Abstract
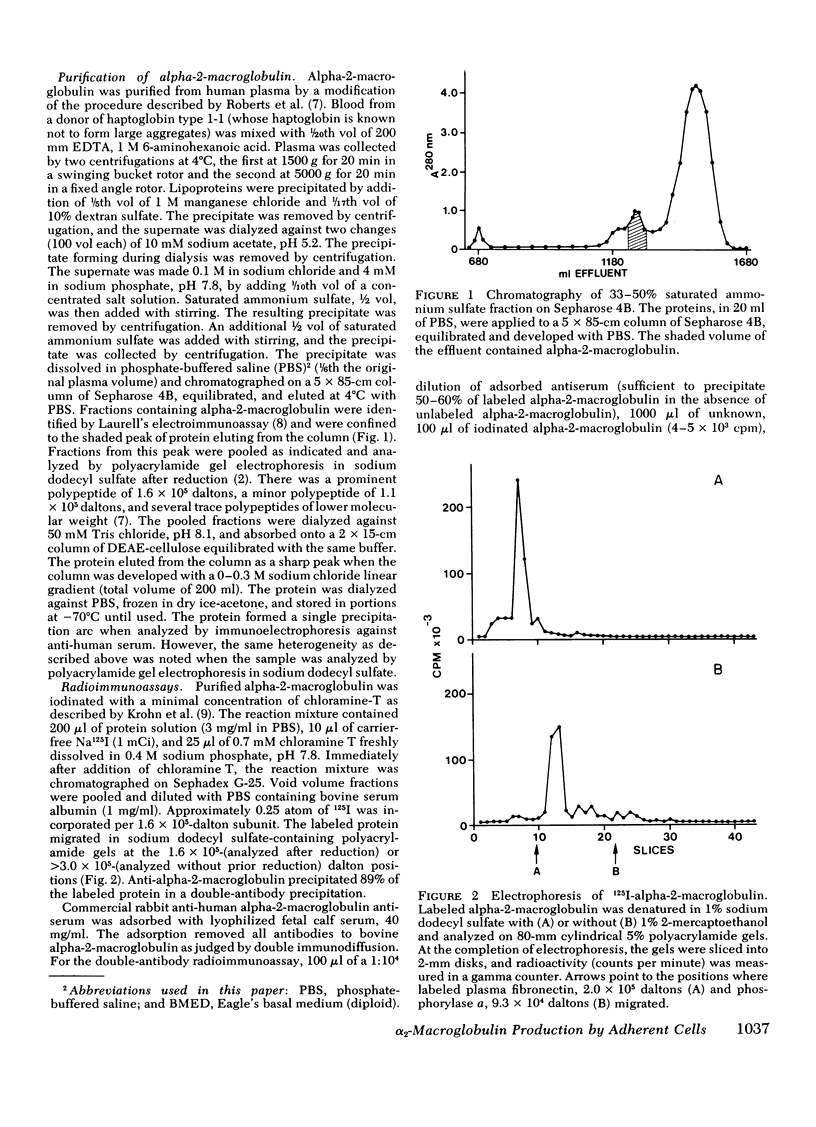
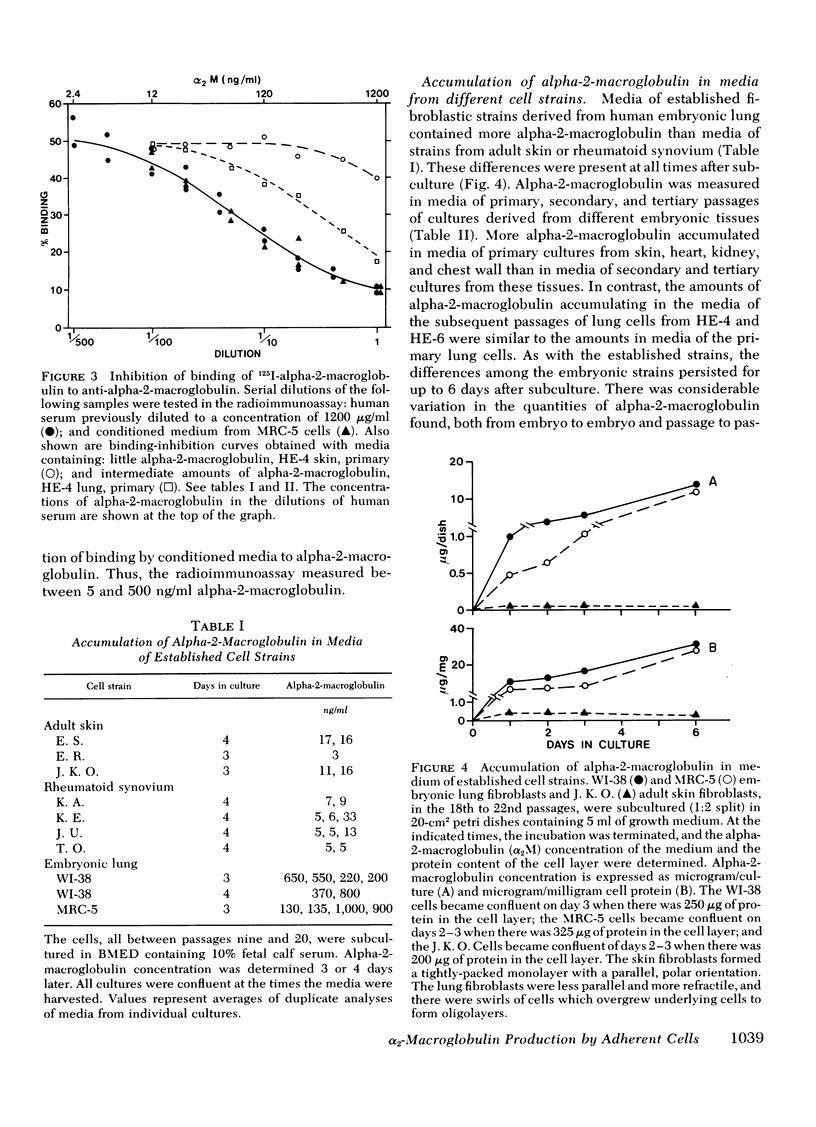
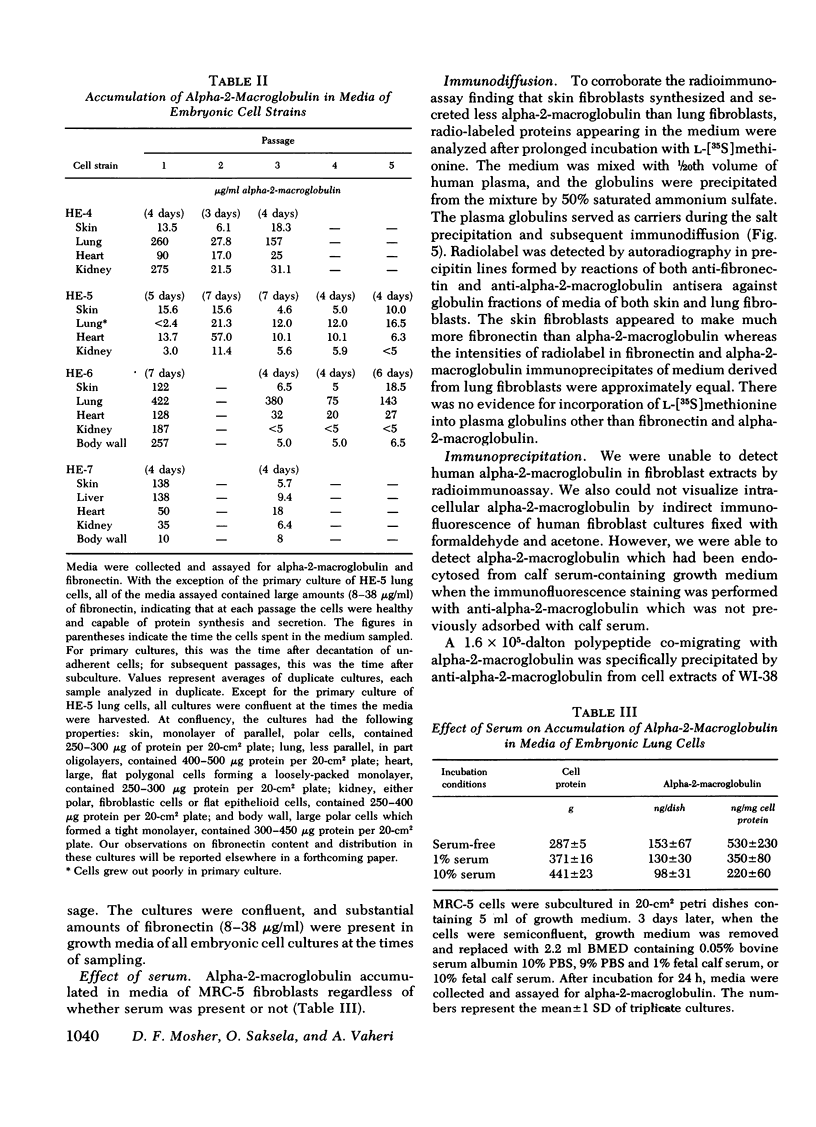
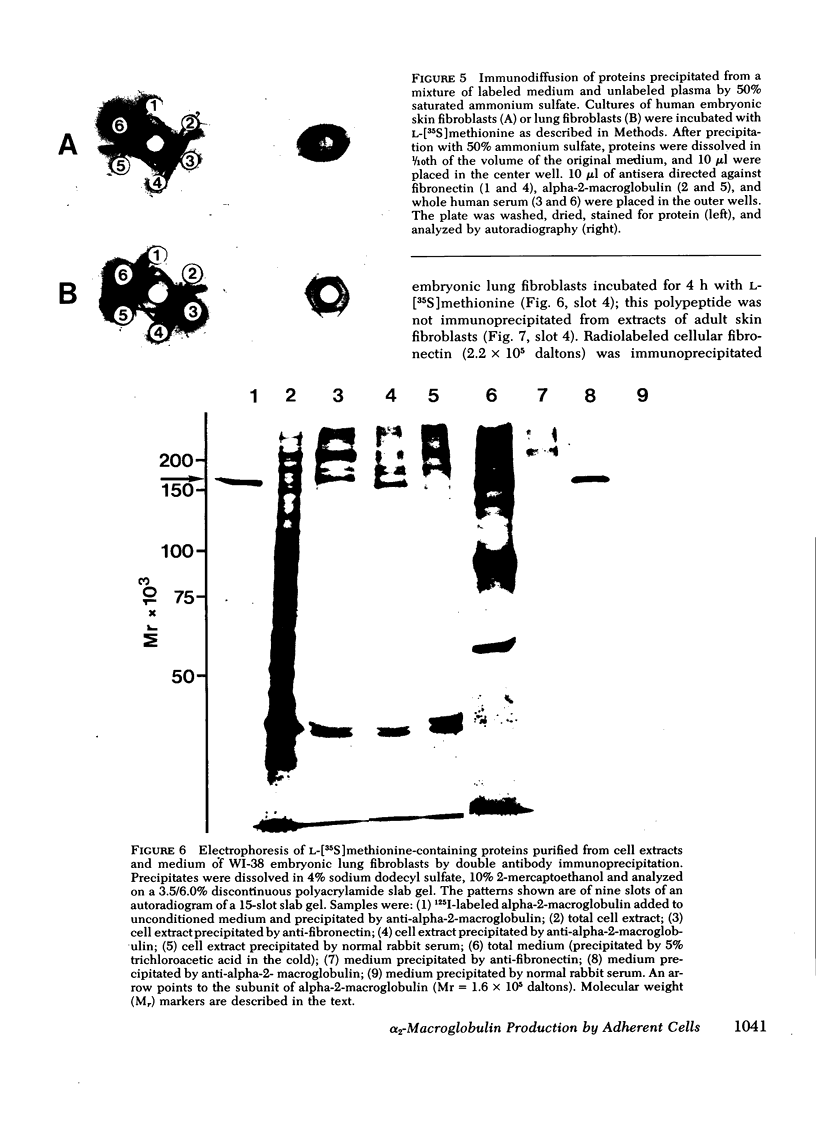
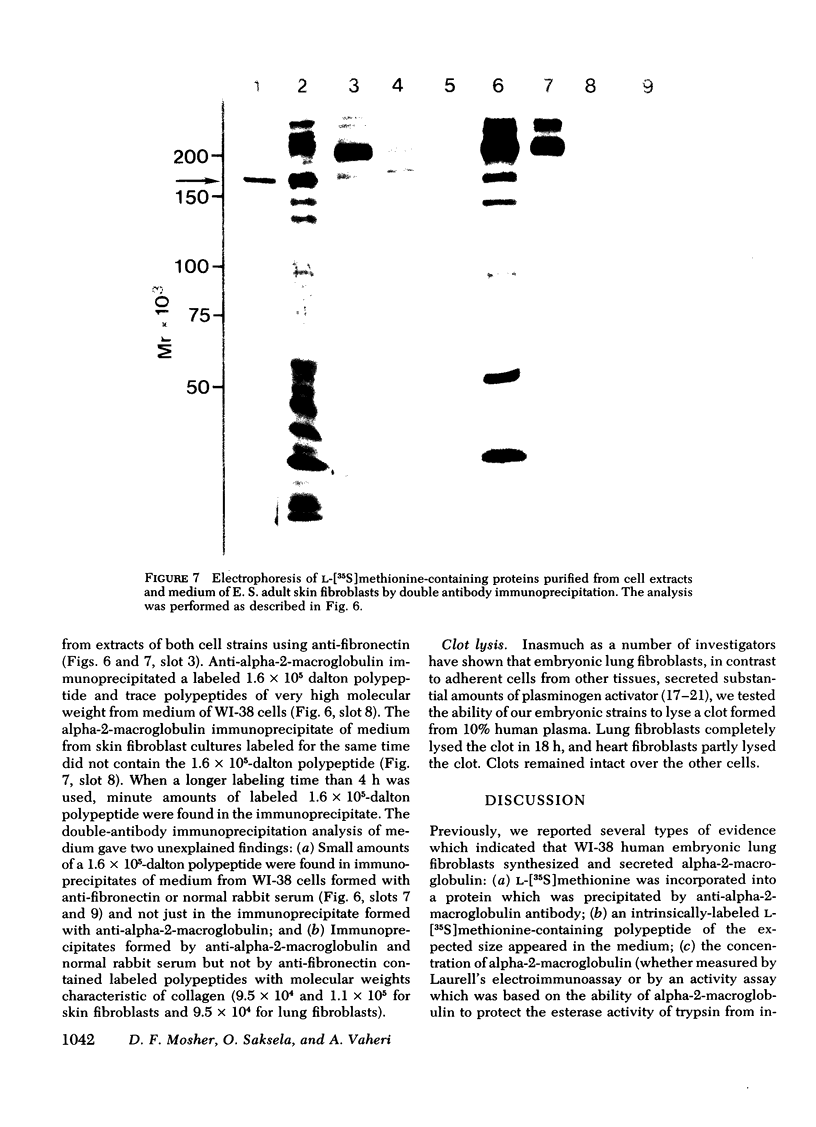
We studied the synthesis and secretion of alpha-2-macroglobulin by cultures of human adherent cells. Much more alpha-2-macroglobulin (measured by radioimmunoassay) accumulated in media of established strains of adherent cells derived from embryonic lung than in media of established strains derived from adult skin or rheumatoid synovium. Alpha-2-macroglobulin accumulated in media of primary cultures of adherent cells from a variety of embryonic tissues. However, the amount of alpha-2-macroglobulin accumulating in media of subsequent passage of these cells declined for all strains except those derived from lung. Immunodiffusion and double-antibody immunoprecipitation studies of cell extracts and media after incubation of cells with l-[35S]methionine supported the radioimmunoassay finding that adherent cells from lung synthesized and secreted more alpha-2-macroglobulin than adherent cells from skin. Intracellular alpha-2-macroglobulin could not be detected by radio-immunoassay or visualized by immunofluorescent microscopy, suggesting that synthesized alpha-2-macroglobulin is rapidly secreted. Plasminogen-rich