Background: Amphetamine affects Caenorhabditis elegans behavior by acting on dopamine transporter (DAT) however residual effects are present in DAT knockouts.
Results: DAT-independent behaviors are eliminated by knocking out the LGC-55 channels.
Conclusion: Amphetamine mediates behavioral effects by acting on both DAT and LGC-55.
Significance: The identification of a novel amphetamine target in C. elegans suggests alternative mechanisms underlying the effects of this psychostimulant.
Keywords: C. elegans, Dopamine Transporters, Electrophysiology, Ion Channels, Membrane Proteins, Amphetamine
Abstract
Amphetamine is a highly addictive psychostimulant, which is thought to generate its effects by promoting release of dopamine through reverse activation of dopamine transporters. However, some amphetamine-mediated behaviors persist in dopamine transporter knock-out animals, suggesting the existence of alternative amphetamine targets. Here we demonstrate the identification of a novel amphetamine target by showing that in Caenorhabditis elegans, a large fraction of the behavioral effects of amphetamine is mediated through activation of the amine-gated chloride channel, LGC-55. These findings bring to light alternative pathways engaged by amphetamine, and urge rethinking of the molecular mechanisms underlying the effects of this highly-addictive psychostimulant.
Introduction
Amphetamine (Amph),2 while being a widely abused psychostimulant drug, is also the most effective medication for the treatment of attention deficit hyperactive disorder (ADHD). However, despite its therapeutic importance and dangers as a recreational drug, the mechanism of action of Amph is still not fully understood. It is thought that Amph and other psychostimulants mediate their behavioral effects through elevation of dopamine (DA) concentrations in the synaptic cleft (1–6). One of the ways Amph increases DA concentration at the synapse is by competing for DA reuptake with the DA transporter (7–9). In addition, several in vitro and in vivo studies have shown that Amph allows the reverse transport of DA through the DA transporter (DAT), thereby causing vesicle-independent DA release into the synaptic cleft (6, 10). Mice lacking the DAT are hyperactive, yet Amph can paradoxically inhibit this hyperactivity (11) and still generate reward responses and increased levels of extracellular DA in the nucleus accumbens (12, 13). These findings suggest that additional targets other than the DAT facilitate these Amph-mediated behavioral effects. Furthermore, acute pharmacological inhibition of DA synthesis in animals lacking the DAT induces transient behavioral phenotypes manifested as severe akinesia and rigidity (14). Surprisingly, these behavioral abnormalities can be reversed by administration of Amph derivatives further supporting that Amph can act physiologically independent of DA and the DAT.
Numerous studies have shown that pharmacological and genetic ablation of the α1-adrenergic receptors hamper Amph-induced locomotor hyperactivity (2, 15–17), suggesting that α1-adrenergic receptors may be a target of Amph. Recently, in vivo and in vitro studies showed that mice overexpressing the metabotropic trace amine-associated receptor type I are hyposensitive to Amph (18), and in vitro studies showed these receptors are directly activated by Amph (19). Taken together, these data suggest that in addition to their interaction with the DAT, Amph produces a number of its behavioral effects through multiple, poorly defined mechanisms.
Here, we show that Amph activates the recently discovered ligand-gated chloride channel LGC-55 to generate behavioral effects in the nematode, Caenorhabditis elegans. Our work identifies a novel target of the psychostimulant amines in C. elegans and urges consideration of the existence of similar channels in mammals.
EXPERIMENTAL PROCEDURES
C. elegans Strains and Growth
Nematodes were grown at 18 °C according to standard protocols (20), except that enriched peptone plates seeded with Escherichia coli strain NA22 were used to grow animals. The WT strain (Bristol N2), the KOs dat-1(ok157)III, lgc-55(n4331)V, lgc-53(n4330)X, ser-2(pk357)X, ser-3(ok1995)I, ser-4(ok512)III, tyra-3(ok325)X, and mod-1(ok103)V animals were obtained from the C. elegans Genetics Center (CGC) at the University of Minnesota, Minneapolis (MN). The dat-1;lgc-55 double KOs were created by crossing the lgc-55(n4331)V with the BY326 strain. The BY326 animals are dat-1 KOs expressing the GFP transcriptional fusion (Pdat-1::GFP), and were kindly donated by Dr. R. Blakely from Vanderbilt University, Nashville, TN. Double deletion of the dat-1;lgc-55 mutants was verified by single and multiple worm PCR.
Behavioral Assays
Both SWIP and head immobilization assays were performed at room temperature (22–24 °C). Statistical analyses were performed with Prism software-5 (GraphPad Software, Inc., San Diego, CA) using one-way ANOVA Bonferroni post-test, otherwise indicated. Data were expressed as mean ± S.E. In each SWIP trial, 8 to 16 age synchronized larva-4 animals were placed in 40 μl of vehicle (200 mm sucrose) with or without Amph (NIDA, Research Triangle Institute) or tyramine (Sigma-Aldrich) in a single well of a Pyrex spot plate (Thermo Fisher Scientific, Waltham, MA). 200 mm sucrose was used as vehicle solution instead of water (21, 22) because of the discrepancy of data obtained by changing type of water. Experiments including tyramine (Sigma) were performed by including 500 μm of ascorbic acid to prevent tyramine oxidation. The same concentration of ascorbic acid was included in the relative controls. Paralyzed animals were counted every minute using an inverted microscope (Carl Zeiss, Inc., Thornwood, NY). The number of paralyzed animals was reported as a percentage of the total number of animals observed in each test ± S.E. As the dat-1 and the dat-1;lgc-55 KO animals showed basal SWIP we calculated the ΔSWIP values as the number of paralyzed animals upon Amph treatment after 10 min minus the number of paralyzed animals upon vehicle treatment. No other mutant tested showed basal SWIP. At least 100 animals were tested per group in at least 5 independent trials.
