and/or radiotherapy, were eligible. We excluded patients who were institutionalized, had dementia or had a prior cancer (other than non-melanoma skin cancer), multiple cancers, as well as males with breast cancer. We also excluded individuals who spoke neither English nor Spanish.
The Institutional Review Boards of the University of Rochester, Colorado Multiple Institutional Review Board, and all participating sites approved study procedures. Study participants provided informed consent and completed a HIPAA release form and permission to view and/or obtain medical records.
Randomization
Participants in both Rochester and Denver were individually randomized, stratified by cancer type. In Rochester, stratification also included recruitment site and randomization was done in blocks of four. An off-site study statistician produced computer generated random numbers and provided randomization assignments in sealed envelopes. Following confirmation of eligibility and informed consent, Research Assistants administered a face-to-face baseline survey to all study patients. They then opened the sealed envelope and notified the participant of their study assignment to receive either PN or usual care.
Intervention
Participants assigned to the intervention arm of the study received care coordination from a patient navigator who acted as a guide and coach during the course of their cancer treatment (15). Navigators were laypersons from the community who were specifically trained in these tasks. Minimal selection criteria included a high school degree, reliable mode of transportation and a current driver’s license. Preference was given to applicants with experience in case management or non-licensed health professionals. Desirable personal qualities in navigators included strong interpersonal and communication skills, fluency in Spanish and English, demonstrated ability to learn, dependability, initiative, as well as passion for and commitment to improving health care for underserved patients.
Intensive training was provided to navigators locally and nationally at baseline and periodically throughout the course of the project (16). Navigators were supervised by experienced managers who met with them regularly. A multidisciplinary team was available to provide expert advice and support as needed. Average caseload of each navigator included 20 cancer patients at a given time.
Navigators at both sites followed protocols developed by the PNRP Steering Committee. Navigation began with a baseline assessment that was typically conducted face-to-face at the inception of PN. Navigators and participants collaboratively identified treatment barriers and strategies to address barriers. Common treatment barriers included financial (insurance, copayments, lost time from work), logistical (transportation, child care), communicational (language, health literacy) and attitudinal (fears and misunderstanding regarding cancer and its treatment) issues (17).
Actions taken by the navigator included: supportive contact with the patient, such as face-to-face meetings, telephone, email or regular mail correspondence; identifying and linking patients to social or financial resources and appropriate community supports; helping with paperwork, obtaining records, scheduling appointments, following-up on test scheduling or results; and accompanying the patient to appointments to help coach as well as providing emotional support (18). Navigators also provided patients with approved educational materials and promoted treatment adherence, particularly appointment keeping. Navigators helped facilitate coordination of care by ensuring that consultation reports, test results, and information on patients’ new prescriptions or prescribed treatments were available to all providers at the time of an appointment. Navigators also encouraged patients to notify practices when they missed a treatment or test or experienced new or changed symptoms, and did so themselves (with patient permission) when the patient failed to do so (19, 20). Depending on the task and patient preference, follow-up navigation was provided in-person, by phone or other means (e.g., electronic mail). For interested patients, navigators coached patients about how to address their questions with their oncologist and/or other providers. Navigators also collaborated with other members of the oncology team, including assisting patients with scheduling appointments as needed, and following-up on missed appointments.
Data collection
Trained research assistants administered surveys in preferred language (English or Spanish) (21). Research assistants also abstracted data from medical records.
Demographic Measures
Socio-demographic and other measures are shown in Table 1. Demographic data were obtained from self-report at the Rochester, NY site and from electronic medical records at the Denver, CO site.
Table 1.