fibrin clots were lysed in culture media of adherent cells from embryonic lung and, to a lesser extent, heart. Adherent cells from other tissues, which produced less alpha-2-macroglobulin, did not lyse fibrin clots. However, all cultures of adherent cells contained pericellular fibronectin, a large, external, transformation-sensitive glycoprotein known to be cleaved by plasmin. We speculate that production of alpha-2-macroglobulin may be a means for protease-secreting normal cells to preserve cell surface integrity and that alpha-2-macroglobulin synthesized locally in lung may protect lung tissues from a variety of proteases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus C. M., Choi T. S., Weintraub D. H., Eisenberg B., Staub H. P., Courey N. G., Foote R. J., Goplerud D., Moesch R. V., Ray M. Studies on the prevention of respiratory distress syndrome of infants due to hyaline membrane disease with plasminogen. Semin Thromb Hemost. 1975 Jul;2(1):42–51. doi: 10.1055/s-0028-1086114. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. G., Harpel P. C. alpha2-Macroglobulin on human vascular endothelium. J Exp Med. 1976 Jul 1;144(1):1–9. doi: 10.1084/jem.144.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernik M. B., Kwaan H. C. Plasminogen activator activity in cultures from human tissues. An immunological and histochemical study. J Clin Invest. 1969 Sep;48(9):1740–1753. doi: 10.1172/JCI106140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Robbins P. W. Effect of proteases on activation of resting chick embryo fibroblasts and on cell surface proteins. Cell. 1975 Oct;6(2):137–147. doi: 10.1016/0092-8674(75)90004-5. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Acs G. Purification and characterization of a cellular fibrinolytic factor associated with oncogenic transformation: the plasminogen activator from SV-40-transformed hamster cells. Biochim Biophys Acta. 1974 Mar 27;340(3):339–347. doi: 10.1016/0005-2787(74)90279-2. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S. PULMONARY EMPHYSEMA AND ALPHA1-ANTITRYPSIN DEFICIENCY. Acta Med Scand. 1964 Feb;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 1969 Aug;48(8):1433–1446. doi: 10.1172/JCI106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., Mosher D., Vaheri A. Cultured human monocytes synthesize and secrete alpha2-macroglobulin. J Exp Med. 1977 Jun 1;145(6):1580–1589. doi: 10.1084/jem.145.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Pierce A. K. Effects of elastase, collagenase, and papain on structure and function of rat lungs in vitro. J Clin Invest. 