Abstract
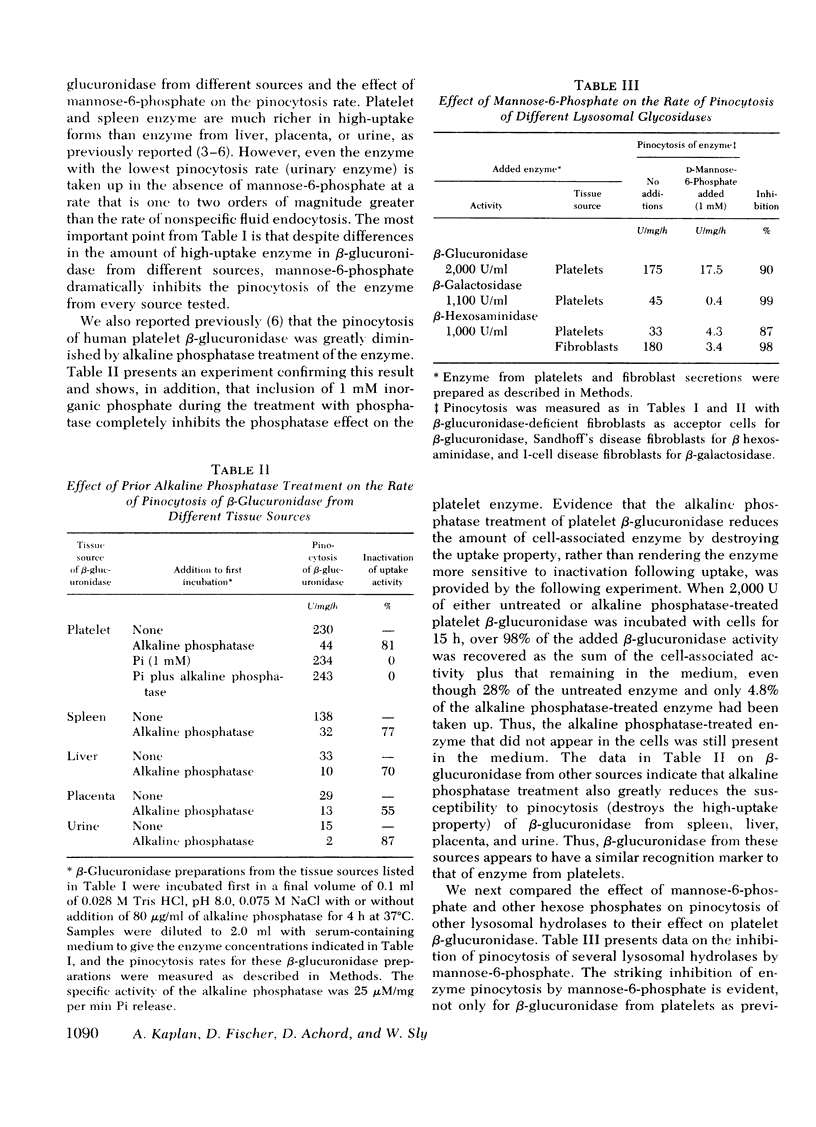
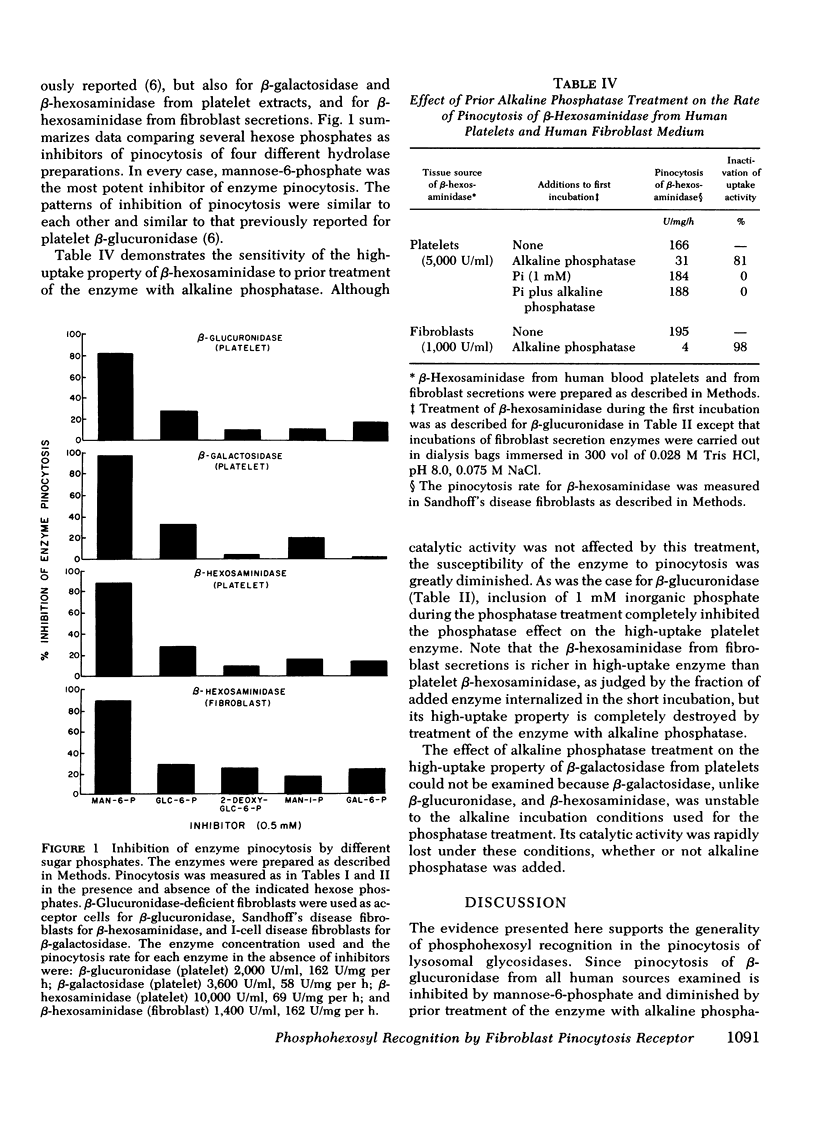
We recently presented data showing that mannose-6-phosphate was a potent competitive inhibitor of pinocytosis of human platelet beta-glucuronidase, and that treatment of "high-uptake" forms of the enzyme with alkaline phosphatase destroyed the high-uptake property of the enzyme without diminishing its catalytic activity. These data indicate that phosphate is a necessary component of the recognition marker on the enzyme for pinocytosis by human fibroblasts, and suggest that the phosphate on high-uptake forms of the enzyme is present as a phosphohexosyl moiety. Results presented here show that mannose-6-phosphate is also a potent inhibitor of pinocytosis of the following enzyme preparations: (a) beta-glucuronidase from human spleen, liver, placenta, and urine; (b) beta-hexosaminidase and beta-galactosidase from human platelets; (c) beta-hexosaminidase from human fibroblast secretions. Alkaline phosphatase treatment of all these enzymes except beta-galactosidase, which was unstable to the incubation conditions and could not be tested, greatly diminished the uptake activity of the enzymes without diminishing their catalytic activity. These results suggest that phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Brot F. E., Glaser J. H., Roozen K. J., Sly W. S., Stahl P. D. In vitro correction of deficient human fibroblasts by beta-glucuronidase from different human sources. Biochem Biophys Res Commun. 1974 Mar 15;57(1):1–8. doi: 10.1016/s0006-291x(74)80349-9. [DOI] [PubMed] [Google Scholar]
- Davis L. G., Costello A. J., Javaid J. I., Brunngraber E. G. 31P nuclear magnetic resonance studies on the phosphoglycopeptides obtained from rat brain glycoprotein. FEBS Lett. 1976 May 15;65(1):35–38. doi: 10.1016/0014-5793(76)80615-1. [DOI] [PubMed] [Google Scholar]