Participant characteristics by group assignment
| Groups | ||
|---|---|---|
| Independent Variable | Navigated (n = 225) | Control (n = 213) |
| Gender | ||
| Female | 89% (200) | 91% (194) |
| Male | 11% (25) | 9% (19) |
| Age Category (years) | ||
| < 40 | 8% (19) | 4% (9) |
| 40 – 49 | 22% (50) | 18% (39) |
| 50 – 59 | 31% (69) | 38% (80) |
| ≥60 | 39% (87) | 40% (85) |
| Race/Ethnicity | ||
| Black | 23% (52) | 17% (37) |
| White | 52% (116) | 61% (129) |
| Hispanic | 21% (47) | 15% (31) |
| Other | 4% (9) | 7% (14) |
| Primary Language | ||
| English | 90% (202) | 92% (195) |
| Other | 10% (23) | 8% (18) |
| Birth Country | ||
| Outside of US | 14% (32) | 15% (31) |
| US | 86% (193) | 85% (182) |
| Education | ||
| < high school | 21% (47) | 20% (41) |
| High school diploma (including equivalency) | 22% (50) | 30% (63) |
| Some college/Vocational school/Associate | 34% (75) | 29% (61) |
| College graduate/Graduate or professional | 23% (52) | 21% (45) |
| Median Household Income by ZIP Code* | ||
| < $30,000 | 20% (44) | 19% (40) |
| $30,000 to $39,999 | 32% (71) | 23% (49) |
| $40,000 to $49,999 | 22% (48) | 23% (49) |
| ≥$50,000 | 26% (57) | 35% (73) |
| Insurance Status Revised | ||
| Uninsured | 18% (40) | 19% (41) |
| Public insurance | 32% (72) | 27% (57) |
| Private insurance | 50% (113) | 54% (115) |
| Employment Status | ||
| No current employment | 63% (142) | 66% (139) |
| Part-time employment | 11% (24) | 9% (19) |
| Full-time employment | 26% (59) | 25% (54) |
| Household Size | ||
| 1 | 22% (49) | 25% (53) |
| 2 | 39% (89) | 38% (82) |
| ≥3 | 39% (87) | 37% (78) |
| Housing Status | ||
| Renting (apartment, home, condo, mobile) | 37% (84) | 30% (64) |
| Own (home, condo, mobile home) | 51% (114) | 60% (126) |
| Other | 12% (26) | 10% (20) |
| Dependents | ||
| 0 | 57% (95) | 57% (88) |
| 1 | 27% (44) | 20% (31) |
| ≥2 | 16% (26) | 23% (35) |
| Distance from treatment facility (miles) | ||
| < 1.5 | 6% (13) | 6% (13) |
| 1.5 – 3.9 | 30% (68) | 26% (56) |
| 4 – 8.4 | 34% (76) | 33% (70) |
| ≥8.5 | 30% (68) | 35% (73) |
| Cancer Type | ||
| Breast | 81% (183) | 80% (170) |
| Colorectal | 19% (42) | 20% (43) |
| Site | ||
| Denver | 27% (60) | 28% (59) |
| Rochester | 73% (165) | 72% (154) |
| Cancer Stage | ||
| Stage 0 | 9% (20) | 9% (18) |
| Stage 1 | 29% (65) | 29% (61) |
| Stage 2 | 33% (72) | 30% (63) |
| Stage 3 | 24% (53) | 26% (55) |
| Stage 4 | 5% (11) | 6 % (12) |
From the U.S. Census 2000 Summary File 3—United States/prepared by the U.S. Census Bureau, 2002.
Study Outcomes Measures
Time to Completion of Treatment
Because a major goal of PN is eliminating or reducing delays in care, the time from cancer diagnosis to the end of primary cancer treatment was the primary outcome of this study. Only participants who received radiation and/or chemotherapy were included for this outcome; patients who had no further treatment after surgery were not considered to require navigation. A research assistant abstracted chart data to assess time from diagnosis to end of primary chemotherapy and/or radiation treatment. If a subject received both radiation and chemotherapy, we used time to completion of chemotherapy, which was longer in all but 10 instances. We also conducted a sensitivity analysis for this outcome in which we stratified groups by radiation only, chemotherapy only and both using time based on completion of final treatment.
