Abstract
Objective
The use of anti-tumor necrosis factor (TNF) agents to treat joint manifestations of sarcoidosis has not been described. We evaluated the efficacy and safety of three such biologics in patients with these symptoms refractory to conventional therapy (nonsteroidal anti-inflammatory drugs, corticosteroids, and/or disease-modifying antirheumatic drugs).
Methods
This retrospective study, covering January 2001 to September 2011, examined clinical–biological parameters collected before anti-TNF treatment (age, sex, duration of disease evolution, drugs taken), and at introduction and under anti-TNF therapy (number of painful and swollen joints, visual analog scale score of global disease activity, disease-activity score of 28 joints with erythrocyte sedimentation rate or C-reactive protein, TNF-antagonist duration). At 3, 6, and 12 months, anti-TNF impact on joints and the therapeutic response according to European League Against Rheumatism criteria used for rheumatoid arthritis were assessed.
Results
Ten patients’ data were evaluated; some of them had received several anti-TNF agents (median [range] duration on each biotherapy was 10 [4–30] months), which enabled analysis of 19 prescriptions. The total duration of anti-TNF exposure was 17.6 patient-years, which was started a median of 3 (0.33–17) years after sarcoidosis diagnosis. The median numbers of painful and swollen joints were 1 (0–28) and 0 (0–9), respectively. Despite rapid efficacy, after 1 year of treatment, clinical (especially joint) and biological parameters were comparable to pretreatment, and only the corticosteroid dose was significantly lower (P=0.03). One case of mild skin toxicity was noted.
Conclusion
TNF antagonists allowed significant steroid sparing and were well tolerated, but do not seem to be effective against sarcoidosis joint involvement.
Keywords: sarcoidosis, anti-TNF, arthritis, adalimumab, etanercept, infliximab
Introduction
Conventional management of sarcoidosis with joint involvement (arthralgias, arthritis) relies on nonsteroidal anti-inflammatory drugs (NSAIDs) for the more moderate forms but sometimes requires corticosteroids.1 Corticoresistance is a sign of poor prognosis that necessitates the prescription of immunomodulators like methotrexate,2 and prognosis is even more dismal when extrapulmonary involvement is also present.3,4 Sarcoidosis is the consequence of an exaggerated monocyte-, macrophage- and T-lymphocyte-mediated immune response to still-unidentified antigens, leading to tumor necrosis factor (TNF) overproduction.5 This excessive synthesis plays a primordial role in the sustained formation of granuloma(s), the main sign of sarcoidosis.5 TNF inhibition may represent a new and relevant therapeutic approach to treat refractory sarcoidosis, notably when its clinical evolution is invalidating and persistent, as TNF plays a preponderant role in sarcoidosis physiopathology.6 At present, the place of biologics, notably TNF antagonists, has not yet been clearly defined, and their use is currently off-label in sarcoidosis. To our knowledge, no study has specifically investigated the efficacy and safety of anti-TNF biotherapies in the context of joint manifestations of sarcoidosis. This study was undertaken to do so.
Patients and methods
Patients
This retrospective, observational study, covering January 2001 to September 2011, included all patients diagnosed with sarcoidosis meeting internationally recognized criteria and treated with TNF antagonist(s) due to articular involvement refractory to conventional therapy (NSAIDs, corticosteroids, and/or disease-modifying antirheumatic drugs [DMARDs]).7 In accordance with French guidelines,8 none of these patients had any contraindications to the use of these agents. Despite the fact that anti-TNF blockers are off-label in France to treat sarcoidosis, their use was decided after a multidisciplinary consensus. All the patients that we described here suffered from a severe clinical disease, required steroids, and/or suffered from methotrexate failure. Two patients with monoarthritis had already received several corticosteroid injections, but with no significant sustained effect. Informed written consent was obtained before anti-TNF prescription. This study conformed with French law and met the criteria of the local ethics committee.