1972 Feb;51(2):288–293. doi: 10.1172/JCI106813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Cross-linking of a major fibroblast surface-associated glycoprotein (fibronectin) catalyzed by blood coagulation factor XIII. Cell. 1976 Sep;9(1):29–35. doi: 10.1016/0092-8674(76)90049-0. [DOI] [PubMed] [Google Scholar]
- Krohn K., Sherman L., Welch M. Studies of radioiodinated fibrinogen. I. Physicochemical properties of the ICl, chloramine-T, and electrolytic reaction products. Biochim Biophys Acta. 1972 Dec 28;285(2):404–413. doi: 10.1016/0005-2795(72)90327-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laug W. E., Jones P. A., Benedict W. F. Relationship between fibrinolysis of cultured cells and malignancy. J Natl Cancer Inst. 1975 Jan;54(1):173–179. doi: 10.1093/jnci/54.1.173. [DOI] [PubMed] [Google Scholar]
- Marco V., Meranze D. R., Yoshida M., Kimbel P. Papain-induced experimental emphysema in the dog. J Appl Physiol. 1972 Sep;33(3):293–299. doi: 10.1152/jappl.1972.33.3.293. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F., Wing D. A. Synthesis and secretion of alpha2-macroglobulin by cultured human fibroblasts. J Exp Med. 1976 Feb 1;143(2):462–467. doi: 10.1084/jem.143.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. M., Fabisch P. H., Sani B. P., Sorof S. Lack of correlation between fibrinolysis and the transformed state of cultured mammalian cells. Biochem Biophys Res Commun. 