Head immobilization assessments were performed on young adults (24 h post-larva 4). Individual animals were transferred into a 60 × 15 mm agar plate supplemented with each drug and 2 mm of glacial acetic acid or 2 mm glacial acetic acid alone. Drug containing plates were prepared by autoclaving 1.7% agar in water, cooling to about 55 °C and adding glacial acetic acid and 30 mm of tyramine or Amph. Animals were observed under a Zeiss stereoscope and scored each minute for 10 min as previous reports showed that lgc-55-mediated head immobilization reached saturation within 5 min (23). Head immobilization was defined as lack of sustained lateral swings of the head (anterior to the posterior pharyngeal bulb).
Oocyte Expression and Electrophysiology
The LGC-55 cDNA cloned in pSGEM vector was a gift of Prof. R. Horvitz, Drs. N. Ringstad and M. Alkema. Complementary RNAs (cRNA) were synthesized using T7 mMESSAGE mMACHINE kit (Ambion). cRNA was then purified and run on denaturating agarose gels for size and integrity verification. cRNA quantification was performed spectroscopically. Stage V-VI oocytes were selected among multi-staged oocytes dissected by 2-hour collagenase (Sigma) treatment (2 mg/ml in Ca2+-free OR2 solution) from Xenopus laevis ovaries. Oocytes were injected with 50 ng/oocyte of cRNA and incubated in OR2 medium, which consists of 82.5 mm NaCl, 2.5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 1 mm Na2HPO4, 0.5 g/liter polyvinyl pyrolidone, and 5 mm HEPES (pH 7.2), supplemented with 0.1 mg/ml penicillin and streptomycin (Invitrogen) and 2 mm Na-pyruvate at 20 °C for 2–3 days before recordings. Currents were measured using a two-electrode voltage-clamp amplifier (GeneClamp 500B; Axon Instruments) and reported as micro ampere (μA) ± S.E. Electrodes (0.2–0.5 MΩ) were filled with 3 m KCl, and oocytes were perfused with a solution containing (in mm) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.2. For current-voltage relationship (I-V) analyses, 500 ms long voltage steps in 20 mV increments were applied from −160 to 100 mV. The holding potential was −30 mV. Reversal potentials were calculated by determining the x intercept of the linear regression line of each I-V curve. In dose-response experiments, currents were normalized to the mean maximum current measured for each drug. Ion selectivity was determined by substituting 96 mm NaCl with 96 mm Na-gluconate or NMDG-Cl. We used the pCLAMP10.3 suite of programs (Axon Instruments) and Origin 8.5 for data acquisition and analysis. Data were acquired and filtered at 1 and 0.2 kHz. Data were collected from 106 oocytes isolated from 4 different frogs. Amph I-V data plots were subtracted by basal currents (no Amph).
RESULTS
Amphetamine Induces Behavioral Effects in Dopamine Transporter Knock-out Animals
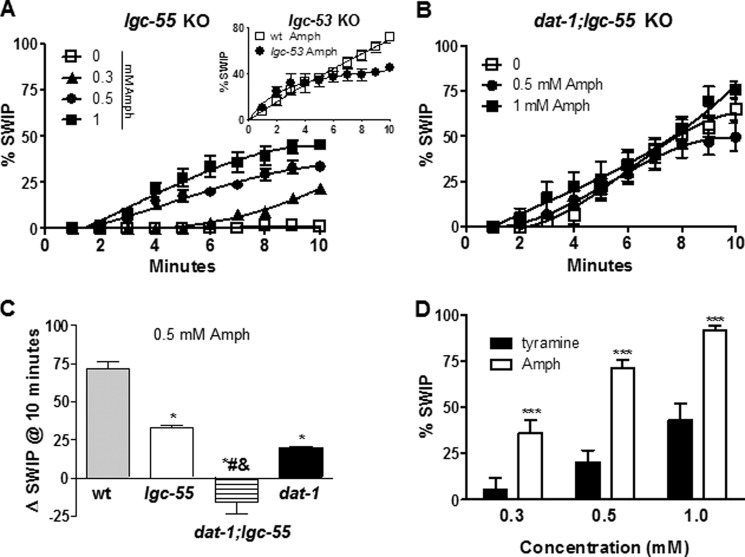
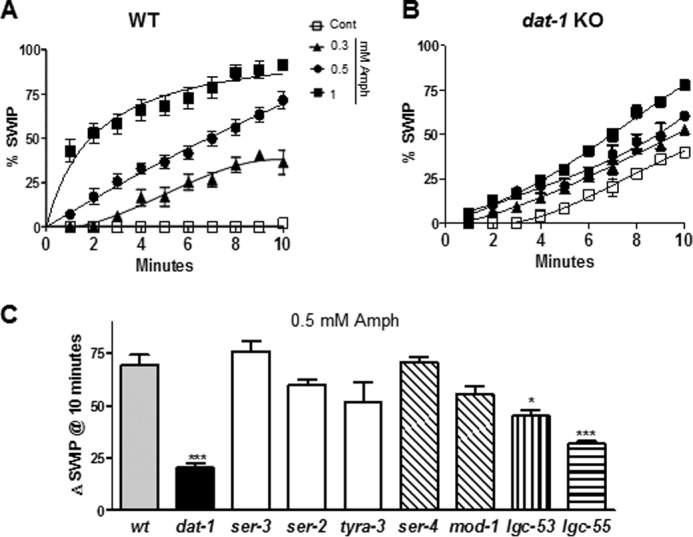
Previously, we reported that Amph-induced DA release through the DAT inhibits C. elegans swimming (21). We named this behavior swimming-induced paralysis (SWIP) (22). WT animals treated for 4 min with 0.1 mm Amph exhibited SWIP, whereas DAT KO animals (dat-1) showed a basal level of SWIP, which could not be further enhanced by exposure to 0.1 mm Amph (21). In this study, we examined the SWIP phenotype using higher (0.3–1 mm) but still physiologically relevant concentrations of Amph (24) for longer treatment periods (10 min). As seen at lower Amph concentrations (21), high concentrations of Amph increased SWIP as a function of exposure time (filled symbols in Fig. 1A). Specifically, 0.3, 0.5, and 1 mm Amph generated 36, 72 and 92% SWIP, respectively after 10 min treatment. All Amph concentrations tested were statistically different with respect to control-treated animals (p < 0.001, two-way ANOVA; n = 456). Interestingly, significant increases in SWIP were also seen in dat-1 KO animals treated with 0.5 and 1 mm Amph (● and ■ in Fig. 1B). These effects were more pronounced after extensive periods of treatment. For instance, 0, 0.3, 0.5, and 1 mm Amph generated 40, 52, 61, and 78% SWIP, respectively after 10 min treatment. Therefore, 0.5 (●) and 1 mm (■) Amph caused a significant increase in SWIP (21 ± 2% and 38 ± 1%, respectively) over dat-1 KO animals treated with vehicle (□; p ≤ 0.001, two-way ANOVA Bonferroni post-test; n = 503). These results reveal that Amph acts on targets other than the DAT to generate SWIP in the dat-1 KO genetic background.