Various clinical parameters were collected before starting treatment: age, sex, and disease duration since the first symptoms and until starting anti-TNF (infliximab, adalimumab or etanercept as first- and second-/third-line choices, respectively). While on TNF antagonist(s), duration of anti-TNF use and concomitant therapies (corticosteroids, methotrexate, NSAIDs) was recorded. To evaluate the clinical efficacy of these biologics, the following indicators were regularly noted during patient follow-up: the numbers of painful and swollen joints; the 28-joint disease-activity score (DAS28) to evaluate peripheral disease involvement, calculated with erythrocyte sedimentation rate (ESR/first hour) or C-reactive protein (CRP); DAS28 decrease ≥1.2 points; the patient’s overall self-assessment of the disease using the visual analog scale (VAS) score; and biological markers (ESR/first hour and CRP concentration). Extra-articular involvement (pulmonary, ocular, neurological, cardiac, cutaneous, muscular) and adverse events or paradoxical side effects until anti-TNF discontinuation were recorded.9
We used the composite DAS28 and European League Against Rheumatism (EULAR) score,10 usually applied to rheumatoid arthritis, as the principal response criterion to anti-TNF at 3, 6, and 12 months and at the end of treatment, and a ≥1.2-point DAS28 decline.
Statistical analyses
The before and after TNF-antagonist therapy medians and range of demographic, clinical, and biological characteristics were compared with Wilcoxon’s paired nonparametric test or McNemar’s test for quantitative or qualitative parameters, respectively. P<0.05 defined significance. Means ± standard deviation and 95% confidence intervals were calculated.
Results
Demographic characteristics
Ten patients (mean age 43 years [standard deviation 7.7], 60% males) were included (Table 1). Six of them had successively received several anti-TNF biologics, enabling analysis of 19 prescriptions of TNF antagonists distributed as follows: eight infliximab (5 mg/kg/6 weeks), eight adalimumab (40 mg/2 weeks), and three etanercept (50 mg/1 week) (Table 2). The overall median anti-TNF-treatment duration was 10 (4–30) months, with respective mean infliximab, adalimumab, and etanercept durations of 13.3, 11.8, and 6.3 months. The total exposure durations were 17.6 patient-years, lasting, respectively, 7.08, 8.9, and 2.1 patient-years for infliximab, adalimumab, and etanercept.
Table 1.
Characteristics of the ten patients with sarcoidosis and evaluation of different parameters before and after 6 months of anti-TNF therapy
| Parameter | Before | After | P-value |
|---|---|---|---|
| Demographic | |||
| Age, years | 43 (36–71) | ||
| Female:male sex ratio | 6:4 | ||
| Disease evolution before anti-TNF, years | 3 (0.33–17) | ||
| Months of anti-TNF therapy | 10 (4–30) | ||
| Joint manifestation | |||
| Painful, n | 1 (0–28) | 1 (0–28) | 0.70 |
| Swollen, n | 0 (0–9) | 0 (0–1) | 0.26 |
| Global VAS score (0–100) | 80 (50–100) | 80 (30–90) | 0.53 |
| Extra-articular involvement | |||
| Pulmonary, n | 9 | 4 | 0.07 |
| Ocular, n | 2 | 0 | 0.47 |
| Biological | |||
| ESR (mm/1st hour) | 6 (1–30) | 6 (4–21) | 0.65 |
| CRP (mg/L) | 6.9 (1–13.6) | 6 (1–17) | 0.20 |
| DAS28 | |||
| With ESR | 3.57 (0.98–5.55) | 3.07 (0.7–5.62) | 0.82 |
| With CRP | 3.55 (2.58–6.05) | 3.44 (1.8–6.05) | 0.63 |
| Concomitant treatments | |||
| NSAIDs, n | 12 | 10 | 0.47 |
| Methotrexate, mg/week | 0 (0–25) | 0 (0–20) | 0.76 |
| Prednisone, mg/day | 5 (0–40) | 0 (0–10) | 0.039 |
Notes: The before and after TNF-antagonist therapy medians (range) of demographic, clinical, and biological characteristics were compared with Wilcoxon’s paired nonparametric test or McNemar’s test for quantitative or qualitative parameters, respectively. P<0.05 defined significance. Results are expressed as medians (range) unless stated otherwise.