Psychological Distress
The Impact of Events scale (IES) was administered in English or Spanish to assess psychological distress three months following initiation of treatment at both sites (and administered more frequently in Rochester). IES is a psychometrically validated 15-item measure that was developed to assess current distress from life events (22, 23). Response options for each item range from “0 = not at all” to “5 = often”. A total scale score for the IES was calculated by adding all individual items. The total IES score could range from a minimum of 0 to a maximum of 75. Interpretation of the Impact of Events total score ranges from less than 9 (no meaningful impact, subclinical), 9 to 25 (may be affected), 26 to 43 (powerful, certainly affected), to greater than 44 (severe impact, capable of altering ability to function). Because of the skewness of the data towards no distress (coefficient of skewness =1.69), we created a dichotomous outcome using <9 (no meaningful distress) vs ≥ 9 (potentially meaningful distress). In a sensitivity analysis, we also analyzed the IES as a continuous outcome.
Satisfaction with Cancer Care
This scale was also administered at both sites at 3 months (and at additional intervals in Rochester) following initiation of treatment. The Patient Satisfaction with Cancer-related Care scale (PSCC) was developed and validated by Jean-Pierre et al as part of the PNRP (24). It is an 18-item measure that assesses patient satisfaction with the cancer care they received. Responses to each item ranged from “1 = not satisfied” to “5 = very satisfied”. A total scale score was calculated for the PSCC by adding scores on all individual items. Because the distribution of the responses was strongly skewed towards higher satisfaction (coefficient of skewness = 0.50), we created a dichotomous outcome using the 25th percentile as a cutoff (<75 v. ≥75). We also analyzed it as a continuous score to confirm the validity of the dichotomous analysis.
Statistical Analysis
We used an intent-to-treat analysis. That is, our analysis was based on initial randomization assignment regardless of receipt or intensity of PN among participants for whom follow-up data were available. We used the F-test from a log-normal regression analysis to assess group-based differences in time to completion of treatment, and chi-square tests for group-based differences in distress and satisfaction. We also completed secondary analyses using stepwise multivariate regression models to assess the effect of PN. We further examined the hypothesis that PN would show greater effects among patients with low income, low education, no insurance, limited English language proficiency, or social isolation by assessing interactions between PN and these factors.
Variance was calculated using the Taylor-series linearization method with study site (Rochester or Denver) as the stratum and practice as the primary sampling unit (PSU) to account for the clustering of patients within site. A p-value of 0.05 or less was considered to be statistically significant. All analyses were conducted using SAS-callable SUDAAN Version 10.0.1 and SAS Version 9.2 on the Windows 7 32-bit platform.
RESULTS
Patient Accrual and Study Flow
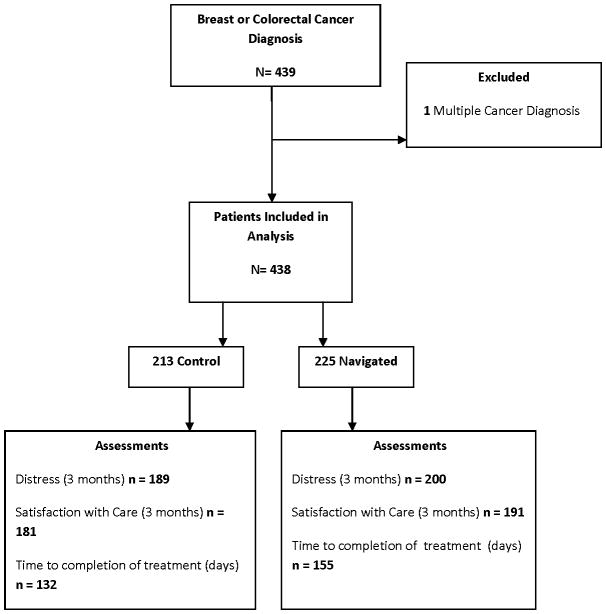
Figure 1 represents a flow chart of our analytic sample. The Rochester site contributed 319 participants and Denver 119 (27%). There were 353 breast cancer patients (all female) and 85 CRC patients (44 male, 41 female), excluding one participant with multiple cancers. Data on time to completion of primary treatment were available for 287 participants, after excluding 151 participants who did not did not require and/or opt for radiation or chemotherapy (many of whom initiated long-term anti-hormonal treatment). Data on distress and satisfaction were available for 389 and 372 participants, respectively (Figure 1).
