Background: Staphylococcal superantigen-like proteins (SSLs) share structural similarity with superantigens but no superantigenic activity. Their functions remained unclear.
Results: SSL10 binds to prothrombin and factor Xa via the γ-carboxyglutamic acid domain. SSL10 inhibits the penultimate step of plasma clotting. SSL10 slightly inhibits clotting by coagulase.
Conclusion: SSL10 inhibits blood coagulation.
Significance: This work presents a novel function of SSLs, disturbing blood coagulation.
Keywords: Bacterial Toxins, Blood Coagulation Factors, Coagulation Factors, Prothrombin, Staphylococcus aureus, Staphylococcal Superantigen-like Protein
Abstract
The staphylococcal superantigen-like protein (SSL) family is composed of 14 exoproteins sharing structural similarity with superantigens but no superantigenic activity. Target proteins of four SSLs have been identified to be involved in host immune responses. However, the counterparts of other SSLs have been functionally uncharacterized. In this study, we have identified porcine plasma prothrombin as SSL10-binding protein by affinity purification using SSL10-conjugated Sepharose. The resin recovered the prodomain of prothrombin (fragment 1 + 2) as well as factor Xa in pull-down analysis. The equilibrium dissociation constant between SSL10 and prothrombin was 1.36 × 10−7 m in surface plasmon resonance analysis. On the other hand, the resin failed to recover γ-carboxyglutamic acid (Gla) domain-less coagulation factors and prothrombin from warfarin-treated mice, suggesting that the Gla domain of the coagulation factors is essential for the interaction. SSL10 prolonged plasma clotting induced by the addition of Ca2+ and factor Xa. SSL10 did not affect the protease activity of thrombin but inhibited the generation of thrombin activity in recalcified plasma. S. aureus produces coagulase that non-enzymatically activates prothrombin. SSL10 attenuated clotting induced by coagulase, but the inhibitory effect was weaker than that on physiological clotting, and SSL10 did not inhibit protease activity of staphylothrombin, the complex of prothrombin with coagulase. These results indicate that SSL10 inhibits blood coagulation by interfering with activation of coagulation cascade via binding to the Gla domain of coagulation factor but not by directly inhibiting thrombin activity. This is the first finding that the bacterial protein inhibits blood coagulation via targeting the Gla domain of coagulation factors.
Introduction
Staphylococcal superantigen-like proteins (SSLs)2 are a family of exoproteins composed of 14 members that have molecular masses of 25–35 kDa produced by a commensal human pathogen, Staphylococcal aureus. SSL1–SSL11 are encoded in the staphylococcal pathogenicity island 2 (SaPI2), and SSL12–SSL14 are in the immune evasion cluster (1). The distinctive features of SSLs are structural similarities with staphylococcal superantigens (TSST-1 (toxic shock syndrome toxin 1) and enterotoxins); i.e. all of them are composed of an N-terminal oligonucleotide/oligosaccharide-binding fold and the C-terminal β-grasp structure (2) despite their sequence similarity of less than 40%, and SSLs do not exhibit superantigenic activity (3–5). Recent studies have revealed that several SSLs interact with host immune factors and disturb their functions: SSL7 binds to IgA and inhibits IgA-FcαR interaction (6); SSL7 binds to complement C5 and inhibits the cleavage of C5 and generation of anaphylatoxin C5a (7); SSL5 binds to PSGL-1 (P-selectin glycoprotein ligand-1), which is expressed on leukocytes and involved in rolling of leukocyte on vascular endothelium (8); SSL5 binds to chemokine and anaphylatoxin receptors and antagonizes these chemoattractants (9); SSL5 binds to platelet receptors GPIbα and GPIIb/IIIa and inhibits aggregation of platelets (10); SSL5 binds to matrix metalloproteinase-9 and inhibits its protease activity and transmigration of neutrophils through Matrigel basement membrane (11); SSL10 binds to CXCR4 and inhibits migration of T cell leukemia (12); SSL10 binds to human IgG, interferes with the interaction between IgG and complement C1q, and inhibits the classical complement activation pathway (13) and Fc receptor-mediated phagocytosis of neutrophils (14); and SSL3 binds to TLR2 (Toll-like receptor 2) and inhibits cytokine production from macrophages in response to TLR2 ligands (15, 16).
We have identified target proteins of SSL3, SSL5, and SSL10 by affinity purification using the Sepharose covalently conjugated with recombinant SSLs from porcine spleen, human neutrophils, and human plasma, respectively (11, 13, 15). In this study, we found that prothrombin is a novel binding protein of SSL10 and SSL10 also bound to factor Xa. We demonstrated that γ-carboxyglutamic acid (Gla) domains of these proteins were responsible for binding and for the SSL10-inhibited blood coagulation.
EXPERIMENTAL PROCEDURES
Reagents
All of the chemical reagents were purchased from Sigma, WAKO Pure Chemicals (Osaka, Japan), and Nacalai Tesque (Kyoto, Japan). Restriction endonucleases and modifying enzymes were from TaKaRa (Osaka, Japan), Roche Applied Science, and Toyobo (Osaka, Japan). Oligonucleotides were supplied by Nihon Gene Laboratories (Sendai, Japan). Glutathione-Sepharose 4B, nickel-Sepharose 6 Fast Flow, NHS-activated Sepharose 4 Fast Flow, and benzamidine-Sepharose 4 Fast Flow were products of GE Healthcare. Human prothrombin was purchased from Calbiochem. Human factor VIIa, IXa, and Xa were products of Hematologic Technologies Inc. (Essex Junction, VT). Human IgG was obtained from serum by the combination of ammonium sulfate precipitation and affinity purification using protein G-Sepharose (GE Healthcare). PS-containing liposomes (phosphatidylcholine/PS/cholesterol = 5.5:1.5:3 molar ratio, 25 nmol of lipid/μl) were prepared by a dry film method as described previously (17).
Preparation of Recombinant SSLs, Coagulase, and SSL-conjugated Sepharose
Recombinant SSLs and SSL-conjugated Sepharose were prepared as described previously (15). Recombinant coagulase (residues 1–325) was produced as N-terminal glutathione S-transferase (GST) fusion protein and then excised using factor Xa. The gene for coagulase was amplified by PCR using genomic DNA of S. aureus (ATCC 277933) as a template and the primers 5′-GGG ATC CCC ATA GTA ACA AAG GAT TAT AGT GGG-3′ (BamHI site underlined) and 5′-GGT CGA CTA TTG TGT TGT TTC TTC AGC TTT AC-3′ (SalI site underlined) and cloned into pGEM-T Easy (Promega, Madison, WI) for DNA sequencing. The DNA fragment of coagulase was excised with BamHI and SalI and cloned into the expression vector pGEX-5X-1 (GE Healthcare). To remove 3 amino acid residues between the factor Xa cleavage recognition sequence and the N terminus of coagulase (Gly-Ile-Pro, which is derived from the multicloning site of pGEX-5X-1), 9 base pairs were deleted by site-directed mutagenesis using oligonucleotides 5′-GGT CGT ATA GTA ACA AAG GAT TAT AGT GGG-3′ and 5′-TAC TAT ACG ACC TTC GAT CAG ATC C-3′. The Escherichia coli strain BL21 was transformed with the resultant construct, and recombinant protein was induced by the culture at 37 °C for 4 h in the presence of 1 mm isopropyl-β-d-thiogalactopyranoside (Wako). The E. coli-produced GST-coagulase was lysed by sonication, and the fusion protein was bound to glutathione-Sepharose 4B. The resin was washed three times and then equilibrated with factor Xa buffer (20 mm Tris-HCl, 50 mm NaCl, 1 mm CaCl2, pH 6.5) and incubated with factor Xa (9.5 μg, 0.22 nmol/250 μl) for 16 h at 25 °C. The reaction mixture was recovered and passed through benzamidine-Sepharose 4 Fast Flow to remove factor Xa. Protein concentration was determined with a BCA protein assay kit (Pierce). The purities of recombinant proteins were estimated to be more than 95% by SDS-PAGE and Coomassie Brilliant Blue (CBB) staining.
Preparation of Mouse Anti-SSL10 Serum
Mouse anti-SSL10 serum was generated by immunizing mice (ICR, male, 6W, Japan SLC, Hamamatsu, Japan) with recombinant SSL10 (50 μg/mouse) as antigen emulsified in Freund's complete adjuvant (BD Biosciences), boosting 2 weeks later with the antigen in incomplete Freund's adjuvant (BD Biosciences), collected by cardiac puncture under anesthesia at 2 weeks postboosting. The serum was pooled from five immunized mice. Anti-SSL10 serum did not cross-react with SSL3, SSL5, and SSL7 in immunoblot analysis.
Preparation of Porcine, Mouse, and Human Plasma
Porcine blood, obtained from a local abattoir, was collected using EDTA (5 mm in final concentration) as an anticoagulant. Mouse blood was collected by cardiac puncture under anesthesia using 3.8% sodium citrate as an anticoagulant. Human blood was donated from healthy donors using 3.8% sodium citrate as an anticoagulant. Plasma was recovered by centrifugation (1,880 × g, 15 min, 4 °C) and stored at −20 °C. To obtain plasma that contains prothrombin without γ-carboxyglutamic acid modification, mice were pretreated with warfarin sodium (Wako) dissolved in drinking water (7.5 mg/liter on the first day and 2.5 mg/liter on the following days), leaving free access to water to the mice.