Abbreviations: TNF, tumor necrosis factor; VAS, visual analog scale; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS, disease-activity score; NSAIDs, nonsteroidal anti-inflammatory drugs.
Table 2.
Distribution of the 19 prescriptions of TNF blockers among the ten patients
| Patients | Sex/age (years) | Sarcoidosis involvement
|
Anti-TNF (prescription in months) | Prednisone (mg/day) | MTX (mg/week) | DAS28 CRP | |
|---|---|---|---|---|---|---|---|
| Articular | Extra-articular | ||||||
| 1 | Woman (57) | Monoarthritis | Pulmonary | Infliximab (30 months) | |||
| Cardiac | Before | 5 | 0 | 3.15 | |||
| Muscular | After | 10 | 15 | 5.54 | |||
| Adalimumab (5 months) | |||||||
| Before | 10 | 15 | 5.54 | ||||
| After | 10 | 0 | 5.61 | ||||
| 2 | Woman (43) | Arthralgias | – | Adalimumab (8 months) | |||
| Before | 5 | 0 | 5.18 | ||||
| After | 0 | 0 | 3.44 | ||||
| Etanercept (7 months) | |||||||
| Before | 0 | 0 | 3.61 | ||||
| After | 5 | 0 | 2.83 | ||||
| Infliximab (10 months) | |||||||
| Before | 5 | 0 | 2.83 | ||||
| After | 0 | 0 | 2.95 | ||||
| 3 | Woman (61) | Arthralgias | Pulmonary | Infliximab (6 months) | |||
| Cutaneous | Before | 12 | 25 | 3.55 | |||
| Muscular | After | 3 | 12.5 | 6.05 | |||
| Adalimumab (12 months) | |||||||
| Before | 3 | 12.5 | 6.05 | ||||
| After | 0 | 0 | 2.55 | ||||
| 4 | Man (38) | Polyarthritis | Pulmonary | Adalimumab (18 months) | |||
| Before | 0 | 0 | 2.72 | ||||
| After | 0 | 0 | 2.13 | ||||
| 5 | Woman (39) | Arthralgias | Pulmonary | Infliximab (15 months) | |||
| Before | 0 | 0 | 5.24 | ||||
| After | 0 | 20 | 5.06 | ||||
| 6 | Man (36) | Monoarthritis | Pulmonary | Adalimumab (7 months) | |||
| Before | 5 | 15 | 2.59 | ||||
| After | 5 | 17.5 | 2.59 | ||||
| 7 | Woman (43) | Oligoarthritis | Pulmonary | Adalimumab (7 months) | |||
| Ocular | Before | 5 | 0 | 4.9 | |||
| After | 0 | 0 | 5.83 | ||||
| Etanercept (8 months) | |||||||
| Before | 5 | 0 | 5.83 | ||||
| After | 5 | 0 | 2.83 | ||||
| Infliximab (10 months) | |||||||
| Before | 5 | 10 | 2.83 | ||||
| After | 0 | 0 | 5.26 | ||||
| 8 | Woman (71) | Arthralgias | Pulmonary | Infliximab (16 months) | |||
| Ocular | Before | 20 | 25 | 2.58 | |||
| After | 10 | 27.5 | 4.91 | ||||
| Adalimumab (16 months) | |||||||
| Before | 10 | 27.5 | 4.91 | ||||
| After | 5 | 20 | 1.91 | ||||
| 9 | Man (39) | Polyarthritis | Cutaneous | Infliximab (11 months) | |||
| Before | 20 | 7.5 | 2.89 | ||||
| After | 0 | 7.5 | 4.3 | ||||
| Etanercept (4 months) | |||||||
| Before | 0 | 7.5 | 3.71 | ||||
| After | 0 | 7.5 | 3.45 | ||||
| Adalimumab (12 months) | |||||||
| Before | 0 | 7.5 | 3.45 | ||||
| After | 0 | 0 | 1.8 | ||||
| 10 | Man (61) | Arthralgia | – | Infliximab (9 months) | |||
| Before | 10 | 0 | 2.86 | ||||
Abbreviations: TNF, tumor necrosis factor; MTX, methotrexate; DAS, disease-activity score; CRP, C-reactive protein.
