Background: The eukaryotic translation initiation factor 2 (eIF2) is a heterotrimeric G-protein.
Results: Cdc123, a conserved cell proliferation protein, binds the unassembled eIF2γ subunit to promote eIF2 complex formation.
Conclusion: Cdc123 is a specific eIF2 assembly factor indispensable for the onset of protein synthesis.
Significance: This study describes a novel step in the eukaryotic translation initiation pathway.
Keywords: Molecular Biology, Protein Assembly, Protein Synthesis, Protein-Protein Interactions, Translation Initiation Factors, Yeast Genetics, Cdc123, eIF2, Translation Initiation
Abstract
The eukaryotic translation initiation factor 2 (eIF2) is central to the onset of protein synthesis and its modulation in response to physiological demands. eIF2, a heterotrimeric G-protein, is activated by guanine nucleotide exchange to deliver the initiator methionyl-tRNA to the ribosome. Here we report that assembly of the eIF2 complex in vivo depends on Cdc123, a cell proliferation protein conserved among eukaryotes. Mutations of CDC123 in budding yeast reduced the association of eIF2 subunits, diminished polysome levels, and increased GCN4 expression indicating that Cdc123 is critical for eIF2 activity. Cdc123 bound the unassembled eIF2γ subunit, but not the eIF2 complex, and the C-terminal domain III region of eIF2γ was both necessary and sufficient for Cdc123 binding. Alterations of the binding site revealed a strict correlation between Cdc123 binding, the biological function of eIF2γ, and its ability to assemble with eIF2α and eIF2β. Interestingly, high levels of Cdc123 neutralized the assembly defect and restored the biological function of an eIF2γ mutant. Moreover, the combined overexpression of eIF2 subunits rescued an otherwise inviable cdc123 deletion mutant. Thus, Cdc123 is a specific eIF2 assembly factor indispensable for the onset of protein synthesis. Human Cdc123 is encoded by a disease risk locus, and, therefore, eIF2 biogenesis control by Cdc123 may prove relevant for normal cell physiology and human health. This work identifies a novel step in the eukaryotic translation initiation pathway and assigns a biochemical function to a protein that is essential for growth and viability of eukaryotic cells.
Introduction
The translation of mRNAs into proteins in eukaryotic cells is initiated in a multistep pathway that requires the coordinated action of several initiation factors (1, 2). Among these, eukaryotic translation initiation factor 2 (eIF2)4 serves as a GTP-dependent carrier of the initiator methionyl-tRNA (3, 4). eIF2 is a heterotrimeric protein complex composed of the nucleotide-binding core subunit eIF2γ to which the smaller eIF2α and eIF2β subunits bind. Following activation by guanine nucleotide exchange, eIF2-GTP recruits the initiator tRNA to the 40S ribosome and participates in scanning of the mRNA. Upon recognition of the start codon, eIF2 is converted into its GDP-bound form, releases the initiator tRNA and dissociates from the ribosome. Rearrangements in the physical contacts of eIF2 are thought to play a central role in translation initiation and selection of the start codon (5, 6).
In addition to its essential function as a carrier of the initiator tRNA, eIF2 is also an important target for the regulation of protein synthesis in response to stress conditions, such as nutrient deprivation or viral infection (7, 8). Under these conditions, eIF2 is phosphorylated on its α-subunit and forms an inactive complex with its guanine nucleotide exchange factor (GEF) eIF2B. The consequent impairment of eIF2 reactivation reduces global translation and reprograms gene expression by enhancing mRNA translation of transcriptional activators of stress adaptation genes (9).
A link of eIF2 to the conserved cell proliferation protein Cdc123 was suggested by several proteomic studies that reported interaction of eIF2γ with Cdc123 in budding yeast (10–14). Cdc123 was first described in a rat fibroblast line carrying a temperature-sensitive allele which blocked the G1-S transition and prevented serum-induced cell cycle entry of quiescent cells (15, 16). More recently, human CDC123 was described as a candidate oncogene in breast cancer (17), a candidate risk locus for type II diabetes (18) and a gene associated with lung function (19). Expression of the human ortholog restored viability to a cdc123 deletion mutant of budding yeast (20) suggesting that the biological function of Cdc123 family members is conserved. However, the molecular mechanism by which Cdc123 acts has remained obscure. Previously, Brenner and co-workers suggested that Cdc123 serves to stabilize eIF2γ in budding yeast by antagonizing the putative ubiquitin ligases Chf1 and Chf2 (20). Since Cdc123 remained essential for cell viability in the absence of Chf1 and Chf2 (20), the proposed role of Cdc123 in stabilizing eIF2γ did not explain the vital necessity of Cdc123. Here we report that Cdc123 makes an essential contribution to the onset of mRNA translation by assembling the eIF2 complex from its three protein subunits.
EXPERIMENTAL PROCEDURES
Yeast Methods
Standard protocols (21) were followed for growth, transformation, mating, sporulation and tetrad dissection of yeast cells. Strains used in this study are isogenic derivatives of either S288C or W303 and are listed in supplemental Table S1. Deletion strains were purchased from EUROSCARF, Frankfurt. Cells were grown in YEP complex medium containing adenine (100 mg/liter), tryptophan (200 mg/liter), and KH2PO4 (10 mm) supplemented with 2% glucose (XYD) or 2% raffinose and 2% galactose (XYRG). Strains carrying episomal plasmids were grown in synthetic complete medium. For growth assays, yeast cells were harvested from exponentially growing cultures, resuspended in H2O at OD600, 1.0, and spotted in 10-fold dilution series on XYD solid media. Plates were incubated for 2–3 days at 25 °C, 30 °C, or 37 °C as indicated.
DNA Constructs and Genetic Manipulations
Genes were amplified from yeast genomic DNA (BY4741) by PCR with primers containing restriction sites for subsequent cloning. The human CDC123 homolog hD123 was amplified from the cDNA clone pEGFP-C3-D123 (22). All PCR-amplified constructs were verified by DNA sequencing (Seqlab). Yeast plasmid constructs were derived from pRS vectors (23). Y2H vectors were pEG202 and pJG4–5 (21), and Escherichia coli expression plasmids were derived from pJOE2955 (24) and pJOE4056.2 (25). For coexpression of mbp- and his6-fusions, we constructed a bicistronic expression plasmid based on pJOE2955. This plasmid contains the rhamnose-inducible promoter prha followed by the coding sequence of a maltose-binding protein (mbp)-GCD11 fusion, a ribosome binding site (RBS) and a his6-CDC123 fusion sequence.
C-terminal epitope fusions of endogenous genes were constructed by PCR-based epitope tagging (26). This strategy was also used for the C-terminal truncation of CDC123 (cdc123Δ327) following the codon for the indicated amino acid (see supplemental Fig. S1).
Temperature-sensitive alleles of CDC123 were created by PCR-mediated random mutagenesis of the coding region, transplacement to the CDC123 locus, and screening of strains for failure to grow at 37 °C. Mutations were determined by plasmid rescue and DNA sequencing. The cdc123-1 allele contains a single point mutation (G847A) resulting in the substitution of a highly conserved amino acid (E283K, see supplemental Fig. S1). For regulated expression, the cdc123-1 allele was placed under the control of the repressible GALL promoter (27).