Preparation of Proteolytic Fragments of Prothrombin and Factor Xa
Prothrombin fragments (fragment 1 + 2 and α-thrombin) were obtained by digestion with factor Xa. Human prothrombin (20 μg, 0.28 nmol) was digested by human factor Xa (5 μg, 0.12 nmol; Hematologic Technologies Inc.) in factor Xa buffer for 16 h at 25 °C. Gla domain-less prothrombin and factor Xa were obtained by limiting digestion with α-chymotrypsin (18). Ten micrograms of prothrombin or factor Xa (0.14 or 0.23 nmol, respectively) was treated with 20 ng of α-chymotrypsin (Sigma) in TBS for 30 min at 37 °C, and digestion was terminated by the addition of 5 μg of aprotinin (Wako).
Isolation and Identification of SSL-binding Proteins from Porcine Plasma
Porcine plasma (15 ml) was mixed with SSL-conjugated Sepharose (10 μl of 50% slurry) and incubated at room temperature for 1 h, and the resin was washed five times with wash buffer (25 mm Tris-HCl, pH 7.5, 0.15 m NaCl, 1% Nonidet P-40). Proteins bound to SSL-conjugated Sepharose were eluted into 20 μl of 2× Laemmli's sample buffer (100 mm Tris-HCl, pH 6.8, 2.0% SDS, 20% glycerol, 0.02% bromphenol blue, 10% 2-mercaptoethanol) (19), separated on SDS-PAGE using a 10% polyacrylamide gel, and stained with CBB R250 (Merck). The protein specifically recovered by SSL-conjugated Sepharose was identified using peptide mass fingerprinting analysis as described previously (11, 13).
Pull-down Analysis
The mixture of human prothrombin (10 μg, 0.14 nmol), IgG (10 μg, 0.067 nmol), and BSA (10 μg, each in 10 μl of PBS) was incubated with SSL10-conjugated Sepharose (2 μl, 50% slurry) at 4 °C for 1 h. The supernatant was recovered by low speed centrifugation and mixed with 5 μl of 4× Laemmli's sample buffer (unbound fraction). The resin was washed twice with 500 μl of wash buffer, and bound proteins were eluted into 20 μl of 2× Laemmli's sample buffer with or without 2-mercaptoethanol (bound fraction). Each fraction was boiled for 5 min and subjected to SDS-PAGE and visualized by CBB staining. Densitometric analysis was performed on the bands corresponding to prothrombin and IgG recovered by SSL10-conjugated beads using ImageJ 1.46r software (National Institutes of Health). The interaction of SSL10 with factor Xa, prothrombin fragments digested by factor Xa, Gla domain-less prothrombin, and Gla domain-less factor Xa was also analyzed by the same procedure. To examine the interaction between SSL10 and prothrombin without modification of glutamate, 2 μl of SSL10-conjugated Sepharose was incubated with 200 μl of normal mice plasma or warfarin-treated mice plasma, and prothrombin bound to the resin was detected by immunoblot using anti-thrombin antibody (thrombin D-15, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), horseradish peroxidase (HRP)-conjugated anti-goat IgG (Jackson ImmunoResearch, West Grove, PA), and ECL Plus Western blotting detection reagents (GE Healthcare). Chemiluminescence was detected using a LAS-3000 Mini Lumino-image Analyzer (LAS-3000, Fujifilm, Tokyo, Japan).
Solid Phase Binding Assay
A 96-well microtiter plate (MaxiSorp, Nalgene, Rochester, NY) was coated with human prothrombin and factors VIIa, IXa, and Xa (6.9 × 10−3 nmol, corresponding to 0.5 μg of prothrombin) at 4 °C for 16 h. After blocking with TBS plus 1% BSA, SSL10 (2.0 × 10−3 nmol, 50 ng in 50 μl) was added to each well and incubated at room temperature for 1 h. After washing with TBS plus 0.05% Tween 20 five times, the well was incubated with a 1:1,000 dilution of mouse anti-SSL10 serum followed by HRP-conjugated anti-mouse IgG (Jackson ImmunoResearch). After washing, the amount of peroxidase bound to each well was determined using the OptEIATM 3,3′,5,5′-tetramethylbenzidine (TMB) substrate reagent set (BD Biosciences) according to the manufacturer's protocol. The absorbance in each well was read at 450 nm/655 nm by using a microplate reader (iMark microplate absorbance reader, Bio-Rad). To examine the effect of Ca2+ on the SSL10-prothrombin interaction, SSL10 was added to the prothrombin-immobilized well in the presence or absence of CaCl2 (0, 1.25, 10, 25, and 50 mm), NaCl (25, 50, and 100 mm), or EDTA (10 mm).
Surface Plasmon Resonance Analysis
Surface plasmon resonance analysis was performed using the ProteOn XPR36 system (Bio-Rad). Recombinant SSL10 was immobilized on a ProteOn GLC sensor chip by amine coupling according to the manufacturer's instructions. Prothrombin or IgG was diluted with 10 mm HEPES plus 0.01% Tween 20 (pH 7.4) and injected into the chip at a flow rate of 100 μl/min. The equilibrium dissociation constants were calculated using ProteOn ManagerTM software.
Examination of Anticoagulant Activity of SSL10
The effect of SSL10 on blood coagulation was examined by a plasma clotting assay. Varying amounts of SSL10 (0–10 μg (0–0.39 nmol)/test tube, dissolved in 10 μl) were added to 100 μl of citrated human plasma and incubated for 5 min at room temperature, added to 10 μl of aPTT reagent (Roche PT reagent, Roche Applied Science), and then incubated for 2 min at 37 °C. After low speed centrifugation, supernatant was transferred to a microtiter plate. The clotting reaction was started by the addition of 10 μl of 100 mm CaCl2, and the absorbance at 415 nm every 10 s was monitored for 6 min using a microplate reader. aPTT was measured using Roche PT reagent according to the manufacturer's protocol except for citrated plasma (0.1 ml), which was incubated with or without SSL10 (10 μg, 0.39 nmol) at 37 °C for 5 min in advance. To examine the effect of SSL10 on plasma clotting induced by factor Xa, citrated human plasma (100 μl) supplemented with PS-containing liposomes (2.5 nmol of lipid) was incubated with SSL10 (10 or 27 μg, 0.39 or 1.1 nmol) for 5 min at room temperature and transferred to the well preadded with factor Xa (0.033 μg, 7.7 × 10−4 nmol) and 100 mm CaCl2 (10 μl), and plasma clotting was monitored as described above. To examine the effect of SSL10 on coagulase-induced clotting, the clotting reaction was started by the addition of recombinant coagulase (500 ng, 13 nmol) to the citrated plasma pretreated with SSL10.
Protease Activity of Prothrombin
The protease activity of thrombin was measured by hydrolysis of chromogenic thrombin substrate peptide. Bovine α-thrombin (Sigma) or supernatant of recalcified plasma was incubated with 0.1 mm chromogenic thrombin substrate (H-Sar-Pro-Arg-p-nitroanilide, HCl, Calbiochem) in the presence or absence of SSL10 at 25 °C in TBS. Hydrolysis of the substrate was measured by recording the absorbance at 415 nm in a microplate reader every 1 min. The concentration of hydrolyzed product was determined from a standard curve of p-nitroaniline concentration versus absorbance at 415 nm. The effect of SSL10 on conversion of prothrombin to the active form was measured by an increment of thrombin activity in recalcified plasma. Citrated plasma (0.05 ml) was preincubated for 5 min at 37 °C in the presence or absence of SSL10. Activation of prothrombin was initiated by adding 0.05 ml of 25 mm CaCl2 and terminated by adding 0.1 ml of 100 mm EDTA. The generated thrombin activity in the supernatant was measured using chromogenic thrombin peptide as described above. To analyze the effect of SSL10 on generation of staphylothrombin by coagulase, prothrombin (1 μg, 0.014 nmol) was incubated with coagulase (0.25 μg, 6.6 × 10−3 nmol) in the presence or absence of SSL10 (2 μg, 0.079 nmol), and then hydrolysis of the substrate was measured by continuously recording the absorbance at 415 nm as described above. The statistical difference was assessed by Student's t test.