FIGURE 1.
Amphetamine induces SWIP in DAT KO animals by recruiting amine-gated channels. A, in wt animals all Amph concentrations tested (filled symbols) generated statistically different SWIP with respect to control-treated animals (□). B, dat-1 KO animals significantly responded to 0.5 (●) and 1 mm (■) Amph with respect to vehicle-containing solution (□). C, bars indicate the ΔSWIP values calculated as the number of paralyzed animals upon 0.5 mm Amph treatment after 10 min minus the number of paralyzed animals upon vehicle treatment. Only dat-1, lgc-53, and lgc-55 KOs showed significant reduction in SWIP with respect to wt animals. *, p ≤ 0.05 and ***, p ≤ 0.0001 both compared with wt animals.
Amphetamine Engages Ligand-gated Ion Channels to Generate SWIP
Previously, in vitro studies showed that Amph activates the trace amine-associated receptors (19). To test whether these receptors were recruited by Amph in our behavioral paradigm, we measured SWIP in animals lacking expression of ser-2, ser-3, or tyra-3, which encode homologues of octopamine and tyramine/octopamine and tyramine receptors, respectively (25, 26). After 10 min, Amph-induced SWIP measured in ser-3, ser-2, and tyra-3 KOs (Fig. 1C, white bars) were not statistically different than those measured in WT animals (Fig. 1C, gray bar), suggesting that these trace amine receptors are not recruited by Amph to cause SWIP. In C. elegans, serotonin signaling inhibits locomotion on solid plates (27). Thus, we investigated whether the metabotropic SER-4 and ionotropic MOD-1 serotonin receptors were involved in Amph-induced SWIP. As shown in Fig. 1C, Amph-induced SWIP was not affected by lack of expression of SER-4 or MOD-1 (oblique line bars). In fact, Amph-induced SWIP measured in SER-4 and MOD-1 KO animals (ser-4 and mod-1) were not statistically different than those measured in WT animals. These data demonstrate that these serotonin receptors are not involved in Amph-induced SWIP.
Recently, the two novel amine-activated receptors, LGC-53 and LGC-55 have been identified in C. elegans (23, 28). These receptors function as ligand-gated ion channels activated by DA (Km = 4.4 μm) and tyramine (Km = 6 μm), respectively. Since the molecular structures of tyramine, DA, and Amph are similar we tested whether Amph-induced SWIP was dependent on LGC-53 and/or LGC-55. As shown in Fig. 1C (vertical line bar), lgc-53 KO (lgc-53) animals showed a 30% reduction in SWIP with respect to WT animals after 10 min of Amph treatment (*, p ≤ 0.05; n = 100). Interestingly, when animals lacking the LGC-55 channels (lgc-55) were tested a stronger (54%) and more significant reduction (***, p ≤ 0.0001, n = 114) in SWIP with respect to WT animals was observed (horizontal bar, Fig. 1C). A further investigation of the lgc-55 KO animals showed that after 2 to 10 min treatment with all Amph concentrations tested (0.3–1 mm), SWIP was significantly reduced (p ≤ 0.0001, two-way ANOVA Bonferroni post-test; n = 425) with respect to WT animals (compare Figs. 2A and 1A). For example, 17 ± 3% and 33 ± 2% lgc-55 KO animals exhibited SWIP after 5 and 10 min of 0.5 mm Amph treatment, whereas at the same time points, WT animals showed 37 ± 3% and 72 ± 4% SWIP, respectively (compare Figs. 2A and 1A). On the other hand, the lgc-53 KOs (●) did not show any difference in Amph-induced SWIP with respect to WT animals (□) after 5 min, 34 ± 5% and 37 ± 3%, respectively (inset, Fig. 2A). Only after 10 min of 0.5 mm Amph treatment the lgc-53 KOs showed a significant 30% reduction in SWIP with respect to WT, 45 ± 3% and 72 ± 4%, respectively (inset Fig. 2A). Because the LGC-55 played a more prominent role in Amph-induced SWIP we focused our studies on the LGC-55 channels.