Yeast-Two-Hybrid Assay
The reporter strain W276 was co-transformed with plasmids derived from pEG202 and pJG4–5 (listed in supplemental Table S2). Transformants were grown in synthetic complete medium with 2% raffinose lacking histidine and tryptophan at 25 °C. Expression of activation domain (AD)-fusions was induced by addition of 2% galactose for 4 h. β-Galactosidase assays were performed as described (21).
GCN4-lacZ Assay
Yeast strains used for GCN4-lacZ assays are haploid progenies of heterozygous diploid strains transformed with reporter plasmids. Strains W10897-W10902 carrying p180 (28) were grown in synthetic complete medium with 2% glucose lacking uracil at 25 °C. Strains W10903-W10906 carrying a LEU2-derivative of p180 (pWS3396) were grown in synthetic complete medium with 2% glucose without leucine at 25 °C and shifted to 30 °C for 4 h before measurement. β-Galactosidase assays (21) were performed in triplicates.
Polysome Profiles
Polysome analysis was done essentially as described (29). 150 ml of yeast culture were harvested in mid-exponential growth phase and washed once in ice cold lysis buffer A (100 mm NaCl, 30 mm MgCl2, 10 mm Tris-HCl, pH 7.5) containing 100 μg/ml cycloheximide. After resuspension in 1 ml of ice cold lysis buffer A containing 100 μg/ml cycloheximide crude cell extracts were prepared. Glass beads and cell debris were removed by centrifugation for 5 min at 2000 × g followed by another 5 min at 7500 × g at 4 °C. 800 μl of supernatant were layered onto a 10–50% sucrose gradient prepared in lysis buffer A without cycloheximide. The gradient was centrifuged at 34,000 rpm for 170 min at 4 °C in a Sorvall TH-641 rotor and subsequently drawn through a spectrophotometer to monitor the A254 nm profile.
Metabolic Labeling
Total protein synthesis was analyzed by metabolic labeling essentially as described (30). Yeast strains grown at 25 °C in synthetic complete glucose (2%) medium lacking cysteine and methionine (SD-CM) were shifted to 30 °C for 4 h. Cultures were diluted to OD600, 0.5 in SD-CM, and 1 μCi/ml 35S-label containing l-[35S]methionine and l-[35S]cysteine (EasyTagTM EXPRESS35S Protein Labeling Mix, PerkinElmer, NEG772002MC) was added together with unlabeled cysteine and methionine (50 μm each). Cultures were incubated at 30 °C, and aliquots were taken at 15-min intervals. Trichloroacetic acid-precipitated 35S-label was quantified by scintillation counting.
Yeast Cell Extracts and Immunoprecipitation
Whole cell extracts were made as described (31). For immunoprecipitations, extracts containing equal amounts of total protein were incubated with specific antibodies for 2 h at 4 °C. After addition of 40 μl of protein A-agarose (Santa Cruz Biotechnology), the samples were incubated for another 2 h at 4 °C. Beads were collected by centrifugation, washed three times with lysis buffer, resuspended in Lämmli sample buffer, and boiled for 5–10 min. The following antibodies were used for immunoprecipitations (see also supplemental Table S3): mouse monoclonal antibody 9E10 for α-myc and rabbit antisera for Sui2 (32) and Sui3 (33). For α-flag immunoprecipitations, extracts were incubated with agarose-conjugated mouse monoclonal α-flag antibody M2 (Sigma-Aldrich) for 2–3 h at 4 °C.
Western Blot Analysis
SDS-PAGE and Western blot analysis were done essentially as described (31). Primary antibodies used in this study are listed in supplemental Table S3, and antisera are described below. The Western blots shown in Figs. 2, A, D, E, and 5D were detected with HRP-conjugated secondary antibodies (31). For detection of the Western blots shown in Figs. 1E, 2C, 3, 4, and 5A IRDye secondary antibodies and an Odyssey Infrared Imaging System (LI-COR Biosciences) were used.
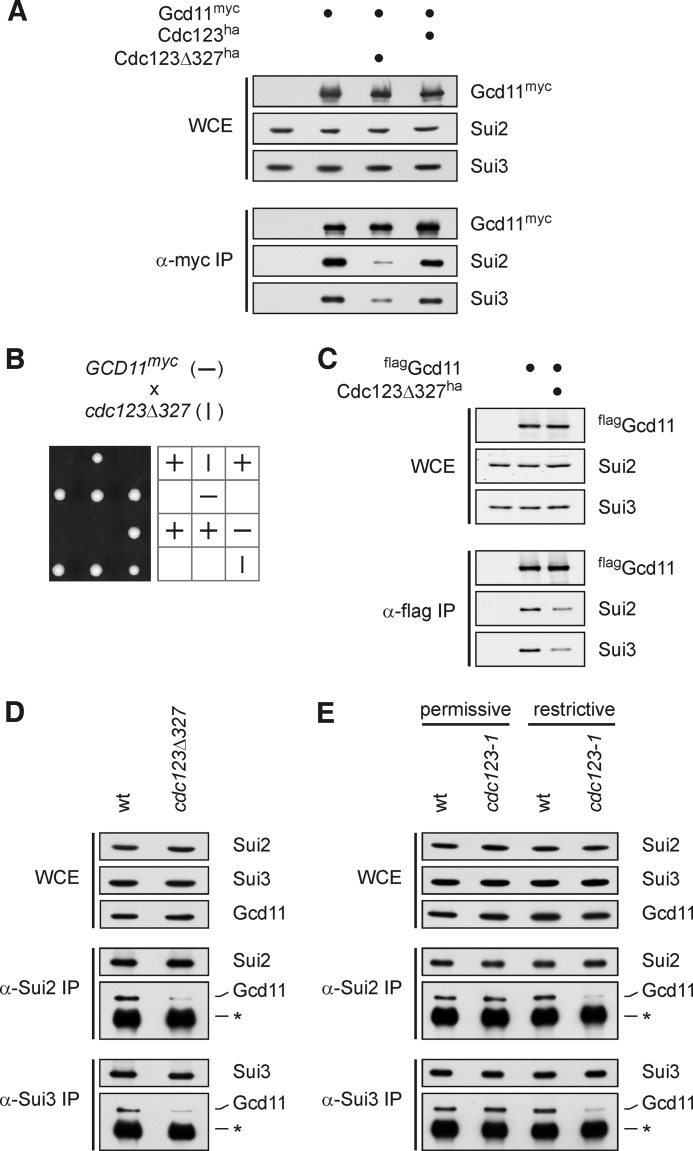
FIGURE 2.
Cdc123 is important for integrity of the eIF2 complex. A and C–E, integrity of the eIF2 complex in cdc123 mutants was analyzed by immunoprecipitation. Levels of eIF2 subunit proteins in WCE and immunoprecipitates (IP) were determined by Western analysis. A, Gcd11-myc was precipitated from a diploid cdc123Δ327 mutant and control strains. B, synthetic lethal interaction of GCD11-myc and cdc123Δ327 alleles. Strains of the indicated genotypes were crossed and the meiotic progeny was analyzed by tetrad dissection. The allele segregation pattern is symbolized by vertical and horizontal lines. C, flag-Gcd11 was precipitated from a haploid cdc123Δ327 mutant and control strains. D and E, Sui2 and Sui3 were precipitated, and associated Gcd11 was analyzed in a cdc123Δ327 mutant strain (D) and in a pGALL-cdc123–1 strain grown in galactose medium at 25 °C (permissive) or shifted to glucose medium at 37 °C for 4 h (restrictive) (E). The asterisk indicates IgG heavy chain.