RESULTS
SSL10 Binds to Prothrombin
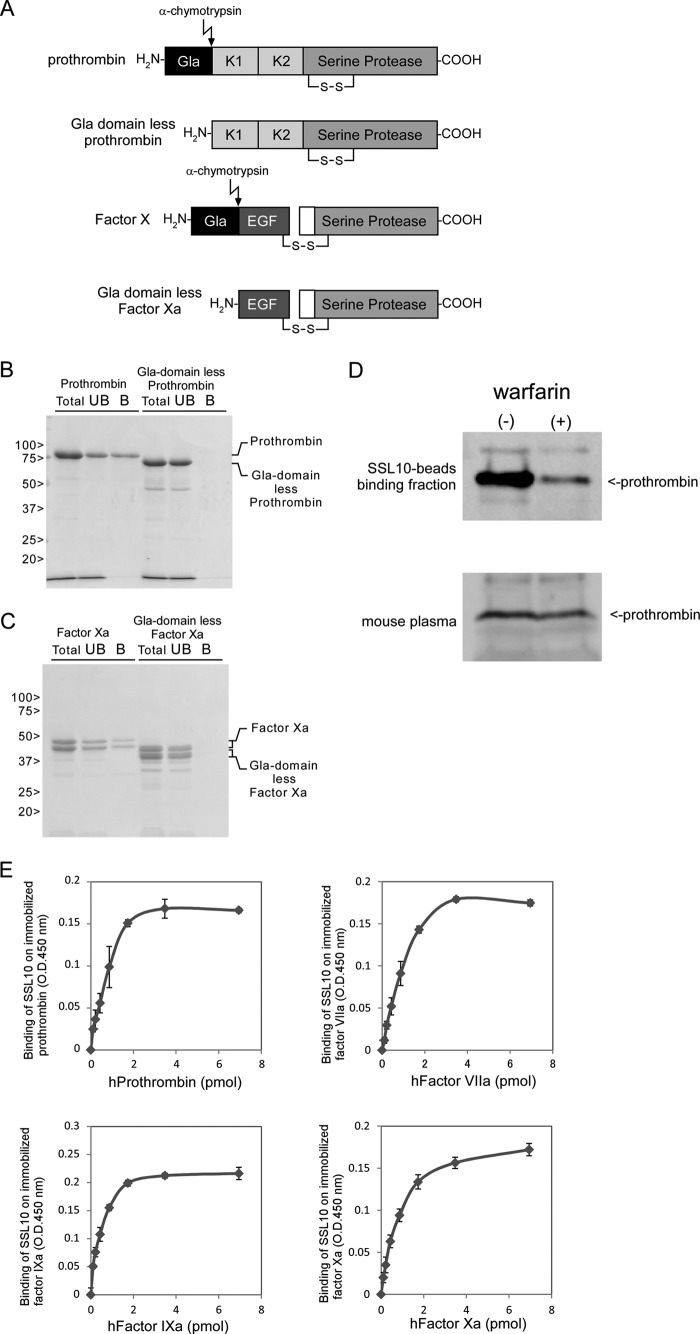
We attempted to isolate novel SSL-binding proteins from porcine plasma by affinity purification using His6-tagged SSL-conjugated Sepharose. As shown in Fig. 1A, an 80-kDa protein was specifically observed in the electrophoretic protein profile recovered by SSL10-conjugated Sepharose fraction but not by SSL7-conjugated Sepharose. SSL7-conjugated Sepharose recovered IgA (65 kDa, heavy chain of IgA in Fig. 1A) as previously reported (6). Other SSL-conjugated Sepharose failed to recover specific proteins from porcine plasma (data not shown). The 80-kDa protein recovered by SSL10-conjugated Sepharose was identified as porcine prothrombin by peptide mass fingerprint analysis using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Mass peaks detected by the MS analysis and the MASCOT search analysis are summarized in Table 1. To examine whether SSL10 recognizes human prothrombin, we performed pull-down analysis using SSL10-conjugated Sepharose and commercially available human prothrombin. SSL10- but not SSL7-conjugated Sepharose recovered human prothrombin from the mixture of human prothrombin and BSA (Fig. 1B). We previously reported that SSL10 binds to human IgG (13), and SSL10-conjugated Sepharose preferentially recovered prothrombin rather than human IgG from the mixture of two proteins; the amounts of prothrombin and IgG (H chain) recovered by SSL10-conjugated Sepharose from the mixture were decreased by 21.7 and 66.0% compared with those from solutions containing each alone, respectively, in densitometric analysis (Fig. 1B). In another independent experiment, the recoveries of prothrombin and IgG from their mixture by the resin were decreased by 17.7 and 49.9% compared with those from solutions containing each alone, respectively (data not shown). In a solid phase binding assay, the amount of SSL10 bound to immobilized prothrombin was increased according to the amount of prothrombin immobilized on the well, and saturation of the binding was observed (Fig. 1C). In surface plasmon resonance analysis, the equilibrium dissociation constant between SSL10 and prothrombin (KD = 1.36 × 10−7 m; Fig. 1D) was smaller than that between SSL10 and IgG (KD = 2.51 × 10−7 m; Fig. 1E). These data indicate that SSL10 is able to bind to porcine and human prothrombin.
FIGURE 1.
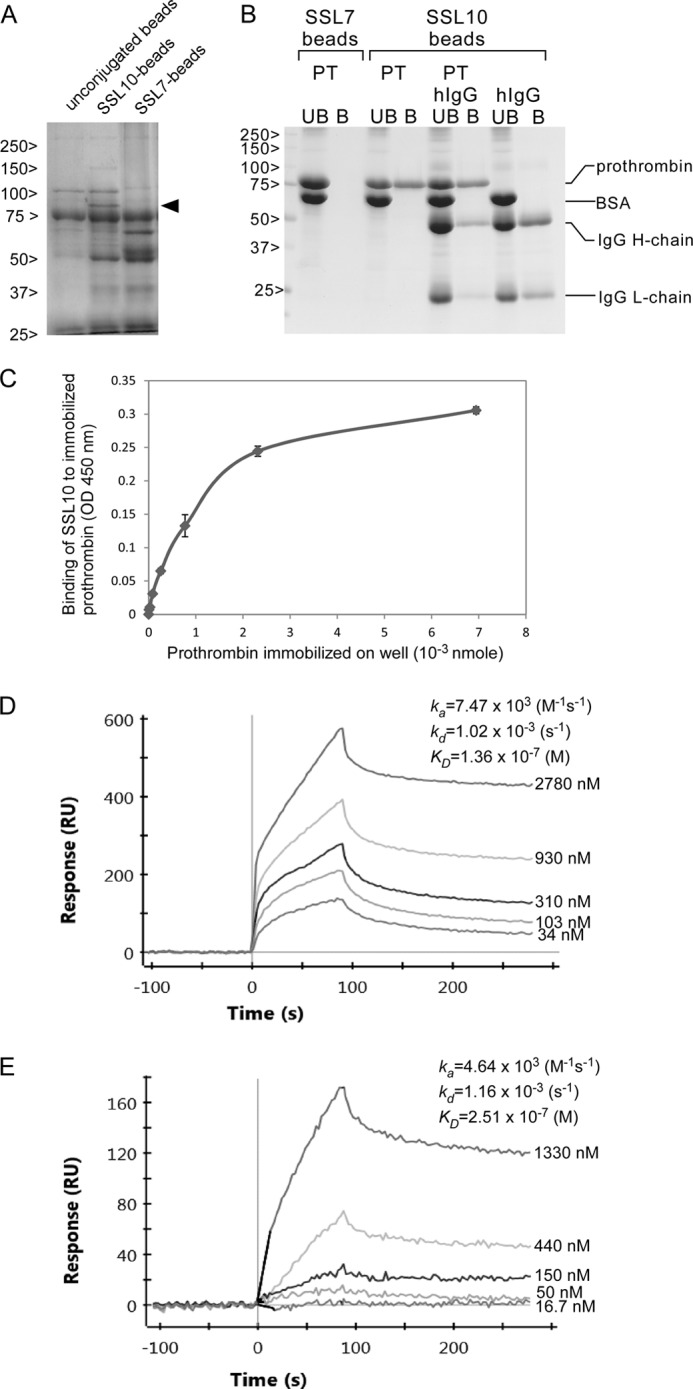
The interaction of SSL10 with prothrombin. A, isolation of SSL10-binding proteins from porcine plasma. Porcine plasma (15 ml) was mixed with SSL7- and SSL10-counjugated Sepharose or unconjugated Sepharose (10 μl, 50% of slurry) and incubated at room temperature for 1 h, and the resins were washed five times with wash buffer. The porcine plasma proteins bound to the resins were separated on SDS-PAGE using 10% polyacrylamide gel and stained with CBB. The protein specifically recovered by SSL10-conjugated Sepharose (80-kDa protein indicated by arrowheads) was subjected to peptide mass fingerprinting analysis. B, pull-down assay. Two microliters of SSL7- or SSL10-conjugated Sepharose (50% slurry) was incubated with a mixture of human IgG (0.067 nmol), human prothrombin (0.14 nmol), and BSA (10 μg) at 4 °C for 1 h. Bound (B) and unbound (UB) fractions were analyzed by SDS-polyacrylamide gel (10%) under reducing conditions followed by CBB staining. The amount of IgG and prothrombin recovered by the resin was estimated by the densitometric analysis on the bands corresponding to them using ImageJ version 1.46r software (National Institutes of Health). C, solid phase binding assay. Human prothrombin (0–6.9 × 10−3 nmol) was adsorbed to a microtiter plate well at 4 °C for 16 h. After blocking with 1% BSA, SSL10 (2.0 × 10−3 nmol in 50 μl) was added to each well and incubated at room temperature for 1 h. The well was reacted with mouse anti-SSL10 serum, followed by HRP-conjugated anti-mouse IgG, and then the amount of HRP bound to the well was determined using TMB substrate reagent. Data are shown as means ± S.D. of triplicates. D and E, sensorgrams of the interaction between prothrombin (D) or IgG (E) and immobilized SSL10. Various concentrations of human prothrombin (2.5–200 μg/ml, 34 to 2.78 × 103 nm) or human IgG (2.5–200 μg/ml, 16.7 to 1.33 × 103 nm) were analyzed by the ProteOn XPR36 system using a ProteOn GLC sensor chip, and the equilibrium dissociation constants (KD) were calculated as described under “Experimental Procedures.” The sensorgrams are representative of three experiments.
TABLE 1.