Before first anti-TNF therapy
Among the ten patients, five had arthralgias without swelling and five had arthritis, including two mono-, one oligo-, and two polyarthritis. Three patients also satisfied the European Spondyloarthropathy Study Group (ESSG) classification criteria.11 None of the patients fulfilled the American College of Rheumatology/EULAR criteria of rheumatoid arthritis. Their median (range) values for all the studied parameters are reported in Table 1. No biological inflammatory syndromes (CRP, ESR) were observed before initiation of TNF blockers. Before the beginning of TNF blockers, according to their DAS28-CRP scores, six patients had low articular involvement (DAS28 ≤3.2), two moderate (3.2< DAS28 ≤5.1), and two active involvement (DAS28 >5.1). Extra-articular involvement was distributed as follows: seven pulmonary, two ocular, one cardiac, and two muscular (Table 2).
The median interval between sarcoidosis diagnosis and start of the first biotherapy was 3 (0–17) years. Five patients did not receive methotrexate during the initiation of the TNFα inhibitor, but had received methotrexate in the previous months. The methotrexate was stopped because of liver cytolysis. One patient also suffered from renal impairment that led to methotrexate discontinuation. Nine patients received combined methotrexate and TNF antagonist, and ten received the biologic alone. Two-thirds of the patients took NSAIDs before starting the anti-TNF.
Under TNF antagonists
No significant anti-TNF impact on articular manifestations (numbers of painful and swollen joints, DAS28 with ESR or CRP, global VAS score), extra-articular involvement (pulmonary, ocular, cardiac, muscular), or biological indicators of inflammatory syndrome were observed (Table 1).
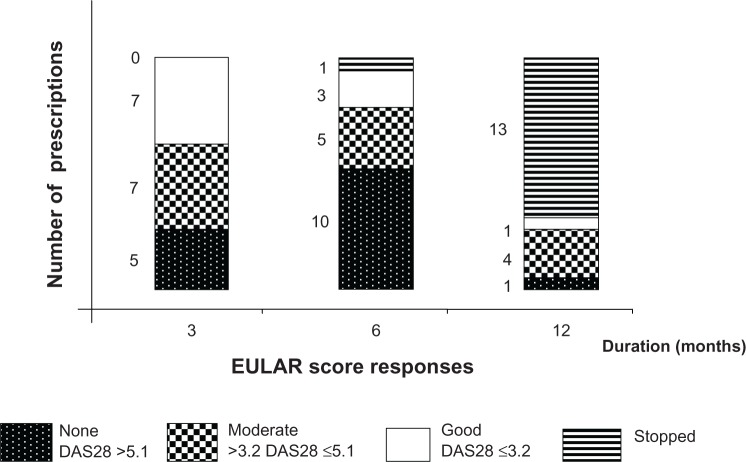
Considering all 19 anti-TNF prescriptions and the EULAR score of clinical response (9), nine achieved satisfactory (DAS28 ≤3.2) and five moderate responses (DAS28 >3.2–≤5.1), whereas five showed no change (DAS28 >5.1). At 6 months, five patients had DAS28 declines >1.2. However, anti-TNF effectiveness, evaluated using the EULAR score after 3, 6, and 12 months, showed initial moderate or satisfactory efficacy (14/19, 73.7%) at 3 months, followed by progressive therapeutic escapes leading to 13 treatment discontinuations because of insufficient responses at 1 year (Figure 1).
Figure 1.
Therapeutic responses evaluated at 3, 6, and 12 months of the according to the EULAR score for the 19 prescribed TNF antagonists. Five patients had DAS28 decreases >1.2 at 6 months.