FIGURE 2.
Amph-induced SWIP depends on both DAT and LGC-55 channels. A, Amph treatments generated reduced SWIP in lgc-55 KOs animals (n = 405) with respect to wt animals (Fig. 1A). Inset, lgc-53 KOs (●) exhibited a significant decrease in SWIP with respect to wt animals (□) only after 8–10 min treatment with 0.5 mm Amph (n = 256). B, in dat-1;lgc-55 double KOs Amph treatments did not generate statistically different SWIP with respect to vehicle treatment (n = 326). C, ΔSWIP calculated after 10 min of 0.5 mm Amph treatment was significantly reduced in lgc-55, dat-1, and dat-1;lgc-55 KOs with respect to wt animals. ΔSWIP was further reduced in dat-1;lgc-55 KOs with respect to dat-1 or lgc-55 single KOs. D, after 10 min, Amph generated SWIP values statistically significant higher than tyramine at all concentrations tested (***, p ≤ 0.001).
Our results suggest that Amph-induced SWIP mainly depends on the DAT (Fig. 1B) and the LGC-55 channel (Fig. 2A). If the DAT and the LGC-55 are independent and parallel targets of Amph, then the effect of each of these targets should be additive. Thus animals lacking both the DAT and the LGC-55 channels should not respond to Amph. To investigate this hypothesis, we generated dat-1;lgc-55 double KO animals and tested them for SWIP behavior. When treated with vehicle for 10 min, the dat-1;lgc-55 double KOs exhibited higher basal SWIP with respect to dat-1 KO animals, 63 ± 5% (n = 102) and 40 ± 2% (n = 137), respectively (compare □ in Figs. 2B and 1B), even though the lgc-55 KO animals did not show basal SWIP. Currently, we do not know the mechanism underling the increase of SWIP in the dat-1;lgc-55 double KOs. Notably though, when treated with 0.5 and 1 mm Amph the dat-1;lgc-55 double KOs did not exhibit further SWIP (● and ■ Fig. 2B). Thus, as summarized in Fig. 2C, the ΔSWIP measured after 10 min of Amph treatments in the lgc-55 (33 ± 5%, n = 100), dat-1 (20 ± 1%, n = 137), or dat-1;lgc-55 (−15 ± 20%, n = 100) KOs were substantially lower than those measured in WT animals (72 ± 12%, *, p ≤ 0.0001; n = 156). Additionally, the ΔSWIP in the dat-1;lgc-55 double KOs was further reduced as compared with the ΔSWIP measured in each single KO (# and & p ≤ 0.0001). Based on these results, we conclude that Amph engages both the DAT and the amine-gated channels, LGC-55 to cause SWIP in C. elegans.
The main endogenous ligand of the LGC-55 is tyramine (23, 28). Thus, we tested animals for SWIP behavior upon treatment with exogenous tyramine. Similarly to Amph, tyramine-induced SWIP increased in a dose-dependent manner (black bars, Fig. 2D). However, after 10 min of 0.3, 0.5, and 1 mm tyramine treatment only 5 ± 6%, 20 ± 6%, and 43 ± 9% animals (n = 300) were paralyzed respectively, in contrast to Amph (n = 356) where 10 min treatment resulted in a significant increase in SWIP (36 ± 7%, 72 ± 4%, and 92 ± 3%, respectively). This result demonstrates that tyramine is less potent than Amph in generating SWIP likely because tyramine engages only the LGC-55 whereas Amph engages both the LGC-55 and DAT. Another possible explanation is that since tyramine is more charged than Amph it may simply permeate the worm cuticle less efficiently than Amph, and thus exhibits reduced apparent potency.