Observed mass peaks detected by the MS analysis and the corresponding amino acid sequences of porcine thrombin
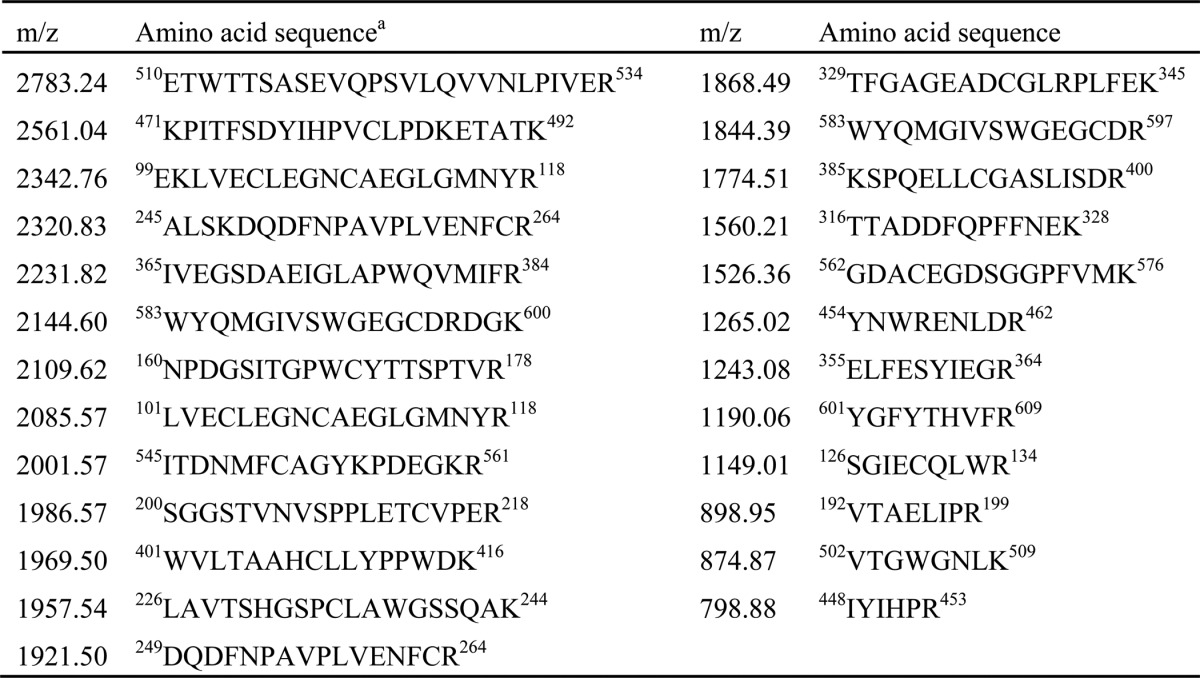
a GenBankTM accession number: Q19AZ8_PIG (prothrombin protein, Sus scrofa). Sequence coverage was 53%. The Mowse score of this analysis was 142.
SSL10 Binds to Fragment 1 + 2 of Prothrombin as Well as Factor Xa
To identify the region of prothrombin responsible for binding to SSL10, we performed pull-down analysis using SSL10-conjugated Sepharose and fragments of prothrombin generated by factor Xa digestion (Fig. 2A). As shown in Fig. 2B, fragment 1 + 2, which is composed of the Gla domain and kringle 1 and 2 (∼27 kDa in unreduced condition) but not the serine protease domain of thrombin (∼35 kDa in unreduced condition) was recovered by SSL10-conjugated Sepharose. Interestingly, factor Xa used for the digestion also bound to SSL10-conjugated Sepharose (Fig. 2B). Factor Xa was independently co-precipitated with SSL10-conjugated Sepharose (Fig. 2B). These data indicate that SSL10 is able to bind to fragment 1 + 2 of prothrombin as well as factor Xa.
FIGURE 2.
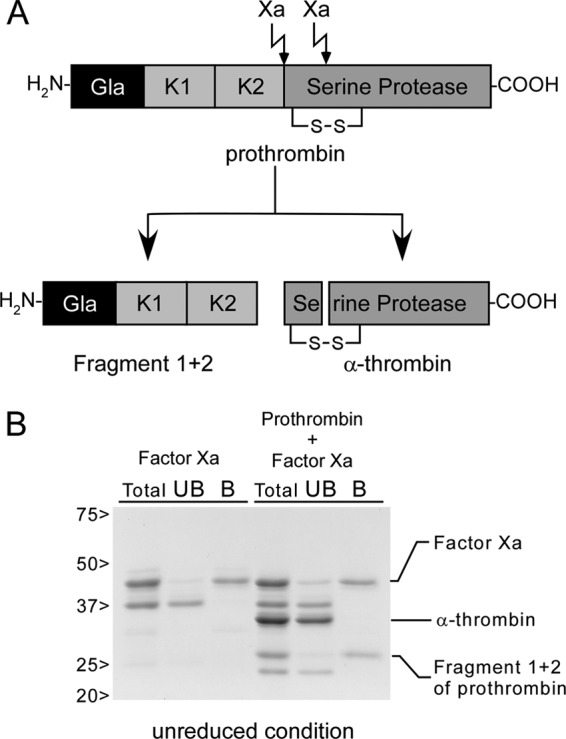
Pull-down analysis using fragments derived from prothrombin and SSL10-conjugated Sepharose. A, fragments of prothrombin generated by digestion with factor Xa. Gla, Gla domain; K1 and K2, kringle 1 and 2 domains. B, SSL10-conjugated Sepharose (2 μl of 50% slurry) was incubated with factor Xa (0.12 nmol) or the digested mixture of prothrombin (0.14 nmol) with factor Xa, and the bound (B) and unbound (UB) fractions were analyzed by SDS-polyacrylamide gel (10%) under unreduced conditions followed by CBB staining.
Gla Domain Is Required for the Interaction between SSL10 and Coagulation Factors
Because the Gla domain is shared among fragment 1 + 2 of prothrombin and factor Xa, we examined the involvement of the Gla domain in the interaction using Gla domain-less prothrombin and factor Xa prepared by limiting digestion of them with α-chymotrypsin (Fig. 3A). Removal of the Gla domain completely abolished binding of prothrombin and factor Xa to SSL10-conjugated Sepharose (Fig. 3, B and C). The Gla domain of coagulation factors contains post-translationally modified γ-carboxyglutamic acids, and an anticoagulation medicine, warfarin, is known to inhibit the post-translational modification by preventing recycle of vitamin K. The recovery of prothrombin from plasma by SSL10-conjugated Sepharose was diminished by the treatment of mice with warfarin (Fig. 3D). Coagulation factors VII and IX are vitamin K-dependent Gla-containing proteins, and SSL10 was able to bind to immobilized factor VIIa and IXa in a manner similar to prothrombin and factor Xa (Fig. 3E). These results indicate that the Gla domain of the coagulation factors is responsible for their binding to SSL10, and modification of γ-carboxyglutamic acid residues in the Gla domain is essential for the interaction.
FIGURE 3.
Involvement of Gla domain and modification of γ-carboxyglutamic acid in the interaction between SSL10 and coagulation factors. A, Gla domain-less prothrombin and factor Xa generated by limiting digestion with α-chymotrypsin. B, prothrombin or Gla domain-less prothrombin (0.14 nmol) were incubated with SSL10-conjugated Sepharose (2 μl of 50% slurry), and the total (T), unbound (UB), and bound (B) fractions were analyzed by SDS-polyacrylamide gel (10%) under reduced conditions followed by CBB staining. C, factor Xa and Gla domain-less factor Xa (0.12 nmol) were incubated with SSL10-conjugated Sepharose, and the total, bound, and unbound fractions were analyzed by SDS-polyacrylamide gel (10%) under unreduced conditions. D, mouse plasma (50 μl) collected from untreated mice or warfarin-treated mice (with free access of mice to water, 7.5 mg/liter on the first day and 2.5 mg/liter on the following 4 days) was incubated with 5 μl of SSL10-conjugated Sepharose (50% of slurry). After washing SSL10-conjugated Sepharose, proteins bound to the resin (top) and mouse plasma (2 μl) (bottom) were separated by SDS-PAGE and detected by immunoblot using anti-prothrombin antibody. E, human factor VIIa, IXa, or Xa (0–6.9 × 10−3 nmol) was adsorbed to a microtiter plate well at 4 °C for 16 h. After blocking with 1% BSA, SSL10 (2.0 × 10−3 nmol in 50 μl) was added to each well and incubated at room temperature for 1 h. The well was reacted with mouse anti-SSL10 serum followed by HRP-conjugated anti-mouse IgG, and then the amount of HRP bound to the well was determined using TMB substrate reagent. Data are shown as means ± S.D. (error bars) of triplicates.
The Interaction between SSL10 and Prothrombin Is Competed by Ca2+
The Gla domain of coagulation factors is known to bind to Ca2+. We analyzed the effect of Ca2+ on the interaction of prothrombin with SSL10. The co-precipitation of prothrombin with SSL10-conjugated Sepharose was decreased in the presence of CaCl2. The inhibitory effect of Ca2+ on SSL10-prothronbin interaction was dose-dependent; the amounts of prothrombin bound to the resin were decreased by 8.94, 44.4, and 56.9% in the presence of 1.25, 10, and 50 mm of Ca2+, respectively, by densitometric analysis (Fig. 4A). In another experiment, the interaction was decreased by 48.2% in the presence of CaCl2 (10 mm) but not affected in the presence of EDTA (10 mm, increased by 1.3%). A similar result was obtained with the solid phase binding assay because the binding of SSL10 to immobilized prothrombin was decreased by 44.1 and 15.8% by the addition of 50 and 10 mm CaCl2, respectively. The binding was slightly and non-significantly decreased by the physiological concentration of Ca2+ (Fig. 4B).