Abbreviations: EULAR, European League Against Rheumatism; DAS, disease-activity score; TNF, tumor necrosis factor.
Moreover, TNF antagonists had no significant impact on methotrexate or NSAID use. In contrast, they allowed significant corticosteroid sparing, according to the number of patients (14 before and nine after), and almost halved the mean prednisone dose (6.3 versus 3.2 mg/day).
Among the 19 anti-TNF prescriptions, three engendered adverse events: two mild infections (vaginal mycosis and bronchitis not requiring hospitalization) and one toxiderma that resolved after treatment withdrawal. No cancer or severe infection occurred under treatment.
No radiological evidence for presence of noncaseating granulomas before or during anti-TNF treatment was observed.
Discussion
Only a small percentage of sarcoidosis patients have persistent joint involvement (15%–25%),12 eg, incapacitating polyarthralgia or mono-/oligoarthritis that requires DMARDs, and the risk of developing or aggravating pulmonary fibrosis is higher when extrapulmonary involvement is uncontrolled.13 Our observations indicate that the different TNF antagonists achieved rapid attenuation of sarcoidosis joint symptoms during the first 3 months, but failed to control any of the studied parameters (number of painful and swollen joints, global VAS, DAS28, and EULAR criteria) thereafter. This rapid regression of manifestations might be explained by a placebo effect or real efficacy that faded over time due to the immunogenicity of the anti-TNF antibodies predominantly prescribed to our study patients.
Pertinently, TNF-antagonist use did obtain significant, albeit moderate, corticosteroid sparing. Moreover, their safety seems good, because no severe adverse events occurred under treatment.
Two double-blinded randomized controlled trials versus placebo evaluated infliximab efficacy against pulmonary sarcoidosis. Rossman et al14 demonstrated improved lung vital capacity under this biologic, but did not assess joint involvement, whereas Hostettler et al’s investigation completing the former showed infliximab efficacy against corticoresistant sarcoidosis, especially for patients with extrapulmonary disease.15 Indeed, six of the eleven patients with extrapulmonary involvement were in remission on biotherapy. Jounieaux et al16 retrospectively examined infliximab efficacy against pulmonary and/or systemic (≥four organs involved) sarcoidosis and found a beneficial response at 13±12 months for 62% of the patients for all organs combined, with 65% efficacy for lung, 67% for skin, and 50% for central nervous system involvement. However, the infliximab effect was not significant for lung involvement. The results of those studies did not consider joint changes under that TNF antagonist.
Judson et al conducted the only trial to evaluate sarcoidosis joint manifestations under infliximab.17 That prospective randomized controlled versus placebo study on 138 patients assessed biologic efficacy against extrapulmonary involvement of sarcoidosis. No satisfactory impact on joint disease was observed at 24 and 48 weeks, even though significant attenuation of involvement for all organs combined was observed at 28 weeks. A weakness of that study was that 20 patients with joint manifestations of sarcoidosis were evaluated with a subjective judgment criterion (the physician’s evaluation). Thus, the effectiveness of TNF-blocking agents in sarcoidosis have not been demonstrated, even in patients with extra pulmonary disease. The results of our study are therefore consistent with the conclusion of a recent review by Maneiro et al18 that stated that there was insufficient evidence to ensure the efficacy of TNF antagonists in sarcoidosis.
Finally, infliximab did not show evidence of its efficiency in articular manifestations of sarcoidosis. This monoclonal antibody directed against TNF may not be active in joint granulomas. The small number of patients in our observation may explain why the DAS28 worsened after infliximab.
Controlled-trial results have demonstrated etanercept inefficacy against respiratory and chronic ocular sarcoidosis involvement.19,20 However, those studies did not address patients’ rheumatological manifestations before and after biotherapy administration. In addition, some monoclonal anti-TNF agents induced sarcoidosis, as we previously described.21 Concerning our three etanercept prescriptions, they were second-/third-line treatments given after monoclonal antibody failure to patients whose clinical pictures were intermediary between sarcoidosis and ankylosing spondyloarthritis, according to ESSG criteria.11 A subanalysis of the different parameters before and after starting anti-TNF identified no significant differences. The efficacies of the soluble receptor and the monoclonal anti-TNF antibodies (infliximab and adalimumab) could not be compared herein because of the small numbers of patients included.