Figure 1.
Participant flow through the study
Participant characteristics
Study participants were predominantly middle aged and older (mean age=57) females (90%). The present sample included a high proportion of racial-ethnic minority patients (44%), and patients with lower educational attainment (less than or equal to high school, 46%). Some did not have health insurance (18%); fewer did not have English as their primary language (9%). Denver participants were more likely to be Latino or uninsured than participants from Rochester. Baseline characteristics were similar between participants randomized to PN or usual care (Table 1).
The majority of participants were diagnosed with breast cancer (80%) due to higher incidence and the presence of designated breast cancer clinics in Rochester that facilitated recruitment. The remainder of the sample (20%) had colorectal cancer. Most participants were diagnosed with stage II or stage III cancer (n=261). Fifty six patients received only chemotherapy, 114 received radiation only, and 117 received both treatments.
Time to Completion of Treatment
A total of 287 participants received chemotherapy or radiation therapy. All completed their treatment. The median time to complete treatment (57 days for intervention and 63 days for control) was not statistically significantly different between the groups (p> 0.05). There were no statistically significant differences when results were stratified by cancer type, stage, or participant characteristics.
Psychological Distress
Participants (n=389) had a median IES total score of 22.3 with a standard error of 1.83 and a range from 18 to 90. The percentage of each group reporting no appreciable psychological distress (IES score < 9) did not significantly differ between the two groups. Results were unchanged in a logistic regression model.
Irrespective of intervention group assignment, subjects were more likely to report distress if they were female, were younger, had low-income, had part-time employment (vs. full-time or no current employment), or a rented residence (vs. owned or other). In contrast, Hispanic patients reported less distress. Similarly, patients who lived alone were less likely to report distress (Table 2). Moreover, we observed a statistically significant interaction between navigation and living alone in terms of distress (p<0.002). Among patients living alone, those assigned to PN were significantly more likely to be distressed.
Table 2.
Multivariate predictors of low psychological distress at 3-months
| Probability of Low Distress (IES < 9)
|
||||
|---|---|---|---|---|
| Independent Variable | Odds Ratio | Lower 95% CI | Upper 95% CI | p-value Wald F |
| Randomization Group | 0.123 | |||
| Control | 1.00 | 1.00 | 1.00 | |
| Navigated | 0.79 | 0.59 | 1.07 | |
| Gender | 0.005 | |||
| Female | 0.48 | 0.29 | 0.80 | |
| Male | 1.00 | 1.00 | 1.00 | |
| Age Categorized | 0.001 | |||
| < 40 | 0.78 | 0.17 | 3.50 | |
| 40 – 49 | 0.39 | 0.14 | 1.09 | |
| 50 – 59 | 0.77 | 0.59 | 1.00 | |
| ≥60 | 1.00 | 1.00 | 1.00 | |
| Race/Ethnicity | 0.001 | |||
| Black | 1.64 | 0.97 | 2.77 | |
| White | 1.00 | 1.00 | 1.00 | |
| Hispanic | 1.84 | 1.38 | 2.47 | |
| Other | 0.55 | 0.21 | 1.44 | |
| Median Household Income by ZIP | 0.011 | |||
| < $30,000 | 0.57 | 0.29 | 1.10 | |
| $30,000 to $39,999 | 2.21 | 1.38 | 3.53 | |
| $40,000 to $49,999 | 1.14 | 0.69 | 1.87 | |
| ≥$50,000 | 1.76 | 0.91 | 3.41 | |
| Employment Status | 0.0101 | |||
| No current employment | 1.27 | 0.64 | 2.53 | |
| Part-time employment | 0.32 | 0.14 | 0.73 | |
| Full-time employment | 1.00 | 1.00 | 1.00 | |
| Household Size | 0.005 | |||
| 1 | 2.28 | 1.39 | 3.72 | |
| 2 | 1.32 | 0.73 | 2.39 | |
| ≥3 | 1.00 | 1.00 | 1.00 | |
| Housing Status | <0.0001 | |||
| Renting | 0.34 | 0.25 | 0.47 | |
| Own | 1.00 | 1.00 | 1.00 | |
| Other | 0.78 | 0.36 | 0.47 | |
Satisfaction with Cancer Care
Among the 372 subjects for whom data were available, the median PSCC score was 81.7 with a standard error of 2.13. In the primary analysis, there was no significant difference in the proportion of PN and control group patients who had a higher satisfaction score (Table 3). However, we observed significant interactions between treatment group and language (p=0.04), educational level (p=0.007), and health insurance (p=0.006). Specifically, being randomized to navigation was associated with significantly greater likelihood of higher satisfaction with cancer care among participants with lower English proficiency (OR 3.75; 95% CI 1.60–8.79), less than a high school education (OR 2.37; 95% CI 1.28–4.40), and no health insurance (OR 2.36; 95% CI 1.41–3.93).