FIGURE 4.
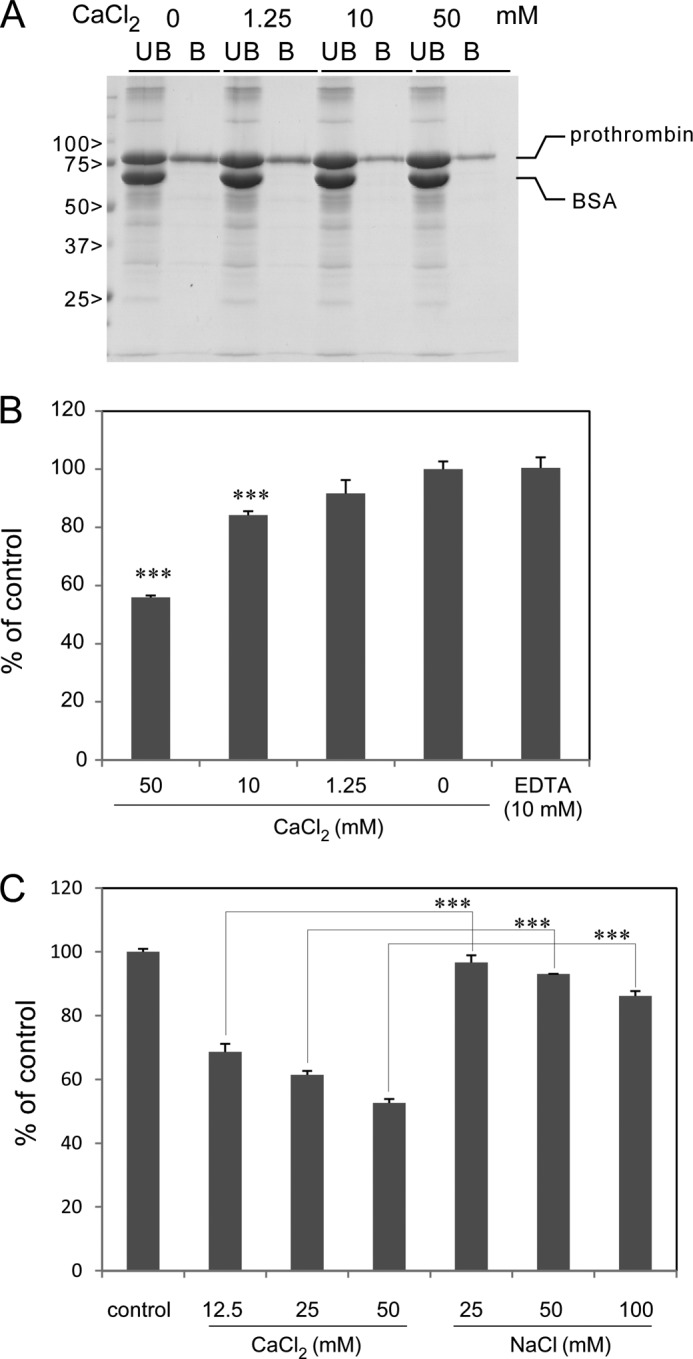
The effect of Ca2+ on SSL10-prothrombin interaction. A, prothrombin (0.14 nmol) was incubated with SSL10-conjugated Sepharose (2 μl, 50% slurry) in the presence or absence of CaCl2 (0–50 mm at final concentration). The bound (B) and unbound (UB) fractions were analyzed by SDS-polyacrylamide gel (10%). The amount of prothrombin recovered by the resin was determined by densitometry on the bands corresponding to prothrombin using ImageJ version 1.46r. B and C, human prothrombin (2.1 × 10−3 nmol) was adsorbed to a microtiter plate well at 4 °C for 16 h. After blocking with 1% BSA, SSL10 (5.9 × 10−3 nmol in 50 μl) was added to each well and incubated at room temperature for 1 h in the presence or absence of CaCl2 (0, 1.25, 10, and 50 mm final concentration) or EDTA (10 mm) in C or of CaCl2 (0, 12.5, 20, and 50 mm final concentration) or NaCl (25, 50 and 100 mm) in D. The well was reacted with mouse anti-SSL10 serum followed by HRP-conjugated anti-mouse IgG, and then the amount of HRP bound to the well was determined using TMB substrate reagent. Data were shown as means ± S.D. (error bars) of triplicates. The statistical difference was assessed by Student's t test. ***, p < 0.01.
Twice the concentration of NaCl slightly inhibited the interaction, but this inhibition was significantly smaller than that by CaCl2 (Fig. 4C). These results indicate that the interaction between SSL10 and prothrombin was competed by Ca2+.
SSL10 Inhibits Plasma Clotting via Inhibiting Activation of Prothrombin but Not Thrombin Activity
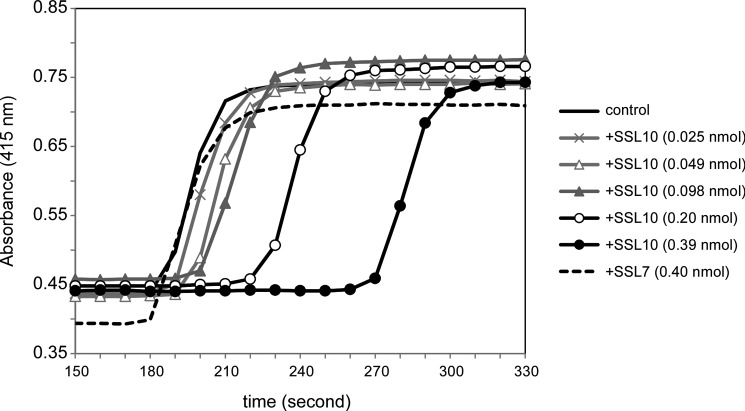
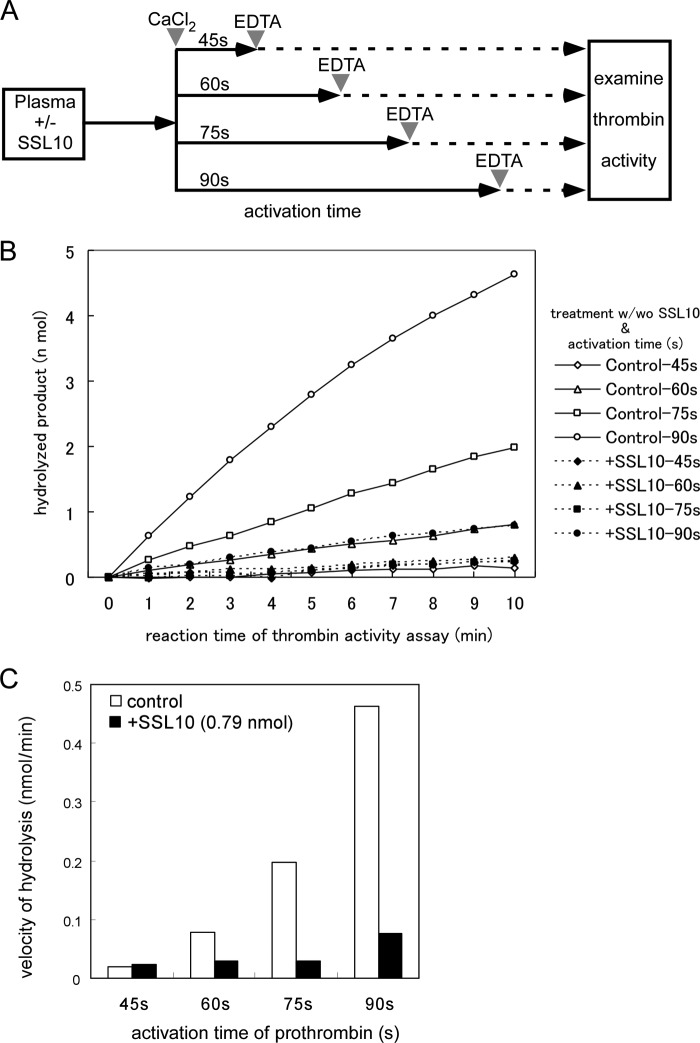
We examined the effect of SSL10 on blood coagulation. Plasma clotting after activation of the intrinsic pathway was prolonged by the addition of SSL10 (0.025–0.39 nmol) to 100 μl of plasma but not SSL7 (Fig. 5). The prolonging effect of SSL10 on plasma clotting was dose-dependent; 0.39, 0.2, and 0.098 nmol of SSL10 prolonged clotting time by 45.1, 20.5, and 7.6%, respectively. The anticoagulant activity of SSL10 was observed by a conventional aPTT method (control value was 38 s; 0.79 nmol of SSL10 prolonged clotting time by 28 s, 73.7%; 0.39 nmol of SSL10 prolonged it by 8 s, 21.1%). On the other hand, SSL10 did not inhibit hydrolysis of thrombin substrate by thrombin, and protease activities of bovine α-thrombin and supernatant of recalcified mouse plasma were not inhibited by a more than 1000-fold molar excess of SSL10 (Fig. 6, A and B). The binding of SSL10 to immobilized bovine α-thrombin used in this study was quite low (less than 10%) as compared with immobilized human prothrombin in solid phase binding analysis (data not shown). Then we examined the temporal generation of thrombin activity in recalcified plasma in the presence or absence of SSL10, as described in the legend to Fig. 7A. The protease activity of thrombin was successively increased after the addition of Ca2+ to citrated plasma; however, it was strongly suppressed in the presence of SSL10 (0.79 nmol) in 100 μl of plasma (Fig. 7, B and C). These results indicate that SSL10 inhibits blood coagulation via diminishing the generation of thrombin but not by inhibiting the protease activity of thrombin.