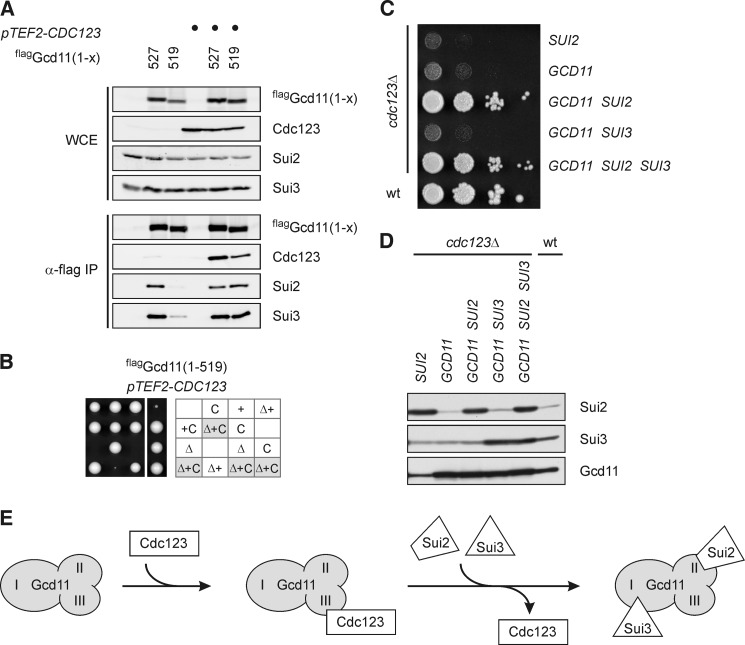
FIGURE 5.
eIF2 complex formation is the essential function of Cdc123. A and B, rescue of Gcd11-(1–519) by overexpression of CDC123. Full-length flag-Gcd11-(1–527) or the truncated version flag-Gcd11(1–519) in the absence or presence of overexpressed Cdc123 (pTEF2-CDC123) were immunoprecipitated and analyzed for interaction of Cdc123, Sui2, and Sui3. Protein levels in WCE and anti-flag immunoprecipitates (α-flag IP) were determined by Western analysis (A). A gcd11Δ/GCD11 heterozygous diploid strain carrying the flag-GCD11-(1–519) and pTEF-CDC123 constructs was sporulated and meiotic progeny was analyzed by tetrad dissection. Δ, gcd11Δ; +, flag-GCD11(1–519); C, pTEF-CDC123 (B). C and D, rescue of a cdc123 deletion mutant by overexpression of eIF2 subunits. Cells were spotted in serial dilutions on an agar plate, and growth was monitored at 30 °C (C). Overexpression of eIF2 subunits was confirmed by Western blotting (D). E, model of Cdc123 function. Through binding to domain III of Gcd11, Cdc123 promotes association of Gcd11 with Sui2 and Sui3, i.e. formation of the heterotrimeric eIF2 protein complex.
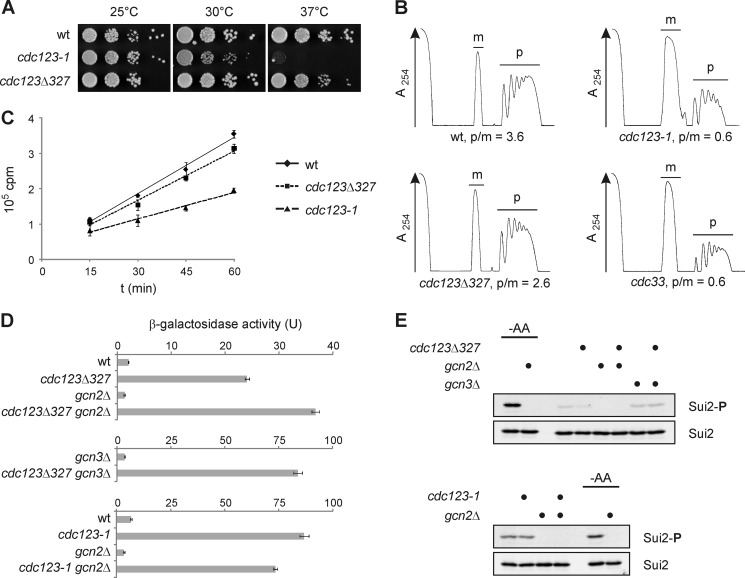
FIGURE 1.
Mutations in CDC123 affect translation initiation and eIF2 function. A, growth of cdc123 mutants. Cells were spotted in serial 10-fold dilutions on agar plates and incubated at the indicated temperatures. See supplemental Fig. S1 for cdc123 mutant alleles. B, polysome profiles of cdc123 mutants. Wild type cells (wt) and a strain expressing low levels of eIF4E (cdc33) were included for comparison. Strains were grown at 25 °C and shifted to 30 °C for 4 h prior to preparation of cell extracts. Monosomes (m) and polysomes (p) were separated in sucrose (10–50%) gradients, and their ratio (p/m) was quantified. RNA was measured at 254 nm (A254). C, global protein synthesis in cdc123 mutants. Wild type cells (wt) and cdc123 mutants were grown at 25 °C and shifted to 30 °C for 4 h prior to addition of [35S]methionine and [35S]cysteine. Incorporation of radiolabeled amino acids into proteins was monitored over time. Shown are mean values and S.D. (n = 3). D, GCN4 expression in cdc123 mutants. GCN4-lacZ reporter levels were measured in strains of the indicated genotype. β-Galactosidase activity is shown as mean and S.D. (n = 3). E, eIF2α-ser52 phosphorylation in cdc123 mutants. An antibody to eIF2α-phospho-ser52 (Invitrogen, 44728G) was used to determine eIF2α phosphorylation (Sui2-P) by Western analysis. As a control, eIF2α-ser52 phosphorylation (Sui2-P) was induced by shifting cells to synthetic medium lacking amino acids (-AA) for 30 min. Total eIF2α levels (Sui2) were analyzed for comparison.
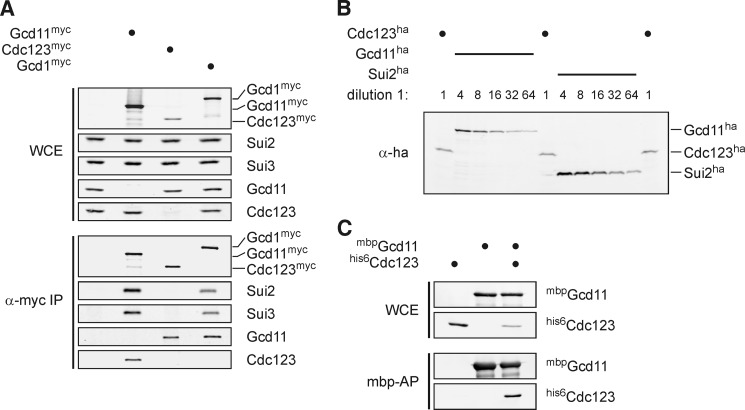
FIGURE 3.