Table 3.
Multivariate predictors of satisfaction with cancer care at 3 months
| Probability of Higher Satisfaction with Care
|
||||
|---|---|---|---|---|
| Independent Variable | Odds Ratio | Lower 95% CI | Upper 95% CI | p-value Wald F |
| Randomization Group | 0.1381 | |||
| Control | 1.00 | 1.00 | 1.00 | |
| Navigated | 1.29 | 0.92 | 1.82 | |
| Gender | 0.8297 | |||
| Female | 0.93 | 0.50 | 1.74 | |
| Male | 1.00 | 1.00 | 1.00 | |
| Age Category (years) | 0.0000 | |||
| < 40 | 0.34 | 0.09 | 1.35 | |
| 40 – 49 | 1.04 | 0.57 | 1.90 | |
| 50 – 59 | 0.71 | 0.45 | 1.12 | |
| ≥60 | 1.00 | 1.00 | 1.00 | |
| Race/Ethnicity | 0.0022 | |||
| Black | 1.75 | 0.75 | 4.08 | |
| White | 2.16 | 0.90 | 5.18 | |
| Hispanic | 0.89 | 0.20 | 4.02 | |
| Other | 1.00 | 1.00 | 1.00 | |
| Education | 0.0000 | |||
| < high school | 0.41 | 0.29 | 0.58 | |
| High school diploma (including equivalency) | 0.47 | 0.39 | 0.57 | |
| Some college/Vocational school/Associate | 0.59 | 0.30 | 1.16 | |
| College graduate/Graduate or professional | 1.00 | 1.00 | 1.00 | |
Sensitivity Analyses
We conducted sensitivity analyses to assess the robustness of our findings. We assessed time to completion of treatment using an analysis stratified by treatment type (chemotherapy only, radiation only, and both). We also assessed distress and satisfaction using continuous measures. Although both sites measured distress and satisfaction at 3 months, only Rochester collected these data at baseline and (depending on the length of the patient’s treatment) at 6, 9 and 12 months. Using the Rochester data, we assessed the impact of controlling for baseline scores as well as longitudinal changes in these scores. In each case, the results were unchanged, i.e., no statistically significant difference between the PN and control groups. Specifically, distress declined from 84% at baseline to 76% at 3-month follow-up among controls and from 90% to 83% among those receiving PN. In contrast, satisfaction declined from 80% at baseline to 71% at 3-month follow-up among controls and from 77% to 72% among those receiving PN.
DISCUSSION
In a randomized controlled trial of patient navigation to reduce barriers to cancer treatment, we observed no overall effect on patients’ time to completion of treatment. Within three months of treatment initiation, we also found no overall effect on psychological distress or satisfaction with care. However, subgroup analysis showed benefits for selected patients. In particular, those with educational, language, and insurance barriers reported greater satisfaction when navigated. These findings, if replicated, suggest that PN may improve experience of care among patients with the greatest needs, which conforms to the original intent of patient navigation (25).
The absence of overall benefit from navigation among breast and colorectal cancer patients is consistent with the findings of Ell et al who observed no significant effect on patient adherence to adjuvant treatment from telephone navigation (compared with information materials) for patients with breast or gynecological cancer (14). However, we observed improved satisfaction among underserved patients, especially among the most vulnerable.