FIGURE 5.
Effect of SSL10 on plasma clotting induced by Ca2+. Plasma was preincubated with varying amount of SSL10 (0.025–0.39 nmol/100 μl of plasma) or SSL7 (0.40 nmol), and then the intrinsic pathway was activated by the addition of aPTT reagent (10 μl). Plasma clotting after the addition of CaCl2 (100 mm, 10 μl) was monitored by measuring the absorbance at 415 nm using a microplate reader. Time course curves are representative of three independent experiments.
FIGURE 6.
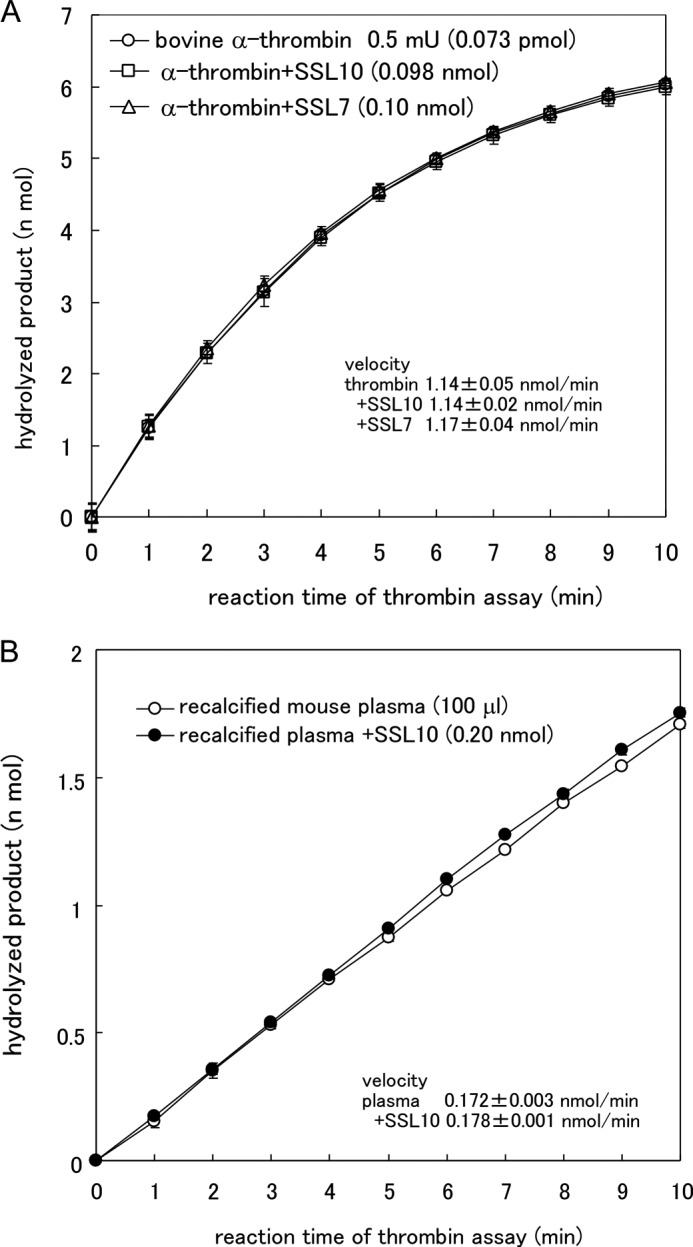
Effect of SSL10 on hydrolysis of thrombin substrate by α-thrombin. A, bovine α-thrombin (0.5 millunits/7.3 × 10−5 nmol; Sigma) was incubated with 0.1 mm of chromogenic thrombin substrate in the presence or absence of SSL7 (0.1 nmol) or SSL10 (0.098 nmol) at 25 °C in TBS. The hydrolyzed product was measured by continuously recording the absorbance at 415 nm in a microplate reader. B, supernatant of recalcified mouse plasma (100 μl) was incubated with the thrombin substrate in the presence or absence of SSL10 (0.20 nmol) at 25 °C in TBS, and the hydrolyzed product was measured using a microplate reader. Data were shown as means ± S.D. (error bars) of triplicates.
FIGURE 7.
The effect of SSL10 on the generation of thrombin activity in recalcified plasma. A, scheme for analyzing the effect of SSL10 on the generation of thrombin activity in recalcified plasma. B, CaCl2 (25 mm, 50 μl) was added to mouse citrated plasma (50 μl) preincubated with or without SSL10 (0.79 nmol). After incubation for the indicated time, activation of prothrombin was terminated by the addition of EDTA (100 mm, 100 μl). The generated thrombin activity in the supernatant was measured using chromogenic thrombin peptide as described under “Experimental Procedures.” B, time course graph for hydrolysis of thrombin substrate by recalcified plasma prepared as shown in A. C, graph for velocity of the hydrolysis of thrombin substrate by recalcified plasma.
SSL10 Attenuates Clotting Induced by the Addition of Xa to Plasma
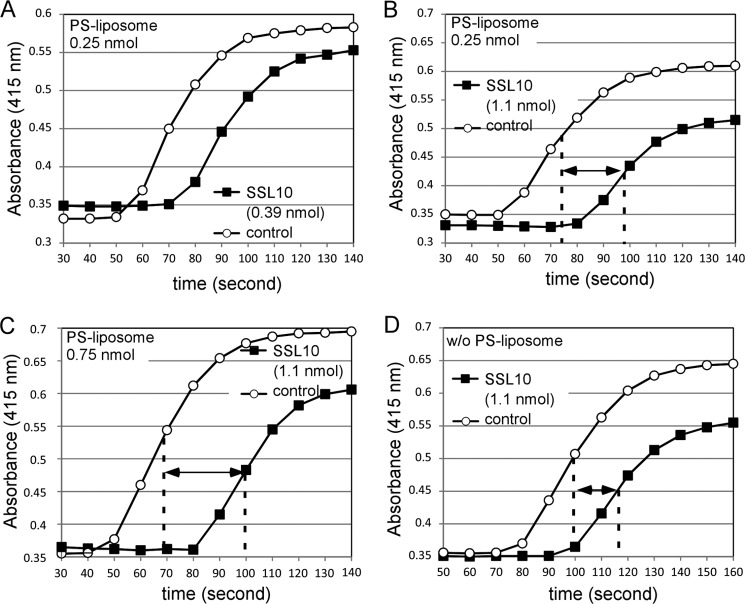
To examine whether SSL10 inhibits conversion of prothrombin to thrombin by factor Xa, the penultimate step of blood coagulation cascade, we observed the clotting induced by the addition of factor Xa and Ca2+ to citrated plasma in the presence or absence of SSL10. Factor Xa (0.077 nmol added to 100 μl plasma) induced plasma clotting in the presence of Ca2+ and PS-containing liposome, and SSL10 (0.39 nmol) delayed the clotting by 20 s, although the delay by SSL10 was shorter than that of aPTT reagent-induced clotting (Fig. 8A). The delay time was increased by a larger amount of SSL10 (1.1 nmol) (Fig. 8B), and the effect of SSL10 on the clotting was dependent on the PS-containing liposomes (Fig. 8, B–D). The addition of phosphatidylcholine liposome did not accelerate the coagulation induced by Xa (data not shown). These results indicate that SSL10 is able to inhibit the conversion of prothrombin to thrombin by factor Xa in plasma, especially on the cell surface membrane.
FIGURE 8.
Effect of SSL10 on plasma clotting induced by factor Xa. SSL10 (0.39 or 1.1 nmol) was added to citrated human plasma (100 μl) supplemented with PS-containing liposomes (2.5–7.5 nmol of lipid), the mixture was incubated for 5 min at room temperature and transferred to the well preadded with factor Xa (7.7 × 10−4 nmol) and 100 mm CaCl2 (10 μl), and plasma clotting was monitored as described under “Experimental Procedures.” A, SSL10 (0.39 nmol) was added to 100 μl of plasma supplemented with 0.25 nmol of PS-containing liposome. B–D, SSL10 (1.06 nmol) was added to 100 μl of plasma supplemented with 0.25 or 0.75 nmol of PS-containing liposome (B or C, respectively) or without liposome (D). The dotted lines in the graphs show the time point of 50% clotting, and the double-headed arrows show the difference of the half-clotting time between control and SSL10.