Cdc123 is sub-stoichiometric to eIF2 subunits and binds unassembled Gcd11. A, binding of Cdc123 to unassembled Gcd11. myc-tagged versions of Gcd11, Cdc123 and the eIF2B-subunit Gcd1 were immunoprecipitated and analyzed for interaction of eIF2 subunits and Cdc123. Protein levels in yeast WCE and anti-myc immunoprecipitates (α-myc IP) were determined by Western analysis. B, cellular abundance of Cdc123 and eIF2 subunits. To compare endogenous protein levels of Cdc123 and eIF2 subunits, ha3 epitope sequences were fused to the chromosomal copies of CDC123, GCD11 (encoding eIF2γ), and SUI2 (encoding eIF2α). Dilution series of whole-cell lysates were analyzed by Western blotting. C, direct binding of Cdc123 to Gcd11. A maltose-binding protein (mbp) fusion of Gcd11 and his6-Cdc123 were co-expressed in E. coli, and mbp-Gcd11 was affinity-purified on amylose beads. Levels of mbp-Gcd11 and his6-Cdc123 in E. coli WCE and mbp-affinity precipitates (mbp-AP) were determined by Western analysis.
FIGURE 4.
Cdc123 binds domain III of Gcd11 to promote eIF2 complex formation and cell viability. A, binding of yeast Cdc123 and the human ortholog hD123 to domain III of Gcd11. Scheme of Gcd11 (top). Indicated are amino acid positions, archaeal homology domains (open boxes) and eukaryotic extensions (gray boxes). Yeast-2-hybrid assays (middle) were performed with DNA-binding domain (BD)-fusions of Cdc123, hD123, Sui2, or Sui3 and transcription activation domain (AD)-fusions of full-length or truncated versions of Gcd11. Expression of the lacZ-reporter was analyzed by assaying β-galactosidase activity. The mean and S.D. are shown (n = 3, for bars marked with an asterisk n = 6). Protein levels of AD-Gcd11 fusions 1–514 and 1–527 were compared by Western blotting and total protein was visualized by Ponceau staining (bottom). B, multiple sequence alignment of the C-terminal region of a/eIF2γ. Indicated are Gcd11 truncations (numbers above the alignment) and the final β-sheet of domain III (arrow) (38). C and D, C-terminal truncations of Gcd11. Flag-Gcd11 and truncated versions were expressed in gcd11Δ/GCD11 heterozygous diploid strains, immunoprecipitated and analyzed for interaction with Cdc123, Sui2, and Sui3. Protein levels in WCE and anti-flag immunoprecipitates (α-flag IP) were determined by Western analysis (C). Strains were sporulated and meiotic progeny was analyzed by tetrad dissection. Δ, gcd11Δ; +, the indicated flag-GCD11-construct (D).
Antisera to Cdc123 and eIF2 Subunits
Rabbit antisera to Sui2 (32), Sui3 (33), and Gcd11 (34) have been described. Additionally, we obtained new rabbit antisera to Cdc123 and each of the eIF2 subunits. His6-fusions of Cdc123, Sui2, and Gcd11 were expressed in E. coli BL21codon+ (Stratagene) and purified with a HIS-SelectTM Cartridge (Sigma-Aldrich). Sui3 was expressed as mbp-fusion and purified using amylose resin (New England Biolabs). The purified proteins were sent to Davids Biotechnology (Regensburg) for production of rabbit antisera. For enhanced specificity, antisera to Cdc123 and Gcd11 were subjected to affinity chromatography. mbp-fusions of Cdc123 and Gcd11 were expressed in E. coli, purified with amylose resin (New England Biolabs), and coupled to AminoLink Resin (Pierce) according to the manufacturer's instructions. Anti-Cdc123 and anti-Gcd11 antisera were loaded onto their corresponding affinity column. After washing with PBS (50 mm sodium phosphate buffer, pH 7.2, 100 mm NaCl), bound antibodies were eluted with 160 mm glycine/HCl (pH 2.8) and pH was immediately adjusted to 7 by addition of sodium phosphate buffer (1 m, pH 8.6). Antibodies were concentrated and transferred to PBS containing 0.05% sodium azide using a centrifugal filter device (Biomax-30 NMWL Membrane, Millipore). Information about the use of specific antisera in particular experiments is included in supplemental Table S3.
Protein Expression in E. coli and Affinity Purification
E. coli strain BL21codon+ (Stratagene) was transformed with the expression plasmids listed in supplemental Table S2. Transformants were inoculated in LB medium with 100 μg/ml ampicillin and 50 μg/ml chloramphenicol, and protein expression was induced with 0.2% rhamnose for 16 h at 25 °C. Cells were resuspended in cold lysis buffer (20 mm Tris/HCl, pH 7.4, 200 mm NaCl, 1 mm EDTA, 1 mm DTT) and lysed by shaking with glass beads in a mixer mill (Retsch) for 5 min at 4 °C. Samples were centrifuged twice for 10 min at 4 °C to remove glass beads and cell debris. For Western blot analysis, the supernatant was mixed with Lämmli sample buffer and boiled for 5 min. For affinity purification of mbp-fusion proteins, equal amounts of total protein were incubated with amylose resin (New England Biolabs) for 3 h at 4 °C. After centrifugation, beads were washed three times with lysis buffer, resuspended in Lämmli sample buffer, and boiled for 5–10 min.
Multiple Sequence Alignment
Amino acid sequences of a/eIF2γ or Cdc123 from different species were aligned with ClustalΩ (35) using default parameters.
RESULTS
Translation Initiation and eIF2 Function Are Compromised in cdc123 Mutants
To address a possible role of Cdc123 in translation initiation, we analyzed the abundance of polysomes in yeast strains impaired in Cdc123 function. Polysomes, i.e. single mRNAs translated by multiple ribosomes, are formed by repeated rounds of translation initiation and therefore serve as a measure of the in vivo initiation frequency. We studied two cdc123 mutant strains (Fig. 1A and supplemental Fig. S1) carrying either the temperature-sensitive cdc123-1 allele or the hypomorphic cdc123Δ327 allele and compared their polysome profiles to a wild type control and a strain with reduced expression of the eIF4E-encoding gene CDC33 (36). The ratio of polysomes to monosomes (p/m) was 3.6 in wild type cells and decreased to 0.6 in the strain with low eIF4E levels (cdc33) (Fig. 1B). The relative amounts of polysomes were reduced (p/m = 2.6) in the hypomorphic cdc123Δ327 strain and very low (p/m = 0.6) in the temperature-sensitive cdc123-1 strain grown at 30 °C for 4 h (Fig. 1B). The observed reduction of polysomes provides biochemical evidence that defects in Cdc123 may impact on the initiation of protein synthesis.
To substantiate this conclusion, we used metabolic labeling to quantify the protein synthesis activity of cdc123 mutants (Fig. 1C). The rate at which the temperature-sensitive cdc123-1 mutant strain incorporated a mixture of [35S]methionine and [35S]cysteine into acid-insoluble material at 30 °C was reduced to about 50% of the wild type control value. A minor reduction of the protein synthesis rate was observed in the hypomorphic cdc123Δ327 strain. These results are in line with the growth properties (Fig. 1A) and polysome levels (Fig. 1B) of these strains and confirm a role of Cdc123 in global protein synthesis.