Amphetamine Activates the LGC-55 Channels
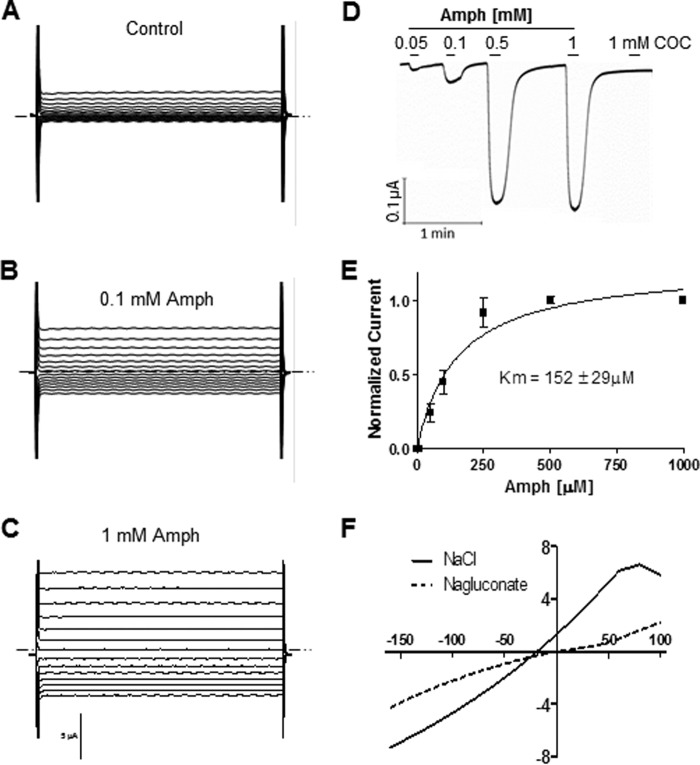
Our data suggest that the effect of Amph on swimming behavior is partly mediated by the LGC-55 channels. One possibility is that Amph directly activates these channels. To test this hypothesis, we performed two-electrode voltage-clamp (TEVC) experiments in Xenopus laevis oocytes expressing the LGC-55. In lgc-55 injected oocytes, Amph perfusion evoked reproducible currents when the oocyte membrane potential was stepped from a holding membrane potential of −30 mV to various test potentials between −160 to 100 mV (Fig. 3, A–C). No current was observed in mock injected oocytes (n = 7). When the membrane potential was clamped at −60 mV, concentration of Amph ranging from 0.05 to 1 mm generated inward currents that reached saturation at 0.5 mm Amph (Fig. 3, D and E). Cocaine, a psychostimulant and blocker of the DAT, failed to elicit any response (n = 5) at concentrations ranging from 0.1 to 1 mm (Fig. 3D), suggesting that the LGC-55 channels are specifically activated by phenylethylamine compounds. At −60 mV, the dose-response curve of Amph-induced currents was well fitted by a Michael-Menten equation with a Km of 152 ± 29 μm (Fig. 3E). This value was higher than the Km = 4.1 ± 1 we calculated for tyramine-induced currents (Fig. 4A; (23, 28)). Moreover, saturating concentrations (250 μm) of tyramine and Amph generated statistically different currents (**, p ≤ 0.001; t test, n = 6) of 3 ± 0.5 and 0.4 ± 0.09 μA, respectively (Fig. 4B). Thus, these data demonstrate that Amph activates the LGC-55 channels with lower affinity and generates smaller currents than tyramine.
FIGURE 3.
Amph activates LGC-55 channels. Representative composite currents recorded in LGC-55 injected oocytes perfused with control solution (A), 0.1 mm (B), and 1 Mm (C) Amph. Currents were stimulated by voltage steps from −160 to + 100 mV in 20-mV increments. D, representative LGC-55 mediated currents generated by Amph and cocaine at −60 mV membrane potential. E, dose-response curve of Amph-induced currents (n = 12). Data points recorded at −60 mV were normalized to the maximal values and fitted to a Michaelis-Menten equation. F, representative current-voltage curve generated by 1 mm Amph after subtraction of basal currents (0 mm Amph) in the presence of 96 (solid line) and 8 (dashed line) mm external chloride.
FIGURE 4.
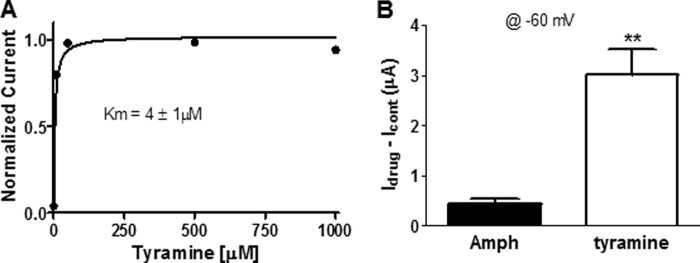
Amph activates smaller LGC-55 currents than tyramine. A, dose-response curve of tyramine-induced currents (n = 4). Data points recorded at −60 mV were normalized to the maximal values and fitted to a Michaelis-Menten equation. B, averaged currents generated by 250 μm of Amph or tyramine when the membrane potential was held at −60 mV.
Since LGC-55 are chloride (Cl−) channels (23, 28), we investigated whether Amph-induced currents were likewise Cl− dependent by analyzing current-voltage relationship under three different conditions. In our standard recording conditions (104 mm extracellular Cl−), currents generated by Amph reversed at −18 ± 3 mV (n = 12, solid line in Fig. 3F). When we substituted the extracellular NaCl with Na-gluconate (96 mm) the Amph-induced currents reversed at −6 ± 4mV (n = 5, dashed line in Fig. 3F). Thus, reducing the extracellular Cl− concentration from 104 to 8 mm produced a significant 12 mV shift of the reversal potential toward more positive values (p ≤ 0.001, t test). On the other hand, complete substitution of extracellular sodium with the large impermeant cation N-methyl-d-glucamine (NMDG) had no significant effect in the reversal potential (−18 ± 3 and −21 ± 5 mV, respectively), suggesting that anions rather than cations are permeable through the LGC-55 channels following Amph activation. Taken together, these results demonstrate that Amph generates Cl− currents by direct activation of the LGC-55 channels. Furthermore, they show that Amph (Fig. 3E) induces maximal activation of the LGC-55 channels within the same range concentrations that induces SWIP in vivo (0.3–1 mm). Thus, we suggest that LGC-55-dependent SWIP is caused by direct activation of these channels.