SSL10 Weakly Attenuates Blood Coagulation by Coagulase
S. aureus is known to produce coagulase, which activates prothrombin non-proteolytically (i.e. forms a complex with prothrombin called staphylothrombin and induces the conformational change of prothrombin to induce conversion of fibrinogen to fibrin). Plasma clotting was induced within 180 s by the addition of 0.013 nmol of recombinant coagulase to citrated plasma (Fig. 9A), which was comparable with the clotting mediated by the intrinsic pathway (i.e. triggered by aPTT reagent and Ca2+ in Fig. 5). The plasma clotting induced by coagulase was slightly delayed by the addition of 0.39 nmol of SSL10, and less than 0.20 nmol of it failed to delay the plasma clotting (Fig. 9A). Plasma clotting initiated by the addition of coagulase (6.6, 3.3, and 1.6 × 10−3 nmol) to 100 μl of citrated plasma in a test tube at 37 °C was terminated within 15, 30, and 45 s, respectively, regardless of the presence or absence of SSL10 (0.39 nmol). We examined whether SSL10 affects the formation of staphylothrombin and protease activity of staphylothrombin. In the presence of SSL10, the increment of thrombin protease activity by coagulase was inhibited by 30% (Fig. 9B). As shown in Fig. 9C, SSL10 did not inhibit hydrolysis of thrombin substrate by staphylothrombin. These results indicate that SSL10 slightly inhibits the formation of staphylothrombin but does not inhibit the protease activity of staphylothrombin, and the effect of SSL10 on coagulation induced by coagulase is minimal.
FIGURE 9.
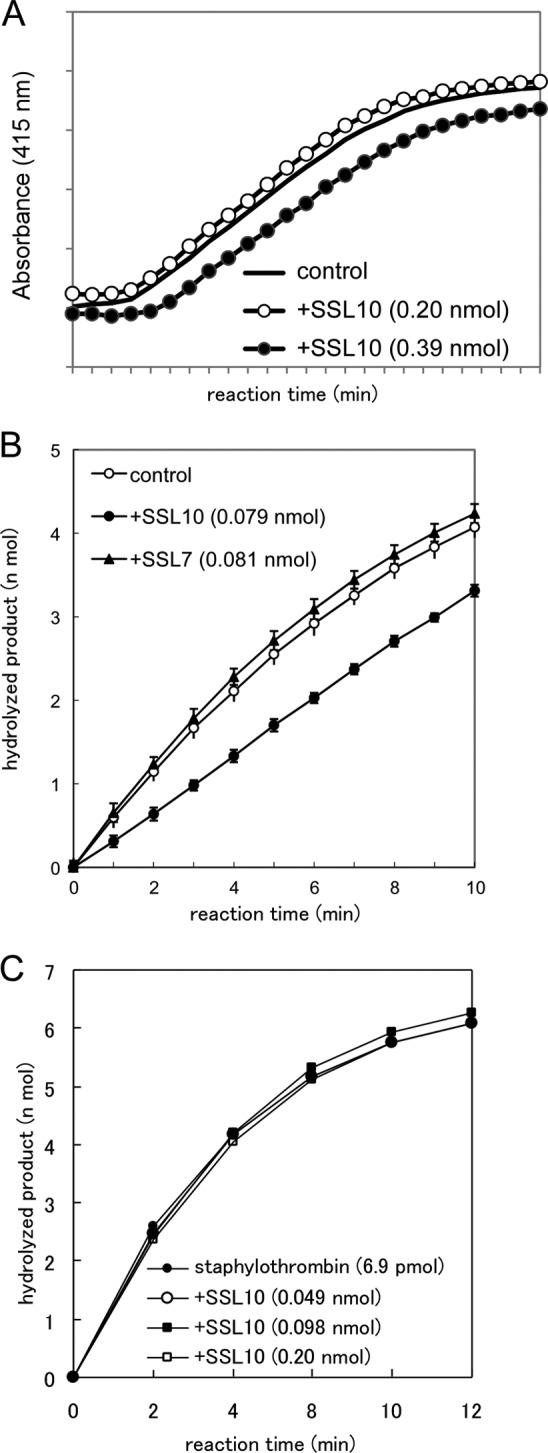
The effect of SSL10 on coagulase and staphylothrombin. A, plasma clotting induced by recombinant coagulase. Plasma clotting was initiated by the addition of recombinant coagulase (0.5 μg) to the citrated plasma pretreated with SSL10 (0.20 and 0.39 nmol) and monitored by measuring the absorbance at 415 nm using a microplate reader. B, generation of staphylothrombin by coagulase. Prothrombin (0.014 nmol) was incubated with coagulase (6.6 pmol) in the presence or absence of SSL10 or SSL7 (0.079 or 0.081 nmol, respectively), and then hydrolysis of the substrate was measured by continuously recording the absorbance at 415 nm in a microplate reader. C, hydrolysis of thrombin substrate by staphylothrombin. Staphylothrombin (prepared from 6.9 pmol of prothrombin) generated by the incubation of prothrombin with recombinant coagulase fragment was incubated with 0.1 mm chromogenic thrombin substrate in the presence or absence of SSL10 (0.049–0.20 nmol) at 25 °C in TBS. Hydrolysis of the substrate was measured by continuously recording the absorbance at 415 nm in a microplate reader. Data in B and C were shown as means ± S.D. (error bars) of triplicates.
DISCUSSION
In this study, we demonstrated that one of the SSL family exoproteins, SSL10, binds to the coagulation factors prothrombin and factor Xa and is able to inhibit blood coagulation. The physiological functions of SSL10 have been revealed (i.e. SSL10 binds to the Fc region of human IgG and inhibits the complement activation through the classical pathway (13) and IgG-Fcγ receptor interaction to inhibit phagocytosis (14), inhibits CXCL12-induced leukemia cell migration by blocking CXCR4 (12), and binds to phosphatidylserine and recognizes apoptotic cells (17)). Taken together, SSL10 seems to possess pleiotropic effects on host immunity and homeostasis.
We could not identify prothrombin as the SSL10-binding protein when we isolated human IgG from human serum using SSL10-conjugated Sepharose (13). That was because the concentration of IgG (7.7–20 mg/ml) was extremely higher than that of prothrombin (0.1–0.15 mg/ml) in plasma, the concentration of prothrombin in serum is less than that in plasma, and SSL10 binds primate IgG but not other mammal IgG (14). Therefore, we were able to isolate prothrombin as SSL10-binding protein using porcine plasma in this study. SSL10 inhibited coagulation of human plasma, indicating that SSL10 acts as anticoagulant in the presence of IgG, and we could not detect other Gla-containing coagulation factors (factors VII, IX, and X) in the SSL10-conjugated Sepharose binding fraction (Fig. 1A), regardless of their binding ability to SSL10 (Fig. 3E). It is likely that their amount was less than that of prothrombin in plasma. In Fig. 1A, 150 kDa of protein also seemed to be specifically recovered by SSL10-conjugated Sepharose; however, its amount was too small to identify the molecule.
SSL10 binds to both prothrombin and factor Xa via their N-terminal Gla domain. Thrombin, the Gla domain-less form of prothrombin, and factor Xa did not bind to SSL10 (Figs. 2B and 3 (B and C)), and SSL10 did not affect the protease activity of thrombin (Fig. 6A). This is reasonable because all of them lose the Gla domain during activation or digestion by protease. SSL10 binds to prothrombin of various species because the Gla domain of prothrombin is highly conserved among species (human versus pig (78%) and human versus mouse (88%)). In Fig. 3, B and C, we could not detect digested Gla domain, because it was too small (the fragment of prothrombin is estimated to be 9 kDa). The viral protein adenovirus serotype 5 hexon protein is reported to bind to the Gla domain and is responsible for the entry of this virus into hepatocytes (20). To our knowledge, this is the first report indicating that the bacterial exoprotein binds to the Gla domain of coagulation factors. There is no homology among them; therefore, each protein binds to the Gla domain of coagulation factors via a distinct mechanism.
Prothrombin existing in plasma from warfarin-treated mice failed to bind to SSL10 (Fig. 3D), and the interaction of SSL10 with prothrombin was attenuated by the excess concentration of Ca2+ (Fig. 4, A–C), suggesting that SSL10 recognizes γ-carboxyglutamic acid in the Gla domain. Because the inhibition by twice the concentration of NaCl was significantly smaller than that by CaCl2 (Fig. 4C), the contribution of the ionic strength of CaCl2 to the inhibition was small. The competitive effect of Ca2+ on SSL10-prothrombin interaction was limited at 1.25 mm of Ca2+; therefore, the interaction between SSL10 and prothrombin will be significant in physiological conditions. We previously reported that SSL10 binds to PS (17). Recombinant GST-annexin V, which interacts with PS, did not bind to SSL10 (data not shown), suggesting that the PS-binding feature does not account for the binding of SSL10 to prothrombin.
The physiological importance of the Gla domain is well established; the domain binds to Ca2+ and is involved in the assembly of coagulation factors on the surface of activated platelets, which is essential for effective activation of prothrombin. In this study, SSL10 delayed blood coagulation induced by the addition of Ca2+ (Fig. 5), and SSL10 abrogated the generation of thrombin activity in recalcified plasma (Fig. 7, B and C), suggesting that SSL10 inhibits blood coagulation via interrupting the activation of prothrombin, the penultimate crucial step of the coagulation cascade. SSL10 inhibited generation of thrombin activity in plasma by the addition of factor Xa and Ca2+, and the inhibitory effect of SSL10 was dependent on the amount of PS-containing liposomes added to the plasma (Fig. 8). In this experiment, the addition of PS-liposome would increase the site of assembly for coagulation factors, and SSL10 may inhibit the assembly of them. Because SSL10 interacted with all of the Gla-containing coagulation factors (prothrombin and factors VII, IX, and X), it is likely that SSL10 also interferes with their functions in coagulation cascade. Indeed, the inhibitory effect of SSL10 on clotting induced by the addition of factor Xa (Fig. 8) was weaker than that on clotting induced by aPTT reagent (Fig. 5). We previously reported that SSL10 is able to bind phosphatidylserine (17). It is possible that SSL10 bridges prothrombin with phospholipid in the absence of Ca2+, but the present data exclude this possibility. The significance of SSL10-phospholipid interaction awaits further study.