- Davis L. G., Javaid J. I., Brunngraber E. G. Identification of phosphoglycoproteins obtained from rat brain. FEBS Lett. 1976 May 15;65(1):30–34. doi: 10.1016/0014-5793(76)80614-x. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Roozen K. J., Brot F. E., Sly W. S. Multiple isoelectric and recognition forms of human beta-glucuronidase activity. Arch Biochem Biophys. 1975 Feb;166(2):536–542. doi: 10.1016/0003-9861(75)90417-8. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Sly W. S. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973 Dec;82(6):969–977. [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Hickman S., Shapiro L. J., Neufeld E. F. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974 Mar 15;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- Hieber V., Distler J., Myerowitz R., Schmickel R. D., Jourdian G. W. The role of glycosidically bound mannose in the assimilation of beta-galactosidase by generalized gangliosidosis fibroblasts. Biochem Biophys Res Commun. 1976 Dec 6;73(3):710–717. doi: 10.1016/0006-291x(76)90868-8. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagunoff D., Nicol D. M., Pritzi P. Uptake of beta-glucuronidase by deficient human fibroblasts. Lab Invest. 1973 Oct;29(4):449–453. [PubMed] [Google Scholar]
- Miller A. L., Frost R. G., O'Brien J. Purification of human liver acid beta-D-galactosidases using affinity chromatography. Anal Biochem. 1976 Aug;74(2):537–545. doi: 10.1016/0003-2697(76)90236-0. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Cantz M. J. Corrective factors for inborn errors of mucopolysaccharide metabolism. Ann N Y Acad Sci. 1971 Jul 6;179:580–587. doi: 10.1111/j.1749-6632.1971.tb46934.x. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Lim T. W., Shapiro L. J. Inherited disorders of lysosomal metabolism. Annu Rev Biochem. 1975;44:357–376. doi: 10.1146/annurev.bi.44.070175.002041. [DOI] [PubMed] [Google Scholar]
- von Figura K., Kresse H. Quantitative aspects of pinocytosis and the intracellular fate of N-acetyl-alpha-D-glucosaminidase in Sanfilippo B fibroblasts. J Clin Invest. 1974 Jan;53(1):85–90. doi: 10.1172/JCI107563. [DOI] [PMC free article] [PubMed] [Google Scholar]