Next we checked for a link of Cdc123 to the initiation factor eIF2 by analyzing expression of GCN4 as a genetic assay of eIF2 activity (28). In response to stress, eIF2 is phosphorylated on serine 52 (Ser-52) of its α-subunit by Gcn2 kinase and consequently forms an inactive complex with its GEF eIF2B. The resulting drop in eIF2 activity attenuates global protein synthesis, but de-represses translation of the GCN4 mRNA. Therefore, in wild type cells, increased expression of GCN4 under stress conditions depends on Gcn2 kinase, eIF2α-ser52 phosphorylation, and Gcn3, a regulatory subunit of eIF2B. GCN4 expression is also up-regulated when subunits of eIF2 or eIF2B are defective due to gene mutations. In these mutant cells, however, elevated expression of GCN4 is independent of Gcn2, eIF2α-ser52 phosphorylation, and Gcn3. Using an established reporter plasmid carrying the 5′- regulatory region of GCN4 fused to the lacZ coding sequence (28), we observed that GCN4 expression was elevated in a cdc123Δ327 mutant strain (Fig. 1D). This increase required neither Gcn2 nor Gcn3. The cdc123-1 allele caused an even more pronounced increase of GCN4 expression, which was again independent of Gcn2. To inspect phosphorylation of eIF2α, we used an antiserum specific for eIF2α-phospho-ser52 to probe cell extracts by Western analysis (Fig. 1E). Unlike amino acid starvation of wild type cells, which caused a prominent, Gcn2-dependent signal, mutations in CDC123 failed to increase eIF2α-ser52 phosphorylation above the low level detected in wild type control cells. Together, these results show that mutations of CDC123 lead to an increase of GCN4 expression that is independent of Gcn2 kinase and eIF2α-ser52 phosphorylation. This phenotype is called general control derepressed (Gcd−) and is a hallmark of defects in either eIF2 subunits or cellular activators of eIF2. The data, therefore, indicate that Cdc123 is needed for the normal function of eIF2.
eIF2 Integrity Is Compromised in cdc123 Mutants
To understand how Cdc123 may support eIF2 function, we analyzed the eIF2 complex in cells carrying the hypomorphic cdc123Δ327 allele. In budding yeast, eIF2α, eIF2β, and eIF2γ are named Sui2, Sui3, and Gcd11, respectively. This nomenclature will be used here to better distinguish between individual subunits and the eIF2 complex. To facilitate eIF2 immunoprecipitation, a myc epitope sequence was fused to the chromosomal copy of GCD11. Western analysis showed that immunoprecipitates of Gcd11-myc from the cdc123Δ327 strain contained much lower levels of Sui2 and Sui3 than precipitates from Cdc123-proficient control strains, while total levels of these proteins detected in whole cell extracts were comparable among the mutant and wild type strains (Fig. 2A). These data suggest that association, but not abundance, of the eIF2 protein subunits is compromised in the cdc123 mutant strain.
In the above analysis, we used diploid strains carrying an untagged GCD11 allele in addition to GCD11-myc, because the combination of the cdc123Δ327 and GCD11-myc alleles rendered haploid cells inviable (Fig. 2B). This synthetic lethal gene interaction indicates that a defect in Cdc123 sensitizes cells to an otherwise negligible alteration of Gcd11 confirming a tight functional link between Cdc123 and eIF2.
To validate the influence of Cdc123 on the eIF2 complex, we repeated the immunoprecipitation experiment with an N-terminally flag epitope-tagged version of Gcd11, which was compatible with the cdc123Δ327 mutation in a haploid strain (Fig. 2C). In addition, we used antisera specific to Sui2 or Sui3 to precipitate the eIF2 complex from untagged haploid strains (Fig. 2D). Again, interaction of Gcd11 with Sui2 and Sui3 was reduced in the cdc123Δ327 mutant relative to the wild type control, while total levels of these proteins were unaffected by the cdc123Δ327 mutation. We also analyzed the eIF2 complex in a strain expressing the temperature-sensitive cdc123-1 allele from the repressible GALL promoter. When grown under permissive conditions (25 °C, galactose medium), precipitates of Sui2 or Sui3 contained Gcd11 at wild type levels. However, following a shift to restrictive conditions (37 °C, glucose medium), amounts of Gcd11, which coprecipitated with Sui2 or Sui3, became almost undetectable in the mutant, but remained high in the control strain (Fig. 2E). Together, these results show that Cdc123 is required for integrity of the eIF2 complex.
Cdc123 Binds Unassembled Gcd11
To gain insight into the mechanism by which Cdc123 acts on the translation initiation factor eIF2, we characterized in more detail the reported physical interaction of Cdc123 with Gcd11 (10–14, 20). In a first step we checked if Cdc123 binds Gcd11 in context of the eIF2 complex. To this end, we immunoprecipitated myc-tagged versions of Gcd11 and Cdc123 along with Gcd1, a subunit of the GEF eIF2B, which associates with the heterotrimeric eIF2 complex (37). The myc epitope sequences were again fused to the chromosomal gene copies to maintain expression at physiological levels. Quantitative Western analysis indicated that cellular amounts of Cdc123 are about 10-fold below those of Gcd11 (Fig. 3, A and B). As expected, precipitates of Gcd11-myc contained Cdc123 as well as Sui2 and Sui3. Likewise, all three eIF2 subunits (Sui2, Sui3, and Gcd11), but not Cdc123, were recovered in precipitates of Gcd1-myc. Precipitates of Cdc123-myc contained Gcd11, however, Sui2 and Sui3 were not detectable. Together these data argue that Cdc123 does not interact with the assembled eIF2 complex, but specifically binds to an unassembled form of Gcd11 that lacks stoichiometric amounts of Sui2 and Sui3.
To ask if Cdc123 and Gcd11 can bind to each other directly, we coexpressed a maltose-binding protein (mbp)-fusion of Gcd11 with a his6-fusion of Cdc123 in E. coli. From a cell lysate, mbp-Gcd11 was affinity purified on amylose beads. By Western analysis, his6-Cdc123 was found to copurify with mbp-Gcd11 (Fig. 3C). Thus, Cdc123 and Gcd11 can interact in the absence of other yeast proteins.
Cdc123 Binds to Domain III of Gcd11 to Promote eIF2 Assembly
In a next step, we set out to define the region of Gcd11 to which Cdc123 binds. By analogy to its archaeal homolog, Gcd11 is thought to consist of three domains: an N-terminal, guanine nucleotide binding domain followed by two smaller, β-barrel domains (4). Domains I and II are the regions through which Gcd11 binds Sui3 and Sui2, respectively. In a yeast two-hybrid screen for interactors of Cdc123, we isolated a clone expressing a small, C-terminal fragment of Gcd11 (amino acids 410–527) containing domain III. Reporter activation by Gcd11-(410–527) even exceeded the signal produced by full-length Gcd11 (Fig. 4A). These results indicate that domain III of Gcd11 is sufficient for binding of Cdc123. To test if domain III was also required for this interaction, we removed 13 amino acids from the C terminus of Gcd11. This truncation is predicted to disrupt the final β-sheet of domain III (Fig. 4B) (38). The C-terminally truncated derivative Gcd11-(1–514) was expressed at wild type levels, but failed to interact with Cdc123 in the two hybrid system (Fig. 4A) indicating that domain III needs to be intact for Gcd11 to interact with Cdc123. Interestingly, this small truncation also abolished the interaction of Gcd11 with Sui2 as well as Sui3 (Fig. 4A). Since neither Sui2 nor Sui3 have been reported to bind to domain III of Gcd11, their reduced interaction with Gcd11-(1–514) was unexpected. Combined with the reduced association of eIF2 subunits in cdc123 mutants (Fig. 2), these data raised the possibility that binding of Cdc123 to domain III of Gcd11 might be required for association of Gcd11 with Sui2 and Sui3.