1974 Nov 27;61(2):621–627. doi: 10.1016/0006-291x(74)91002-x. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Harpel P. C. Platelet alpha2-macroglobulin and alpha1-antitrypsin. J Biol Chem. 1976 Aug 10;251(15):4512–4521. [PubMed] [Google Scholar]
- Nagy B., Ban J., Brdar B. Fibrinolysis associated with human neoplasia: production of plasminogen activator by human tumours. Int J Cancer. 1977 May 15;19(5):614–620. doi: 10.1002/ijc.2910190504. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Hynes R. O., Franks L. M., Hemmings V. J. Surface proteins and fibrinolytic activity of cultured mammalian cells. Cancer Res. 1976 Apr;36(4):1475–1480. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Tunstall A. M., James K. Association of alpha-macroglobulins with lymphoid cells. Nat New Biol. 1973 Nov 21;246(151):78–81. doi: 10.1038/newbio246078a0. [DOI] [PubMed] [Google Scholar]
- Tunstall A. M., James K. Preliminary studies on the synthesis of alpha2-macroglobulin by human lymphocytes in vitro. Clin Exp Immunol. 1974 Aug;17(4):697–701. [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- WILLIAMS C. A., Jr, ASOFSKY R., THORBECKE G. J. PLASMA PROTEIN FORMATION IN VITRO BY TISSUES FROM MICE INFECTED WITH STAPHYLOCOCCI. J Exp Med. 1963 Aug 1;118:315–326. doi: 10.1084/jem.118.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]