Our findings of no overall benefit contrast with benefits observed in most randomized trials of PN for cancer screening (3–9), and diagnostic follow-up on abnormal cancer screening tests (10–13). These seemingly conflicting findings may reflect better targeting of PN when used for screening or follow-up on abnormal screening. When used for those purposes, PN typically targets patients who have failed to receive a particular intervention. That is, eligibility for enrollment is often based on demonstrated need. In contrast, in our trial and the one conducted by Ell et al (14), navigation was provided to all eligible (intervention group) patients. While both our study populations were socially disadvantaged, we did not specifically target patients who had not adhered to their cancer treatment or who had dropped out of treatment due to various barriers. Greater attention from providers to disadvantaged patients with diagnosed cancer (such as social work services or care coordinators in cancer centers) may explain these findings, and why traditional social risk factors (age, race, ethnicity, education, income, language, or insurance) were not associated with longer time to treatment. In addition, increasing attention to patients’ socioeconomic needs at both study sites may have led to more assistance from social work and other institutional resources that may have mitigated the impact of PN.
Our finding of improvement in experience with health care among patients with selected risk factors has plausibility based on our theoretical model for navigation (1). Navigators were trained to address educational, language, and insurance barriers. Conceivably, addressing these risk factors yielded improved satisfaction with cancer care. The finding that patients living alone experienced greater distress when navigated was unexpected and warrants replication.
These findings come with important limitations. First, we aggregated findings from two trials that used the same design and measures to increase our power to detect effects. The two sites differed in the frequency of assessment of distress and satisfaction and the two study populations differed in rates of no insurance and ethnicity. Thus, we controlled for site effects and also conducted sensitivity analyses that controlled for baseline scores and assessed longer follow-up. In these sensitivity analyses we observed the same findings. Second, while the positive findings are consistent with our original PNRP secondary hypothesis, they reflect secondary analyses and should be replicated. Third, we examined PN during care for only two types of cancer and most of our participants were women with breast cancer. We do not know whether our findings would be generalizable to other cancers. Last, we studied navigation provided in only two communities and do not know whether these findings generalize to other parts of the country.
In conclusion, PN during cancer treatment did not affect time to completion of treatment, satisfaction with cancer care, or distress in the overall population. However, selected subgroups may benefit in terms of patient satisfaction. The association of PN with increased distress among those living alone was unexpected and requires further study.
Acknowledgments
Funder: This study was supported by the Center to Reduce Cancer Health Disparities of NCI (3U01CA116924-05S)
Footnotes
Conflict of interest: All of the authors report no conflicts of interest
References
- 1.Freund KM, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett E, et al. National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008;113:3391–9. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells KJ, Battaglia TA, Dudley DJ, Garcia R, Greene A, Calhoun E, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor VM, Jackson JC, Yasui Y, Nguyen TT, Woodall E, Acorda E, et al. Evaluation of a cervical cancer control intervention using lay health workers for Vietnamese American women. Am J Public Health. 2010;100:1924–9. doi: 10.2105/AJPH.2009.190348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TT, McPhee SJ, Gildengorin G, Nguyen T, Wong C, Lai KQ, et al. Papanicolaou testing among Vietnamese Americans: results of a multifaceted intervention. Am J Prev Med. 2006;31:1–9. doi: 10.1016/j.amepre.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed NU, Haber G, Semenya KA, Hargreaves MK. Randomized controlled trial of mammography intervention in insured very low-income women. Cancer Epidem Biomar. 2010;19:1790–8. doi: 10.1158/1055-9965.EPI-10-0141. [DOI] [PubMed] [Google Scholar]