To confirm and extend these findings, we constructed a series of Gcd11 truncations (Fig. 4B). These constructs were tagged with an N-terminal flag epitope to compare their protein interaction pattern by immunoprecipitation (Fig. 4C). To ask if the truncated versions retained their biological function, we also checked their ability to complement a GCD11 gene deletion (Fig. 4D). Gcd11 tolerated the removal of 4 amino acids from the C terminus, since precipitates of flag-Gcd11-(1–523) contained Sui2, Sui3 as well as Cdc123 at levels comparable to the full length Gcd11 construct. Moreover, flag-Gcd11-(1–523) behaved like full-length Gcd11 in restoring growth of an otherwise inviable gcd11 deletion mutant. Removal of 8 amino acids, however, had a major impact on Gcd11 function. Levels of Sui2, Sui3 as well as Cdc123 that coprecipitated with flag-Gcd11-(1–519) were severely reduced and cells which expressed this construct as sole source of Gcd11 grew very poorly. Finally, removal of 13 amino acids rendered Gcd11 nonfunctional. Flag-Gcd11-(1–514) neither coprecipitated Cdc123 nor Sui2 nor Sui3 (Fig. 4C), consistent with the two-hybrid results (Fig. 4A). In addition, expression of flag-Gcd11-(1–514) failed to restore viability of a gcd11 deletion mutant (Fig. 4D). Thus, the essential biological function of Gcd11 and its association with Sui2 and Sui3 strictly correlate with binding of Cdc123 to Gcd11.
The data support a model in which Cdc123 serves to assemble the heterotrimeric eIF2 complex through binding to domain III of unassembled Gcd11. According to this model, eIF2 assembly defects associated with a domain III truncation of Gcd11 are caused by impaired binding of Cdc123. To verify this view, we asked if elevated levels of Cdc123 can restore the function of a domain III-truncated version of Gcd11. Indeed, flag-Gcd11-(1–519) precipitated wild type levels of Sui2 and Sui3 when Cdc123 was overexpressed, while only minor amounts of Sui2 and Sui3 associated with flag-Gcd11-(1–519) when Cdc123 was expressed at its physiological level (Fig. 5A). In addition, elevated levels of Cdc123 suppressed the severe growth defect of a mutant strain whose GCD11 gene is replaced by the flag-GCD11-(1–519) construct (Fig. 5B). Thus, the elevated abundance of Cdc123 neutralized the assembly defect and restored the biological function of a C-terminally truncated mutant version of Gcd11. These results, therefore, provide evidence for the ability of Cdc123 to promote assembly of the eIF2 complex.
We finally addressed the question if eIF2 assembly might be the essential cellular function of Cdc123. To test this idea, we asked if viability of a cdc123 deletion mutant can be restored by overexpression of eIF2 subunits (Fig. 5, C and D). In fact, elevated expression of either Sui2 or Gcd11 was sufficient to restore viability the cdc123 deletion mutant, but growth of these cells was severely compromised. Overexpression of Sui3 failed to rescue and did not improve growth when combined with Sui2 or Gcd11. However, the combined overexpression of Sui2 and Gcd11 caused a strong synergistic effect and allowed cdc123 deletion cells to grow almost as fast as wild type cells. Thus, Cdc123 is no longer essential for cell viability when Sui2 and Gcd11 are both expressed at high levels suggesting that eIF2 assembly is the mechanism behind the vital necessity of Cdc123.
DISCUSSION
This work describes assembly of eIF2 from its three protein subunits as a new step of translation initiation and identifies the conserved cell proliferation protein Cdc123 as the requisite assembly factor. We propose that eIF2 is formed in an ordered pathway (Fig. 5E) initiated by binding of Cdc123 to domain III of unassembled Gcd11(eIF2γ). Possibly by inducing an allosteric transition to make binding sites on domains I and II accessible, Cdc123 enables Gcd11(eIF2γ) to associate with Sui2(eIF2α) and Sui3(eIF2β). Binding of Sui2(eIF2α) might precede and allow for the binding of Sui3(eIF2β), since high levels of Sui2(eIF2α), but not Sui3(eIF2β), could compensate for the absence of Cdc123 (Fig. 5C). Stepwise, protein-assisted assembly might enable eIF2 to reach an elaborate structure suitable for multiple specific contacts during the initiation cycle (6). Our results are consistent with the view that Cdc123 serves to assemble newly synthesized eIF2 subunits. The existence of a dedicated assembly factor, however, raises the question if eIF2 might disassemble at some step of the initiation cycle. Disassembly coupled to start codon recognition to support efficient release of the initiator methionyl-tRNA would be an intriguing possibility. Future studies of Cdc123 promise to shed new light on the translation initiation pathway.
Cdc123 and its mammalian orthologs were originally described as cell proliferation factors required for the G1/S transition of the cell cycle (15, 20). We propose that the observed cell cycle arrest in cdc123 mutants is a consequence of reduced translation initiation rates, because the G1/S transition is especially sensitive to defects in protein synthesis (39). Indeed, several translation initiation factors were initially identified as cell division cycle mutants in yeast, for example the cap-binding protein eIF4E encoded by CDC33 (40) or the eIF3 subunit Prt1 encoded by CDC63 (41).
The molecular function of Cdc123 is most likely conserved among eukaryotic organisms including humans, since expression of the human Cdc123 ortholog (hD123) complemented a cdc123 gene deletion of budding yeast (20). Moreover, hD123 interacted with Gcd11(eIF2γ) in a yeast-two-hybrid system and, consistent with the results for yeast Cdc123, domain III of Gcd11(eIF2γ) was necessary and sufficient for this interaction (Fig. 4A).
eIF2 and its control by phosphorylation and guanine nucleotide exchange are critical to various cellular processes and dysfunction of eIF2 is the cause of human diseases (42, 43). Cdc123-mediated biogenesis of eIF2 might provide additional opportunities for regulation. Indeed, Cdc123 is a low abundance protein in yeast (Fig. 3, A and B) and subject to phosphorylation and ubiquitylation in human cells (44, 45). Moreover, human Cdc123 is encoded by a candidate disease risk locus (17–19). Thus, regulation of eIF2 biogenesis via Cdc123 may define a new pathway of protein homeostasis control relevant to human health.
Supplementary Material
Acknowledgments
We thank A. Weissgerber for performing the Y2H screen; W. Hausner, D. Peterhoff, and W. Mages for help with the metabolic labeling experiment; B. Castilho, T. Donahue, and E. Hannig for providing antisera; A. Hinnebusch and C. Höög for providing plasmids p180 and pEGFP-C3-D123, respectively; T. von der Haar for providing the YTH3 yeast strain; J. Medenbach and O. Stemmann for comments on the manuscript.

This article contains supplemental Tables S1–S3 and Fig. S1.
- eIF
- eukaryotic translation initiation factor
- BD
- binding domain
- AD
- activation domain
- WCE
- whole-cell extract
- Cdc
- cell division cycle
- GEF
- guanine nucleotide exchange factor.