Corticosteroid sparing is essential in chronic pathologies to contain the occurrence of iatrogenic complications, like Cushing’s syndrome, diabetes, or steroid-induced osteoporosis.22 Jounieaux et al observed corticosteroid sparing, estimated at 2.8 mg/day, close to that observed herein (3.1 mg/day).16 Baughman et al did not find a decline of the corticoid dose (topical or systemic) after adding etanercept to the regimen.19 Our patients’ methotrexate and NSAID use also diminished, but not significantly, by 8.8% and 8.3%, respectively.
Our patients’ mild inflammatory syndromes also decreased nonsignificantly after the adjunction of anti-TNF, with CRP and ESR being 19.8% and 12.6% lower (P=0.2 and 0.65), respectively.
The small sample size of patients did not allow us to study the dose-dependent response to TNF inhibition in sarcoidosis, since all but one of the patients received a dose of 5 mg/kg of infliximab (only one patient began the infliximab treatment with a 3 mg/kg starting dose that was enhanced to 5 mg/kg after 3 months).
We observed good safety of the different biotherapies, with three side effects for a total duration of exposure of 17.6 patient-years, with mild toxiderma the only notable toxicity under infliximab. In contrast, Jounieaux et al reported that 13 of 31 patients developed adverse events, seven severe, sometimes requiring infliximab withdrawal.16 This difference is probably attributable to the different population they studied: longer-standing (9 years) and systemic (a mean of four organs involved) sarcoidosis. In their multicenter study, Rossman et al evaluated the contribution of infliximab against corticoresistant pulmonary sarcoidosis14: four of 19 patients had severe disease. In a study evaluating etanercept efficacy against pulmonary sarcoidosis, two of the 17 patients included developed severe side effects (lymphoma and plasmacytoma).14
One of the limits of our study was the small number of patients included retrospectively. However, this population had sarcoidosis characterized by quasi-exclusive joint involvement, sometimes diagnosed with sarcoidosis and ankylosing spondyloarthritis overlap syndrome. This association has already been described.22–25 Inflammatory joint pain has already been described as the most frequent articular involvement of sarcoidosis.25 We assessed disease activity with the composite EULAR score usually used for rheumatoid arthritis, because no validated score is available to evaluate the evolution of the inflammatory joint activity of sarcoidosis under treatment. Moreover, calculating a composite index (DAS28) in a group of patients with low ESR and CRP and low frequency of clinically overt arthritis is probably not the most relevant method of evaluation.
To our knowledge, this is the only study to address specifically the efficacy and safety of TNF antagonists (infliximab, adalimumab, and etanercept) in the context of sarcoidosis joint involvement. However, to assess objectively anti-TNF efficacy for this rare indication, a prospective randomized controlled trial on a larger population will be necessary.