- 6.Sung JF, Blumenthal DS, Coates RJ, Williams JE, Alema-Mensah E, Liff JM. Effect of a cancer screening intervention conducted by lay health workers among inner-city women. Am J Prev Med. 1997;13:51–7. [PubMed] [Google Scholar]
- 7.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–84. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 8.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82:216–24. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasser KE, Murillo J, Medlin E, Lisboa S, Valley-Shah L, Fletcher RH, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastani R, Mojica CM, Berman BA, Ganz PA. Low-income women with abnormal breast findings: results of a randomized trial to increase rates of diagnostic resolution. Cancer Epidem Biomar. 2010;19:1927–36. doi: 10.1158/1055-9965.EPI-09-0481. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell AE, Jo AM, Crespi CM, Sudan M, Bastani R. Peer navigation improves diagnostic follow-up after breast cancer screening among Korean American women: results of a randomized trial. Cancer Cause Control. 2010;21:1931–40. doi: 10.1007/s10552-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelstad LP, Stewart S, Otero-Sabogal R, Leung MS, Davis PI, Pasick RJ. The effectiveness of a community outreach intervention to improve follow-up among underserved women at highest risk for cervical cancer. Prev Med. 2005;41:741–8. doi: 10.1016/j.ypmed.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Yabroff KR, Kerner JF, Mandelblatt JS. Effectiveness of interventions to improve follow- up after abnormal cervical cancer screening. Prev Med. 2000;31:429–39. doi: 10.1006/pmed.2000.0722. [DOI] [PubMed] [Google Scholar]
- 14.Ell K, Vourlekis B, Xie B, Nedjat-Haiem FR, Lee PJ, Muderspach L, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115:4606–15. doi: 10.1002/cncr.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendren S, Griggs JJ, Epstein RM, Humiston S, Rousseau S, Jean-Pierre P, et al. Study protocol: a randomized controlled trial of patient navigation-activation to reduce cancer health disparities. BMC Cancer. 2010;10:551. doi: 10.1186/1471-2407-10-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calhoun EA, Whitley EM, Esparza A, Ness E, Greene A, Garcia R, et al. A national patient navigator training program. Health Promot Pract. 2010;11:205–15. doi: 10.1177/1524839908323521. [DOI] [PubMed] [Google Scholar]
- 17.Hendren S, Chin N, Fisher S, Winters P, Griggs J, Mohile S, et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc. 2011;103:701–10. doi: 10.1016/s0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jean-Pierre P, Hendren S, Fiscella K, Loader S, Rousseau S, Schwartzbauer B, et al. Understanding the processes of patient navigation to reduce disparities in cancer care: perspectives of trained navigators from the field. J Cancer Educ. 2011;26:111–20. doi: 10.1007/s13187-010-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll JK, Humiston SG, Meldrum SC, Salamone CM, Jean-Pierre P, Epstein RM, et al. Patients’ experiences with navigation for cancer care. Patient Educ Couns. 2010;80:241–7. doi: 10.1016/j.pec.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yosha AM, Carroll JK, Hendren S, Salamone CM, Sanders M, Fiscella K, et al. Patient navigation from the paired perspectives of cancer patients and navigators: a qualitative analysis. Patient Educ Couns. 2011;82:396–401. doi: 10.1016/j.pec.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean-Pierre P, Fiscella K, Winters P, Paskett E, Wells K, Battaglia TA, et al. Psychometric validation and reliability analysis of a Spanish version of the patient satisfaction with cancer-related care measure: a patient navigation research program study. Support Care Cancer. 2011 doi: 10.1007/s00520-011-1297-3. Pub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundin EC, Horowitz MJ. Horowitz’s Impact of Event Scale Evaluation of 20 Years of Use. Psychosom Med. 2003;65:870–6. doi: 10.1097/01.psy.0000084835.46074.f0. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz MJ, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Jean-Pierre P, Fiscella K, Freund KM, Clark J, Darnell J, Holden A, et al. Structural and reliability analysis of a patient satisfaction with cancer-related care measure: a multisite patient navigation research program study. Cancer. 2011;117:854–61. doi: 10.1002/cncr.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman HP. Patient navigation: a community centered approach to reducing cancer mortality. J Cancer Educ. 2006;21:S11–S14. doi: 10.1207/s15430154jce2101s_4. [DOI] [PubMed] [Google Scholar]