REFERENCES
- 1. Jackson R. J., Hellen C. U., Pestova T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hinnebusch A. G., Lorsch J. R. (2012) The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb. Perspect. Biol. 4, a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lorsch J. R., Dever T. E. (2010) Molecular view of 43 S complex formation and start site selection in eukaryotic translation initiation. J. Biol. Chem. 285, 21203–21207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmitt E., Naveau M., Mechulam Y. (2010) Eukaryotic and archaeal translation initiation factor 2: a heterotrimeric tRNA carrier. FEBS Lett. 584, 405–412 [DOI] [PubMed] [Google Scholar]
- 5. Hinnebusch A. G. (2011) Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. 75, 434–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aitken C. E., Lorsch J. R. (2012) A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 19, 568–576 [DOI] [PubMed] [Google Scholar]
- 7. Gebauer F., Hentze M. W. (2004) Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 5, 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holcik M., Sonenberg N. (2005) Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6, 318–327 [DOI] [PubMed] [Google Scholar]
- 9. Hinnebusch A. G. (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59, 407–450 [DOI] [PubMed] [Google Scholar]
- 10. Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., Adams S. L., Millar A., Taylor P., Bennett K., Boutilier K., Yang L., Wolting C., Donaldson I., Schandorff S., Shewnarane J., Vo M., Taggart J., Goudreault M., Muskat B., Alfarano C., Dewar D., Lin Z., Michalickova K., Willems A. R., Sassi H., Nielsen P. A., Rasmussen K. J., Andersen J. R., Johansen L. E., Hansen L. H., Jespersen H., Podtelejnikov A., Nielsen E., Crawford J., Poulsen V., Sørensen B. D., Matthiesen J., Hendrickson R. C., Gleeson F., Pawson T., Moran M. F., Durocher D., Mann M., Hogue C. W., Figeys D., Tyers M. (2002) Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 [DOI] [PubMed] [Google Scholar]
- 11. Gavin A. C., Aloy P., Grandi P., Krause R., Boesche M., Marzioch M., Rau C., Jensen L. J., Bastuck S., Dümpelfeld B., Edelmann A., Heurtier M. A., Hoffman V., Hoefert C., Klein K., Hudak M., Michon A. M., Schelder M., Schirle M., Remor M., Rudi T., Hooper S., Bauer A., Bouwmeester T., Casari G., Drewes G., Neubauer G., Rick J. M., Kuster B., Bork P., Russell R. B., Superti-Furga G. (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 [DOI] [PubMed] [Google Scholar]
- 12. Krogan N. J., Cagney G., Yu H., Zhong G., Guo X., Ignatchenko A., Li J., Pu S., Datta N., Tikuisis A. P., Punna T., Peregrín-Alvarez J. M., Shales M., Zhang X., Davey M., Robinson M. D., Paccanaro A., Bray J. E., Sheung A., Beattie B., Richards D. P., Canadien V., Lalev A., Mena F., Wong P., Starostine A., Canete M. M., Vlasblom J., Wu S., Orsi C., Collins S. R., Chandran S., Haw R., Rilstone J. J., Gandi K., Thompson N. J., Musso G., St Onge P., Ghanny S., Lam M. H., Butland G., Altaf-Ul A. M., Kanaya S., Shilatifard A., O'Shea E., Weissman J. S., Ingles C. J., Hughes T. R., Parkinson J., Gerstein M., Wodak S. J., Emili A., Greenblatt J. F. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 [DOI] [PubMed] [Google Scholar]
- 13. Collins S. R., Kemmeren P., Zhao X. C., Greenblatt J. F., Spencer F., Holstege F. C., Weissman J. S., Krogan N. J. (2007) Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol. Cell Proteomics 6, 439–450 [DOI] [PubMed] [Google Scholar]
- 14. Yu H., Braun P., Yildirim M. A., Lemmens I., Venkatesan K., Sahalie J., Hirozane-Kishikawa T., Gebreab F., Li N., Simonis N., Hao T., Rual J. F., Dricot A., Vazquez A., Murray R. R., Simon C., Tardivo L., Tam S., Svrzikapa N., Fan C., de Smet A. S., Motyl A., Hudson M. E., Park J., Xin X., Cusick M. E., Moore T., Boone C., Snyder M., Roth F. P., Barabási A. L., Tavernier J., Hill D. E., Vidal M. (2008) High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohno K., Okuda A., Ohtsu M., Kimura G. (1984) Genetic analysis of control of proliferation in fibroblastic cells in culture. I. Isolation and characterization of mutants temperature-sensitive for proliferation or survival of untransformed diploid rat cell line 3Y1. Somat Cell Mol. Genet. 10, 17–28 [DOI] [PubMed] [Google Scholar]
- 16. Okuda A., Kimura G. (1996) An amino acid change in novel protein D123 is responsible for temperature-sensitive G1-phase arrest in a mutant of rat fibroblast line 3Y1. Exp. Cell Res. 223, 242–249 [DOI] [PubMed] [Google Scholar]
- 17. Adélaïde J., Finetti P., Bekhouche I., Repellini L., Geneix J., Sircoulomb F., Charafe-Jauffret E., Cervera N., Desplans J., Parzy D., Schoenmakers E., Viens P., Jacquemier J., Birnbaum D., Bertucci F., Chaffanet M. (2007) Integrated profiling of basal and luminal breast cancers. Cancer Res. 67, 11565–11575 [DOI] [PubMed] [Google Scholar]
- 18. Zeggini E., Scott L. J., Saxena R., Voight B. F., Marchini J. L., Hu T., de Bakker P. I., Abecasis G. R., Almgren P., Andersen G., Ardlie K., Boström K. B., Bergman R. N., Bonnycastle L. L., Borch-Johnsen K., Burtt N. P., Chen H., Chines P. S., Daly M. J., Deodhar P., Ding C. J., Doney A. S., Duren W. L., Elliott K. S., Erdos M. R., Frayling T. M., Freathy R. M., Gianniny L., Grallert H., Grarup N., Groves C. J., Guiducci C., Hansen T., Herder C., Hitman G. A., Hughes T. E., Isomaa B., Jackson A. U., Jørgensen T., Kong A., Kubalanza K., Kuruvilla F. G., Kuusisto J., Langenberg C., Lango H., Lauritzen T., Li Y., Lindgren C. M., Lyssenko V., Marvelle A. F., Meisinger C., Midthjell K., Mohlke K. L., Morken M. A., Morris A. D., Narisu N., Nilsson P., Owen K. R., Palmer C. N., Payne F., Perry J. R., Pettersen E., Platou C., Prokopenko I., Qi L., Qin L., Rayner N. W., Rees M., Roix J. J., Sandbaek A., Shields B., Sjogren M., Steinthorsdottir V., Stringham H. M., Swift A. J., Thorleifsson G., Thorsteinsdottir U., Timpson N. J., Tuomi T., Tuomilehto J., Walker M., Watanabe R. M., Weedon M. N., Willer C. J., Illig T., Hveem K., Hu F. B., Laakso M., Stefansson K., Pedersen O., Wareham N. J., Barroso I., Hattersley A. T., Collins F. S., Groop L., McCarthy M. I., Boehnke M., Altshuler D. (2008) Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 40, 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soler Artigas M., Loth D. W., Wain L. V., Gharib S. A., Obeidat M., Tang W., Zhai G., Zhao J. H., Smith A. V., Huffman J. E., Albrecht E., Jackson C. M., Evans D. M., Cadby G., Fornage M., Manichaikul A., Lopez L. M., Johnson T., Aldrich M. C., Aspelund T., Barroso I., Campbell H., Cassano P. A., Couper D. J., Eiriksdottir G., Franceschini N., Garcia M., Gieger C., Gislason G. K., Grkovic I., Hammond C. J., Hancock D. B., Harris T. B., Ramasamy A., Heckbert S. R., Heliövaara M., Homuth G., Hysi P. G., James A. L., Jankovic S., Joubert B. R., Karrasch S., Klopp