S. aureus, but not other weakly pathogenic staphylococci, produces the extracellular protein coagulase, which binds to prothrombin and forms an enzymatically active complex called staphylothrombin (21). Coagulase is thought to be responsible for the virulence of S. aureus (22). SSL10 did not inhibit the proteolytic activity of staphylothrombin, mildly attenuated the formation of staphylothrombin, and inhibited plasma clotting induced by coagulase. However, the anti-coagulant effect was slight as compared with that on the intrinsic pathway. These results suggest that the inhibitory effect of SSL10 on staphylococcal coagulase is limited. Probably, SSL10 is able to disturb physiological blood coagulation with minimal effect on coagulase. S. aureus produces a series of proteins that affect coagulation and fibrinolysis (i.e. coagulase, von Willebrand factor-binding protein (23), staphylokinase (24), and SSL10). The regulation of or interference with coagulation and fibrinolysis would be beneficial for S. aureus.
The target proteins of the SSL family found so far are mostly immune molecules, suggesting that the SSL family contributes to the immune evasion of S. aureus. The present findings suggest the idea that the SSL family is a wide spectrum disturbing factor against not only host immunity but also homeostasis. Fourteen SSLs share low amino acid homology; therefore, each SSL would have a corresponding binding partner, and the functions of most SSL proteins remain uncharacterized. Moreover, several SSLs have multiple binding partners (e.g. SSL5 (binds to PSGL-1, chemoattractants receptors, matrix metalloproteinase-9, GPIbα, and GPIIb/IIIa), SSL7 (IgA and C5) and SSL10 (CXCR4, IgG, phosphatidylserine, and prothrombin)). The identification of all of the SSL target molecules will contribute to the understanding of the virulence and pathogenesis of S. aureus.
Acknowledgments
We are grateful to the Toyota meat inspection center for providing porcine blood. We thank Akari Hanai, Chizuko Murase, Takuya Okumura, Natsuko Yamaoka, and Ayako Saito for technical support. We thank Takeshi Kumagai (Bio-Rad Laboratories, Japan) for valuable technical advice in surface plasmon resonance analysis.
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Ohyama Health Foundation; and the Takeda Science Foundation.
- SSL
- staphylococcal superantigen-like protein
- aPTT
- activated partial thromboplastin time
- CBB
- Coomassie Brilliant Blue
- Gla
- γ-carboxyglutamic acid
- PS
- phosphatidylserine
- TMB
- 3,3′,5,5′-tetramethylbenzidine.
REFERENCES
- 1. Postma B., Poppelier M. J., van Galen J. C., Prossnitz E. R., van Strijp J. A., de Haas C. J., van Kessel K. P. (2004) Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 172, 6994–7001 [DOI] [PubMed] [Google Scholar]
- 2. Williams R. J., Ward J. M., Henderson B., Poole S., O'Hara B. P., Wilson M., Nair S. P. (2000) Identification of a novel gene cluster encoding staphylococcal exotoxin-like proteins. Characterization of the prototypic gene and its protein product, SET1. Infect. Immun. 68, 4407–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fitzgerald J. R., Reid S. D., Ruotsalainen E., Tripp T. J., Liu M., Cole R., Kuusela P., Schlievert P. M., Järvinen A., Musser J. M. (2003) Genome diversification in Staphylococcus aureus. Molecular evolution of a highly variable chromosomal region encoding the staphylococcal exotoxin-like family of proteins. Infect. Immun. 71, 2827–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Shangiti A. M., Naylor C. E., Nair S. P., Briggs D. C., Henderson B., Chain B. M. (2004) Structural relationships and cellular tropism of staphylococcal superantigen-like proteins. Infect. Immun. 72, 4261–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arcus V. L., Langley R., Proft T., Fraser J. D., Baker E. N. (2002) The three-dimensional structure of a superantigen-like protein, SET3, from a pathogenicity island of the Staphylococcus aureus genome. J. Biol. Chem. 277, 32274–32281 [DOI] [PubMed] [Google Scholar]
- 6. Langley R., Wines B., Willoughby N., Basu I., Proft T., Fraser J. D. (2005) The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc α RI binding and serum killing of bacteria. J. Immunol. 174, 2926–2933 [DOI] [PubMed] [Google Scholar]
- 7. Bestebroer J., Aerts P. C., Rooijakkers S. H., Pandey M. K., Köhl J., van Strijp J. A., de Haas C. J. (2010) Functional basis for complement evasion by staphylococcal superantigen-like 7. Cell Microbiol 12, 1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bestebroer J., Poppelier M. J., Ulfman L. H., Lenting P. J., Denis C. V., van Kessel K. P., van Strijp J. A., de Haas C. J. (2007) Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109, 2936–2943 [DOI] [PubMed] [Google Scholar]
- 9. Bestebroer J., van Kessel K. P., Azouagh H., Walenkamp A. M., Boer I. G., Romijn R. A., van Strijp J. A., de Haas C. J. (2009) Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood 113, 328–337 [DOI] [PubMed] [Google Scholar]
- 10. de Haas C. J., Weeterings C., Vughs M. M., de Groot P. G., Van Strijp J. A., Lisman T. (2009) Staphylococcal superantigen-like 5 activates platelets and supports platelet adhesion under flow conditions, which involves glycoprotein Ibα and αIIbβ3. J. Thromb. Haemost. 7, 1867–1874 [DOI] [PubMed] [Google Scholar]
- 11. Itoh S., Hamada E., Kamoshida G., Takeshita K., Oku T., Tsuji T. (2010) Staphylococcal superantigen-like protein 5 inhibits matrix metalloproteinase 9 from human neutrophils. Infect. Immun. 78, 3298–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walenkamp A. M., Boer I. G., Bestebroer J., Rozeveld D., Timmer-Bosscha H., Hemrika W., van Strijp J. A., de Haas C. J. (2009) Staphylococcal superantigen-like 10 inhibits CXCL12-induced human tumor cell migration. Neoplasia 11, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Itoh S., Hamada E., Kamoshida G., Yokoyama R., Takii T., Onozaki K., Tsuji T. (2010) Staphylococcal superantigen-like protein 10 (SSL10) binds to human immunoglobulin G (IgG) and inhibits complement activation via the classical pathway. Mol. Immunol. 47, 932–938 [DOI] [PubMed] [Google Scholar]
- 14. Patel D., Wines B. D., Langley R. J., Fraser J. D. (2010) Specificity of staphylococcal superantigen-like protein 10 toward the human IgG1 Fc domain. J. Immunol. 184, 6283–6292 [DOI] [PubMed] [Google Scholar]
- 15. Yokoyama R., Itoh S., Kamoshida G., Takii T., Fujii S., Tsuji T., Onozaki K. (2012) Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infect. Immun. 80, 2816–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bardoel B. W., Vos R., Bouman T., Aerts P. C., Bestebroer J., Huizinga E. G., Brondijk T. H., van Strijp J. A., de Haas C. J. (2012) Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J. Mol. Med. 90, 1109–1120 [DOI] [PubMed] [Google Scholar]
- 17. Itoh S., Yokoyama R., Murase C., Takii T., Tsuji T., Onozaki K. (2012) Staphylococcal superantigen-like protein 10 (SSL10) binds to phosphatidylserine and apoptotic cells. Microbiol. Immunol. 56, 363–371 [DOI] [PubMed] [Google Scholar]
- 18. Dode C., Rabiet M. J., Bertrand O., Labie D., Elion J. (1980) Characterization of a proteolytically modified form of human prothorombin. Biochem. Biophys. Res. Commun. 94, 660–666 [DOI] [PubMed] [Google Scholar]
- 19. Laemmli U. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 20. Waddington S. N., McVey J. H., Bhella D., Parker A. L., Barker K., Atoda H., Pink R., Buckley S. M., Greig J. A., Denby L., Custers J., Morita T., Francischetti I. M., Monteiro R. Q., Barouch D. H., van Rooijen N., Napoli C., Havenga M. J., Nicklin S. A., Baker A. H. (2008) Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132, 397–409 [DOI] [PubMed] [Google Scholar]
- 21. Kawabata S., Morita T., Iwanaga S., Igarashi H. (1985) Enzymatic properties of staphylothrombin, an active molecular complex formed between staphylocoagulase and human prothrombin. J. Biochem. 98, 1603–1614 [DOI] [PubMed] [Google Scholar]
- 22. McAdow M., Missiakas D. M., Schneewind O. (2012) Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections. J. Innate Immun. 4, 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kroh H. K., Panizzi P., Bock P. E. (2009) Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc. Natl. Acad. Sci. U.S.A. 106, 7786–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lijnen H. R., Van Hoef B., De Cock F., Okada K., Ueshima S., Matsuo O., Collen D. (1991) On the mechanism of fibrin-specific plasminogen activation by staphylokinase. J. Biol. Chem. 266, 11826–11832 [PubMed] [Google Scholar]