Abstract
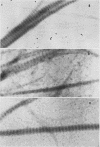
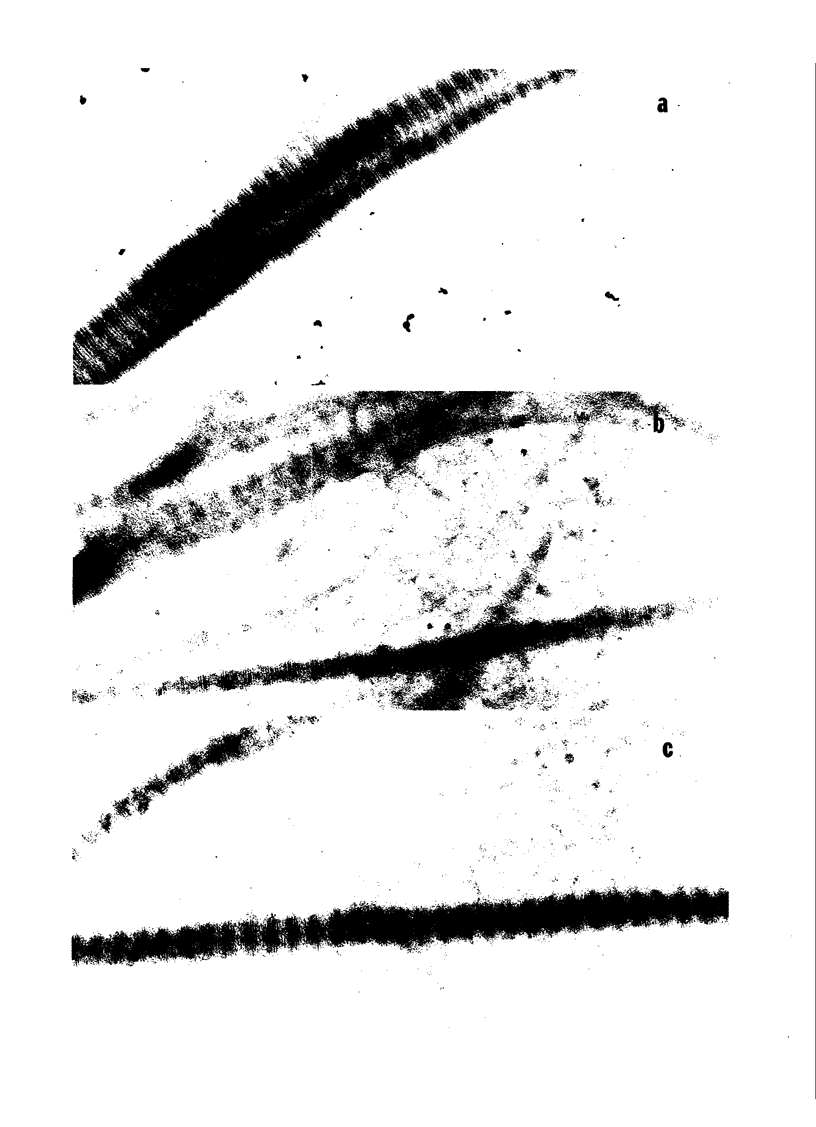
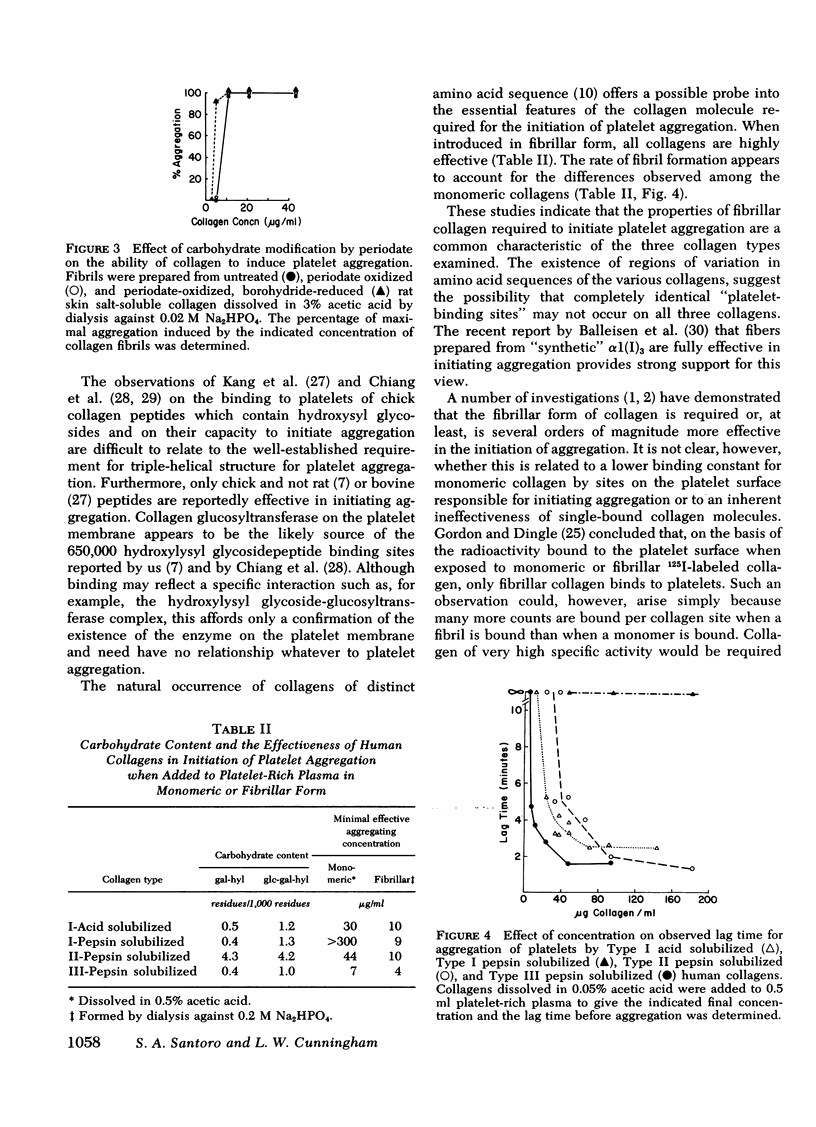
We have shown previously that periodate oxidation of collagen carbohydrate does not affect its ability to aggregate platelets. We now describe an additional characterization of periodate-modified collagen which demonstrates that collagen devoid of intact carbohydrate is fully capable of fibril formation, and we confirm its capacity to initiate platelet aggregation. Furthermore, we demonstrate that the platelet aggregating abilities of Types I, II, and III fibrillar collagen are quite similar despite differences in carbohydrate content and amino acid sequence. We also demonstrate that monomeric, pepsin-solubilized Type I human collagen is ineffective inhibiting aggregation by performed fibrils derived from the same molecule, thus establishing that the affinity of platelets for collagen depends upon prior polymerization of collagen. We interpret these and other findings to demonstrate that the hydroxylysyl glycoside regions of collagen are not highly specific sites involved in platelet-collagen interactions leading to "physiological" aggregation, and that the possibility must be considered that multiple interactions involving collagen sites of comparatively low structural specificity may be the initiating events in release of platelet ADP and the ensuing aggregation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balleisen L., Gay S., Marx R., Kühn K. Comparative investigation on the influence of human and bovine collagen types I, II and III on the aggregation of human platelets. Klin Wochenschr. 1975 Oct 1;53(19):903–905. doi: 10.1007/BF01468982. [DOI] [PubMed] [Google Scholar]
- Balleisen L., Marx R., Kühn K. Platelet-collagen interaction. The influence of native and modified collagen (Type I) on the aggregation of human platelets. Haemostasis. 1976;5(3):155–164. doi: 10.1159/000214131. [DOI] [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Platelet collagen adhesion characterization of collagen glucosyltransferase of plasma membranes of human blood platelets. Biochim Biophys Acta. 1971 Dec 21;252(3):533–545. doi: 10.1016/0304-4165(71)90156-5. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Gordon J. L., MacIntyre D. E. Platelet-aggregating activity of type I and type III collagens from human aorta and chicken skin. Biochem J. 1976 Dec 15;160(3):647–651. doi: 10.1042/bj1600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H. R. Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with alpha chymotrypsin-digested subendothelium. Thromb Haemost. 1977 Feb 28;37(1):1–16. [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Bensusan H. B. On the role of the collagen carbohydrate residues in the platelet. Collagen interaction. Biochim Biophys Acta. 1976 Aug 24;444(1):43–52. doi: 10.1016/0304-4165(76)90222-1. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Bensusan H. B. The role of collagen quaternary structure in the platelet: collagen interaction. J Clin Invest. 1974 Dec;54(6):1480–1487. doi: 10.1172/JCI107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. R., Gross J. High-resolution analysis of the modified quarter-stagger model of the collagen fibril. Biopolymers. 1974 May;13(5):931–941. doi: 10.1002/bip.1974.360130509. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Beachey E. H., Kang A. H. Binding of collagen alpha1 chains to human platelets. J Clin Invest. 1977 Mar;59(3):405–411. doi: 10.1172/JCI108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T. M., Beachey E. H., Kang A. H. Interaction of a chick skin collagen fragment (alpha1-CB5) with human platelets. Biochemical studies during the aggregation and release reaction. J Biol Chem. 1975 Sep 10;250(17):6916–6922. [PubMed] [Google Scholar]
- Crothers D. M., Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972 Mar;9(3):341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D., Segrest J. P. The isolation of identical hydroxylysyl glycosides from hydrolysates of soluble collagen and from human urine. J Biol Chem. 1967 May 25;242(10):2570–2571. [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Dingle J. T. Binding of radiolabelled collagen to blood platelets. J Cell Sci. 1974 Oct;16(1):157–166. doi: 10.1242/jcs.16.1.157. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Simons E. R., Chesney C. M., Colman R. W. The effect of chemical or enzymatic modifications upon the ability of collagen to form multimers and to initiate platelet aggregation. Thromb Res. 1975 Jul;7(1):113–122. doi: 10.1016/0049-3848(75)90129-2. [DOI] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Jaffe R., Deykin D. Evidence for a structural requirement for the aggregation of platelets by collagen. J Clin Invest. 1974 Mar;53(3):875–883. doi: 10.1172/JCI107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Urban C. L., Barber A. J. Enzymatic basis for platelet: collagen adhesion as the primary step in haemostasis. Nat New Biol. 1971 Nov 3;234(44):5–7. doi: 10.1038/newbio234005a0. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Beachey E. H., Katzman R. L. Interaction of an active glycopeptide from chick skin collagen (alpha 1-CB5) with human platelets. J Biol Chem. 1974 Feb 25;249(4):1054–1059. [PubMed] [Google Scholar]
- Menashi S., Harwood R., Grant M. E. Native collagen is not a substrate for the collagen glucosyltransferase of platelets. Nature. 1976 Dec 16;264(5587):670–672. doi: 10.1038/264670a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Puett D., Wasserman B. K., Ford J. D., Cunningham L. W. Collagen-mediated platelet aggregation. Effects of collagen modification involving the protein and carbohydrate moieties. J Clin Invest. 1973 Oct;52(10):2495–2506. doi: 10.1172/JCI107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Stark M., Miller E. J., Kühn K. Comparative electron-microscope studies on the collagens extracted from cartilage, bone, and skin. Eur J Biochem. 1972 May;27(1):192–196. doi: 10.1111/j.1432-1033.1972.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wojtecka-Lukasik E., Sopata I., Wize J., Skonieczna M., Niedzwiecka-Namyslowska I., Kowalski E. Adhesion of platelets to collagen devoid of telopeptides. Thromb Diath Haemorrh. 1967 Aug 15;18(1-2):76–79. [PubMed] [Google Scholar]