N., Koch B., Kritchevsky S. B., Launer L. J., Liu Y., Loehr L. R., Lohman K., Loos R. J., Lumley T., Al Balushi K. A., Ang W. Q., Barr R. G., Beilby J., Blakey J. D., Boban M., Boraska V., Brisman J., Britton J. R., Brusselle G. G., Cooper C., Curjuric I., Dahgam S., Deary I. J., Ebrahim S., Eijgelsheim M., Francks C., Gaysina D., Granell R., Gu X., Hankinson J. L., Hardy R., Harris S. E., Henderson J., Henry A., Hingorani A. D., Hofman A., Holt P. G., Hui J., Hunter M. L., Imboden M., Jameson K. A., Kerr S. M., Kolcic I., Kronenberg F., Liu J. Z., Marchini J., McKeever T., Morris A. D., Olin A. C., Porteous D. J., Postma D. S., Rich S. S., Ring S. M., Rivadeneira F., Rochat T., Sayer A. A., Sayers I., Sly P. D., Smith G. D., Sood A., Starr J. M., Uitterlinden A. G., Vonk J. M., Wannamethee S. G., Whincup P. H., Wijmenga C., Williams O. D., Wong A., Mangino M., Marciante K. D., McArdle W. L., Meibohm B., Morrison A. C., North K. E., Omenaas E., Palmer L. J., Pietilainen K. H., Pin I., Pola Sbreve Ek O., Pouta A., Psaty B. M., Hartikainen A. L., Rantanen T., Ripatti S., Rotter J. I., Rudan I., Rudnicka A. R., Schulz H., Shin S. Y., Spector T. D., Surakka I., Vitart V., Volzke H., Wareham N. J., Warrington N. M., Wichmann H. E., Wild S. H., Wilk J. B., Wjst M., Wright A. F., Zgaga L., Zemunik T., Pennell C. E., Nyberg F., Kuh D., Holloway J. W., Boezen H. M., Lawlor D. A., Morris R. W., Probst-Hensch N., Kaprio J., Wilson J. F., Hayward C., Kahonen M., Heinrich J., Musk A. W., Jarvis D. L., Glaser S., Jarvelin M. R., Ch Stricker B. H., Elliott P., O'Connor G. T., Strachan D. P., London S. J., Hall I. P., Gudnason V., Tobin M. D. (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat. Genet. 43, 1082–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bieganowski P., Shilinski K., Tsichlis P. N., Brenner C. (2004) Cdc123 and checkpoint forkhead associated with RING proteins control the cell cycle by controlling eIF2γ abundance. J. Biol. Chem. 279, 44656–44666 [DOI] [PubMed] [Google Scholar]
- 21. Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (2005) Current Protocols in Molecular Biology (Harkins E. W., ed), John Wiley & Sons, Inc [Google Scholar]
- 22. Hoja M. R., Wahlestedt C., Höög C. (2000) A visual intracellular classification strategy for uncharacterized human proteins. Exp. Cell Res. 259, 239–246 [DOI] [PubMed] [Google Scholar]
- 23. Sikorski R. S., Hieter P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiese A., Wilms B., Syldatk C., Mattes R., Altenbuchner J. (2001) Cloning, nucleotide sequence and expression of a hydantoinase and carbamoylase gene from Arthrobacter aurescens DSM 3745 in Escherichia coli and comparison with the corresponding genes from Arthrobacter aurescens DSM 3747. Appl. Microbiol. Biotechnol. 55, 750–757 [DOI] [PubMed] [Google Scholar]
- 25. Wegerer A., Sun T., Altenbuchner J. (2008) Optimization of an E. coli L-rhamnose-inducible expression vector: test of various genetic module combinations. BMC Biotechnol. 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 27. Mumberg D., Müller R., Funk M. (1994) Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hinnebusch A. G. (1985) A hierarchy of trans-acting factors modulates translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol. Cell Biol. 5, 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sagliocco F. A., Moore P. A., Brown A. J. (1996) Polysome analysis. Methods Mol. Biol. 53, 297–311 [DOI] [PubMed] [Google Scholar]
- 30. Carr-Schmid A., Valente L., Loik V. I., Williams T., Starita L. M., Kinzy T. G. (1999) Mutations in elongation factor 1β, a guanine nucleotide exchange factor, enhance translational fidelity. Mol. Cell Biol. 19, 5257–5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwab M., Neutzner M., Möcker D., Seufert W. (2001) Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20, 5165–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cigan A. M., Pabich E. K., Feng L., Donahue T. F. (1989) Yeast translation initiation suppressor sui2 encodes the α subunit of eukaryotic initiation factor 2 and shares sequence identity with the human α subunit. Proc. Natl. Acad. Sci. U.S.A. 86, 2784–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hashimoto N. N., Carnevalli L. S., Castilho B. A. (2002) Translation initiation at non-AUG codons mediated by weakened association of eukaryotic initiation factor (eIF) 2 subunits. Biochem. J. 367, 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hannig E. M., Cigan A. M., Freeman B. A., Kinzy T. G. (1993) GCD11, a negative regulator of GCN4 expression, encodes the γ subunit of eIF-2 in Saccharomyces cerevisiae. Mol. Cell Biol. 13, 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., Higgins D. G. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. von der Haar T., McCarthy J. E. (2002) Intracellular translation initiation factor levels in Saccharomyces cerevisiae and their role in cap-complex function. Mol. Microbiol. 46, 531–544 [DOI] [PubMed] [Google Scholar]
- 37. Alone P. V., Dever T. E. (2006) Direct binding of translation initiation factor eIF2gamma-G domain to its GTPase-activating and GDP-GTP exchange factors eIF5 and eIF2B epsilon. J. Biol. Chem. 281, 12636–12644 [DOI] [PubMed] [Google Scholar]
- 38. Schmitt E., Blanquet S., Mechulam Y. (2002) The large subunit of initiation factor aIF2 is a close structural homologue of elongation factors. EMBO J. 21, 1821–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jorgensen P., Tyers M. (2004) How cells coordinate growth and division. Curr. Biol. 14, R1014–1027 [DOI] [PubMed] [Google Scholar]
- 40. Brenner C., Nakayama N., Goebl M., Tanaka K., Toh-e A., Matsumoto K. (1988) CDC33 encodes mRNA cap-binding protein eIF-4E of Saccharomyces cerevisiae. Mol. Cell Biol. 8, 3556–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanic-Joyce P. J., Johnston G. C., Singer R. A. (1987) Regulated arrest of cell proliferation mediated by yeast prt1 mutations. Exp. Cell Res. 172, 134–145 [DOI] [PubMed] [Google Scholar]
- 42. Scheper G. C., van der Knaap M. S., Proud C. G. (2007) Translation matters: protein synthesis defects in inherited disease. Nat. Rev. Genet. 8, 711–723 [DOI] [PubMed] [Google Scholar]
- 43. Borck G., Shin B. S., Stiller B., Mimouni-Bloch A., Thiele H., Kim J. R., Thakur M., Skinner C., Aschenbach L., Smirin-Yosef P., Har-Zahav A., Nürnberg G., Altmüller J., Frommolt P., Hofmann K., Konen O., Nürnberg P., Munnich A., Schwartz C. E., Gothelf D., Colleaux L., Dever T. E., Kubisch C., Basel-Vanagaite L. (2012) eIF2γ mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol. Cell 48, 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim W., Bennett E. J., Huttlin E. L., Guo A., Li J., Possemato A., Sowa M. E., Rad R., Rush J., Comb M. J., Harper J. W., Gygi S. P. (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.