Amphetamine Mimics the Neurotransmitter Tyramine in C. elegans Behavior
Previous reports showed that LGC-55 has an important biological function in C. elegans (23). When activated by tyramine, the LGC-55 channels hyperpolarize head neurons and muscles causing suppression of head movements. To test whether Amph, like tyramine, inhibited head movement through the LGC-55, we measured the percentage of animals showing head immobilization in WT and lgc-55 KO animals treated with 30 mm tyramine or Amph for 5 min (Fig. 5). Similarly to tyramine (23) Amph caused head immobilization in 100% WT animals (Fig. 5A). Importantly, in the lgc-55 KOs Amph caused head immobilization only in 25% ± 13 animals, and tyramine completely failed to block head movements (Fig. 5B). Last, we measured head immobilization in the dat-1;lgc-55 double KOs, and we found that Amph induced head immobilization only in 15 ± 10% dat-1;lgc-55 KOs and tyramine completely failed to block head movements (Fig. 5C). These results demonstrate that the synthetic phenylethylamine Amph, like the physiological tyramine, activates the LGC-55 channels to inhibit head movements in C. elegans. Moreover, these results confirm previous data establishing that tyramine acts exclusively on the LGC-55 to inhibit head movements (23), and suggest that Amph in part acts on other targets as we measured a residual head immobilization both in lgc-55 and dat-1;lgc-55 KO animals treated with Amph. Note that the concentrations of Amph and tyramine used in this assay are higher than those used in SWIP assay. One possible explanation is that drugs are absorbed more readily through the mouth when animals are treated in water than through the cuticle when animals are treated on agar plates.
FIGURE 5.
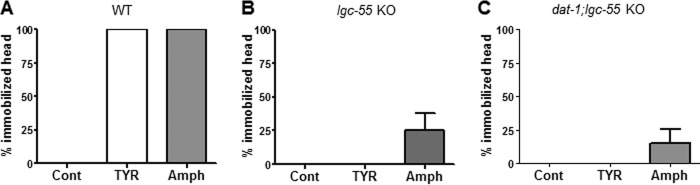
Amph causes head immobilization in a LGC-55-dependent manner. A, Wt (A), lgc-55 (B), and dat-1;lgc-55 (C) animals did not show head immobilization when tested in control plates (n = 18, 20, and 26, respectively). A, 30 mm of tyramine or Amph induced head immobilization in 100% wt animals (n = 20 and 38, respectively) after 5 min treatment. B, 30 mm of tyramine or Amph induced head immobilization in 0% (n = 20) and 25% lgc-55 KO (n = 35) animals, respectively. C, 30 mm of tyramine or Amph induced head immobilization in 0% (n = 28) and 15% dat-1;lgc-55 KO (n = 33) animals, respectively.
DISCUSSION
Amph is a synthetic psychostimulant which is believed to cause its behavioral effects by increasing extracellular DA content. In vitro and in vivo studies have demonstrated that Amph competes for reuptake of DA and promotes reverse transport of DA through DAT into the synapse (6). Recent behavioral studies have also shown that mice overexpressing DAT exhibit a 3-fold increase in sensitivity to Amph with respect WT animals (29). These data unequivocally demonstrate the important role played by DAT in Amph actions. However, in mice lacking the DAT as well as the ability to synthesize DA, Amph still produces behavioral effects (12, 14). While, compensatory changes in DAT KOs likely account for these phenomena (30), an alternative explanation for this finding is that mechanisms involving targets and molecules other than the DAT and DA itself are responsible for some of the effects generated by Amph. In fact, a number of studies showed that the α1-adrenergic and trace amine-associated receptors are also activated by Amph to generate behavioral effects suggesting that Amph acts on multiple targets. Here, we used C. elegans as a model system to search for alternative Amph targets both at the molecular and behavioral level.
Previous studies support that genes that are involved in dopaminergic transmission are highly conserved from worm to man (31, 32). These include genes that encode biosynthetic enzymes, transporter systems, and receptors. Also, previous reports showed that DA and Amph inhibit C. elegans locomotor activity (21, 27). While this behavior may seem in contrast to what occurs in humans, this is likely the consequence of the specific behavioral output for which the C. elegans dopaminergic system is needed. Indeed, release of DA is associated with food encounter and is required to slow down the worm to facilitate feeding (27).
The in vivo results presented in this study (Figs. 1 and 2) together with our previously published data (21) show that Amph engages both the LGC-55 channels and the DAT to generate locomotor defects in C. elegans. Also, our in vitro data demonstrated that Amph 1) increases the extracellular DA levels through the DAT, as assayed by amperometric DA currents (21), and 2) activates the LGC-55 channels as measured by TEVC (Fig. 3). These data suggest that two mechanisms, DA efflux and LGC-55 activity, underlie the behavioral effects caused by Amph. This conclusion was supported by our data showing that whereas the single dat-1 or lgc-55 KO animals respond to Amph albeit to lesser degree, the dat-1;lgc-55 double KO animals do not respond at all to Amph treatments (Fig. 2, B and C).