Footnotes
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Baughman RP, Costabel U, Du Bois RM. Treatment of sarcoidosis. Clin Chest Med. 2008;29:533–548. doi: 10.1016/j.ccm.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Sato A, Toyoshima M, et al. Weekly low-dose methotrexate therapy for sarcoidosis. Intern Med. 1994;33:437–440. doi: 10.2169/internalmedicine.33.437. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane JT. Prognosis in sarcoidosis. Br Med J (Clin Res Ed) 1984;26:1557–1558. doi: 10.1136/bmj.288.6430.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anholt LM, Roberts H. Sarcoidosis and polyarthritis. Can Med Assoc J. 1965;93:293–297. [PMC free article] [PubMed] [Google Scholar]
- 5.Seitzer U, Swider C, Stüber F, et al. Tumour necrosis factor promoter gene polymorphism in sarcoidosis. Cytokine. 1997;9:787–790. doi: 10.1006/cyto.1997.0224. [DOI] [PubMed] [Google Scholar]
- 6.Antoniu SA. Targeting the TNF-alpha pathway in sarcoidosis. Expert Opin Ther Targets. 2010;14:21–29. doi: 10.1517/14728220903449244. [DOI] [PubMed] [Google Scholar]
- 7.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 8.Pham T, Bachelez H, Berthelot JM, et al. TNF alpha antagonist therapy and safety monitoring. Joint Bone Spine. 2011;78(Suppl 1):15–185. doi: 10.1016/S1297-319X(11)70001-X. [DOI] [PubMed] [Google Scholar]
- 9.Fouache D, Goëb V, Massy-Guillemant N, et al. Paradoxical adverse events of anti-tumour necrosis factor therapy for spondyloarthropathies: a retrospective study. Rheumatology (Oxford) 2009;48:761–764. doi: 10.1093/rheumatology/kep083. [DOI] [PubMed] [Google Scholar]
- 10.van der Heijde DM, van ‘t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579–581. [PubMed] [Google Scholar]
- 11.Dougados M, Van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 12.Visser H, Vos K, Zanelli E, et al. Sarcoid arthritis: clinical characteristics, diagnostic aspects, and risk factors. Ann Rheum Dis. 2002;61:499–504. doi: 10.1136/ard.61.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gran JT, Bøhmer E. Acute sarcoid arthritis: a favourable outcome? A retrospective survey of 49 patients with review of the literature. Scand J Rheumatol. 1996;25:70–73. doi: 10.3109/03009749609069210. [DOI] [PubMed] [Google Scholar]
- 14.Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:201–208. [PubMed] [Google Scholar]
- 15.Hostettler KE, Studler U, Tamm M, Brutsche MH. Long-term treatment with infliximab in patients with sarcoidosis. Respiration. 2012;83:218–224. doi: 10.1159/000328738. [DOI] [PubMed] [Google Scholar]
- 16.Jounieaux F, Chapelon C, Valeyre D, et al. Infliximab treatment for chronic sarcoidosis – a case series. Rev Mal Respir. 2010;27:685–692. doi: 10.1016/j.rmr.2010.06.011. French. [DOI] [PubMed] [Google Scholar]
- 17.Judson MA, Baughman RP, Costabel U, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008;31:1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 18.Maneiro JR, Salgado E, Gomez-Reino JJ, Carmona L. Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthitis Rheum. 2012;42:89–103. doi: 10.1016/j.semarthrit.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Baughman RP, Lower EE, Bradley DA, Raymond LA, Kaufman A. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest. 2005;128:1062–1047. doi: 10.1378/chest.128.2.1062. [DOI] [PubMed] [Google Scholar]
- 20.Utz JP, Limper AH, Kalra S, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124:177–185. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- 21.Josse S, Klemmer N, Moreno-Swirc S, Goëb V, Lequerré T, Vittecoq O. Infliximab induced skin and pulmonary sarcoidosis in a rheumatoid arthritis patient. Joint Bone Spine. 2009;76:718–719. doi: 10.1016/j.jbspin.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res. 1999;14:1061–1066. doi: 10.1359/jbmr.1999.14.7.1061. [DOI] [PubMed] [Google Scholar]
- 23.El Ouazzani FZ, Tahiri L, Akasbi N, Kadi N, Harzy T. Sarcoidosis and ankylosing spondylitis: a rare association. Pan Afr Med J. 2011;8:23. doi: 10.4314/pamj.v8i1.71080. French. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochi S, Nanki T, Kaneko H, et al. Successful treatment of ankylosing spondylitis coexisting with pulmonary sarcoidosis by infliximab. Clin Exp Rheumatol. 2009;27:698–699. [PubMed] [Google Scholar]
- 25.Alaoui FZ, Talaoui M, Benamour S. Osteo-articular manifestations of sarcoidosis. Presse Med. 2012;15(34):19–24. doi: 10.1016/s0755-4982(05)83878-3. French. [DOI] [PubMed] [Google Scholar]