Previously, Pirri et al. demonstrated that in C. elegans the endogenous amine tyramine causes head immobilization through activation of the LGC-55 channels (23). Our data show that Amph similarly to tyramine caused head immobilization in a LGC-55 dependent manner (Fig. 5). These data further support that Amph acts on LGC-55 channels to mediate behaviors in C. elegans. Importantly, despite the higher affinity of LGC-55 channels for tyramine (Km = 4 μm; Fig. 4A) as compared with Amph (Km = 158 μm; Fig. 3E), and the larger currents generated by tyramine (Fig. 4B), Amph induces SWIP in a higher percentage of animals than tyramine (Fig. 2D). These results further underscore that Amph acts on both the LGC-55 and DAT to induce SWIP.
Ringstad et al. showed that the LGC-53 channels are specifically activated by DA (28). In fact, oocytes injected with lgc-53 generated Cl− currents when perfused with 10 μm DA, whereas 10 μm tyramine produce no effect. Our data show that the lgc-53 KOs exhibited 30% reduction in Amph-induced SWIP with respect to WT only after prolonged treatments (10 min), but no difference was observed up to 7 min treatments (inset, Fig. 2A). We speculate here that the tardive and reduced effect of Amph seen in the lgc-53 KOs is due to the increase of extracellular DA released through DAT, which subsequently activates the LGC-53 channels. We understand that at this point this interpretation is pure speculation. However, our explanation is in part supported by the fact that genetic depletion of DAT and LGC-55 is enough to eliminate Amph-induced SWIP in the dat-1;lgc-55 double KOs (Fig. 2B), suggesting that the LGC-53 are not directly activated by Amph.
The ligand-gated ion channels are known to mediate fast chemical to electrical transduction throughout the nervous system. Our electrophysiology data showed that the LGC-55 channels generated Cl− currents when activated by Amph (Fig. 3). Because the Cl− equilibrium potential is usually close to or more negative than the cell resting potential, we can speculate that Amph causes membrane hyperpolarization resulting in an inhibitory effect in the LGC-55-expressing cells. This idea suggests that the LGC-55 channels have an important role in regulating neuronal transmission. Indeed, their activation correlates with a behavioral response in C. elegans (Figs. 2 and 5). We now plan to initiate cell-specific rescue experiments to identify, which cells expressing LGC-55 are responsible for the Amph-induced SWIP phenotype or how their hyperpolarization mediates these effects.
In conclusion, we have identified new targets of the psychostimulant Amph. Specifically, we showed that the effects of Amph on C. elegans swimming induced paralysis (SWIP) are in part mediated by the newly identified amine-gated channels LGC-55. We further showed that Amph directly activates LGC-55 channels expressed in Xenopus oocytes and that Amph mimics the natural neurotransmitter tyramine in mediating LGC-55-dependent behaviors. Thus Amph, besides inducing DA release through the DAT as shown both in mammals and C. elegans, also activates the LGC-55 channels in C. elegans. Both pathways, when activated, generate a behavioral phenotype in C. elegans (SWIP). Moreover, we demonstrate that C. elegans SWIP is a unique behavioral paradigm to search for Amph targets. In fact, using the genetic tools offered by C. elegans we were able to perform behavioral screens in multiple mutants. This same approach would have been extremely costly and time-consuming if done in mammalian mutants.
Our data reveal that Amph activation of LGC-55 channels is within the same range of concentrations relevant in drug addiction but are orders of magnitude higher than what would be expected in an ADHD patient taking therapeutic Amph (5–10 μm). Indeed, Amph Km for LGC-55, as measured in Xenopus oocytes, is 152 μm and is consistent with the concentrations of Amph found in the brain of tolerant addicts (hundreds μm, (24)). Thus, if LGC-55 homologues exist in humans, our data may have an impact in the process of designing new therapies to treat addiction to Amph. Interestingly, a preliminary screen through the human protein database has revealed the existence of 4 orphan proteins sharing 30–45% identity with the LGC-55 at the amino acid level. Whether these receptors are activated by Amph needs to be established by future studies. If one or more of these receptors are found to be activated by Amph, they could provide a novel therapeutic target with great promise given the many links between aminergic signaling, mental disorders and addiction.
Acknowledgments
We thank Robert Horvitz, Niels Ringstad, and Mark Alkema for the LGC-55 cDNA and Randy Blakely for the BY326 animals. We also thank Ying Wang for technical support, and Keith Henry, Peter Larsson, and Roxanne Vaughan for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R21 DA024797 (to L. C.) and Grants RGS-09-043-01-DDC5 and R01NS070969 (to L. B.).
- Amph
- amphetamine
- DA
- dopamine
- DAT
- dopamine transporter
- SWIP
- swimming-induced paralysis.
REFERENCES
- 1. Amara S. G., Sonders M. S. (1998) Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol. Depend. 51, 87–96 [DOI] [PubMed] [Google Scholar]
- 2. Darracq L., Blanc G., Glowinski J., Tassin J. P. (1998) Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J. Neurosci. 18, 2728–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zahniser N. R., Larson G. A., Gerhardt G. A. (1999) In Vivo Dopamine Clearance Rate in Rat Striatum: Regulation by Extracellular Dopamine Concentration and Dopamine Transporter Inhibitors. J. Pharmacol. Exp. Ther. 289, 266–277 [PubMed] [Google Scholar]
- 4. Budygin E. A., Kilpatrick M. R., Gainetdinov R. R., Wightman R. M. (2000) Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci. Lett. 281, 9–12 [DOI] [PubMed] [Google Scholar]
- 5. Sabeti J., Gerhardt G. A., Zahniser N. R. (2003) Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J. Pharmacol. Exp. Ther. 305, 180–190 [DOI] [PubMed] [Google Scholar]
- 6. Sulzer D., Sonders M. S., Poulsen N. W., Galli A. (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 75, 406–433 [DOI] [PubMed] [Google Scholar]
- 7. Ritz M. C., Lamb R. J., Goldberg S. R., Kuhar M. J. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 8. Ritz M. C., Lamb R. J., Goldberg S. R., Kuhar M. J. (1988) Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog. Neuropsychopharmacol Biol. Psychiatry. 12, 233–239 [DOI] [PubMed] [Google Scholar]
- 9. Zaczek R., Culp S., De Souza E. B. (1991) Interactions of [3H]amphetamine with rat brain synaptosomes. II. Active transport. J. Pharmacol. Exp. Ther. 257, 830–835 [PubMed] [Google Scholar]
- 10. Fog J. U., Khoshbouei H., Holy M., Owens W. A., Vaegter C. B., Sen N., Nikandrova Y., Bowton E., McMahon D. G., Colbran R. J., Daws L. C., Sitte H. H., Javitch J. A., Galli A., Gether U. (2006) Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429 [DOI] [PubMed] [Google Scholar]
- 11. Gainetdinov R. R., Wetsel W. C., Jones S. R., Levin E. D., Jaber M., Caron M. G. (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283, 397–401 [DOI] [PubMed] [Google Scholar]
- 12. Carboni E., Spielewoy C., Vacca C., Nosten-Bertrand M., Giros B., Di Chiara G. (2001) Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J. Neurosci. 21, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Budygin E. A., Brodie M. S., Sotnikova T. D., Mateo Y., John C. E., Cyr M., Gainetdinov R. R., Jones S. R. (2004) Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc. Natl. Acad. Sci. 101, 7781–7786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sotnikova T. D., Beaulieu J. M., Barak L. S., Wetsel W. C., Caron M. G., Gainetdinov R. R. (2005) Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drouin C., Darracq L., Trovero F., Blanc G., Glowinski J., Cotecchia S., Tassin J. P. (2002) Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J. Neurosci. 22, 2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickinson S. L., Gadie B., Tulloch I. F. (1988) α1- and α2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology 96, 521–527 [DOI] [PubMed] [Google Scholar]
- 17. Blanc G., Trovero F., Vezina P., Hervé D., Godeheu A. M., Glowinski J., Tassin J. P. (1994) Blockade of prefronto-cortical α1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical d-amphetamine injection. Eur. J. Neurosci. 6, 293–298 [DOI] [PubMed] [Google Scholar]
- 18. Revel F. G., Meyer C. A., Bradaia A., Jeanneau K., Calcagno E., André C. B., Haenggi M., Miss M. T., Galley G., Norcross R. D., Invernizzi R. W., Wettstein J. G., Moreau J. L., Hoener M. C. (2012) Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology 37, 2580–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bunzow J. R., Sonders M. S., Arttamangkul S., Harrison L. M., Zhang G., Quigley D. I., Darland T., Suchland K. L., Pasumamula S., Kennedy J. L., Olson S. B., Magenis R. E., Amara S. G., Grandy D. K. (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic Acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 60, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 20. Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carvelli L., Matthies D. S., Galli A. (2010) Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol. Pharmacol. 78, 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDonald P. W., Hardie S. L., Jessen T. N., Carvelli L., Matthies D. S., Blakely R. D. (2007) Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J. Neurosci. 27, 14216–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pirri J. K., McPherson A. D., Donnelly J. L., Francis M. M., Alkema M. J. (2009) A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron 62, 526–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tallóczy Z., Martinez J., Joset D., Ray Y., Gácser A., Toussi S., Mizushima N., Nosanchuk J. D., Goldstein H., Loike J., Sulzer D., Santambrogio L. (2008) Methamphetamine inhibits antigen processing, presentation and phagocytosis. PLoS Pathog. 4, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chase D. L., Koelle M. R. (2007) Biogenic amine neurotransmitters in C. elegans. WormBook 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsalik E. L., Niacaris T., Wenick A. S., Pau K., Avery L., Hobert O. (2003) LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263, 81–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sawin E. R., Ranganathan R., Horvitz H. R. (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 [DOI] [PubMed] [Google Scholar]
- 28. Ringstad N., Abe N., Horvitz H. R. (2009) Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science 325, 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salahpour A., Ramsey A. J., Medvedev I. O., Kile B., Sotnikova T. D., Holmstrand E., Ghisi V., Nicholls P. J., Wong L., Murphy K., Sesack S. R., Wightman R. M., Gainetdinov R. R., Caron M. G. (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc. Natl. Acad. Sci. U.S.A. 105, 4405–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou F. C., Lesch K. P., Murphy D. L. (2002) Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 942, 109–119 [DOI] [PubMed] [Google Scholar]
- 31. McDonald P. W., Jessen T., Field J. R., Blakely R. D. (2006) Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol. Neurobiol. 26, 593–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schafer W. R. (2004) Addiction research in a simple animal model: the nematode Caenorhabditis elegans. Neuropharmacology 47, 123–131 [DOI] [PubMed] [Google Scholar]