Background: The ability to distinguish similar experiences is a critical aspect of memory.
Results: Contextual discrimination involves LTP promoted by calcium-permeable AMPA-type glutamate receptors, RAS-GRF1 and p38 MAP kinase.
Conclusion: A newly discovered, LTP-supporting, signaling pathway contributes to a key component of memory formation.
Significance: Defects in this signaling pathway may contribute to cognitive loss associated with neurological disorders.
Keywords: Glutamate Receptors Ionotropic (AMPA, NMDA); Neurobiology; Neurons; p38 MAPK; RAS; Signal Transduction; Synaptic Plasticity; RAS-GRF; Hippocampus
Abstract
RAS-GRF1 is a guanine nucleotide exchange factor with the ability to activate RAS and RAC GTPases in response to elevated calcium levels. We previously showed that beginning at 1 month of age, RAS-GRF1 mediates NMDA-type glutamate receptor (NMDAR)-induction of long term depression in the CA1 region of the hippocampus of mice. Here we show that beginning at 2 months of age, when mice first acquire the ability to discriminate between closely related contexts, RAS-GRF1 begins to contribute to the induction of long term potentiation (LTP) in the CA1 hippocampus by mediating the action of calcium-permeable, AMPA-type glutamate receptors (CP-AMPARs). Surprisingly, LTP induction by CP-AMPARs through RAS-GRF1 occurs via activation of p38 MAP kinase rather than ERK MAP kinase, which has more frequently been linked to LTP. Moreover, contextual discrimination is blocked by knockdown of Ras-Grf1 expression specifically in the CA1 hippocampus, infusion of a p38 MAP kinase inhibitor into the CA1 hippocampus, or the injection of an inhibitor of CP-AMPARs. These findings implicate the CA1 hippocampus in the developmentally dependent capacity to distinguish closely related contexts through the appearance of a novel LTP-supporting signaling pathway.
Introduction
It is well known that the hippocampus plays a critical role in processing contextual information relating to episodic memory. Moreover, the contribution of the hippocampus to learning and memory develops late among brain regions, appearing first ∼21 days after birth in mammals (1). Among this category of functions, the hippocampus is particularly important for discriminating similar contexts, which requires processing of not only spatial but also temporal and relational information (2). In fact, clearly distinct roles for the dentate gyrus and CA3 and CA1 regions of the hippocampus in this form of learning have been identified (3). However, the biochemical underpinnings of these functions remain poorly understood. Likely candidates include signaling components that regulate synaptic plasticity in each of these hippocampus segments.
One class of signaling molecules that have been implicated in synaptic plasticity consists of the RAS-GRF1 and RAS-GRF2 guanine nucleotide exchange factors (4). These proteins have the capacity to activate RAS and RAC GTPases through their CDC25 and DH domains, respectively. Both proteins can be activated by calcium-dependent binding of calmodulin to their IQ motifs. Beginning at ∼1 month of age, RAS-GRF1 mediates NMDA receptor (NMDAR)2-induced LTD, whereas RAS-GRF2 mediates NMDAR-induced LTP in the CA3/CA1 synapse of the hippocampus (5). At this site, RAS-GRF2 mediates LTP induced specifically by NMDARs that contain GluN2A subunits (6). Consistent with their defective LTP, RAS-GRF2 knock-out mice display defective contextual fear conditioning memory.3 In contrast, RAS-GRF1 mice display more subtle fear memory defects in that contextual fear memory is weakly defective (7, 8), but contextual discrimination is completely lost (7). How RAS-GRF1 contributes to contextual discrimination is not known.
MAP kinases play integral roles in neuronal functions related to synaptic plasticity associated with learning and memory (9). ERK MAP kinase in synapses of mature animals can be activated by a variety of upstream receptors, including the Trk family of tyrosine kinase receptors (10) and both NMDA-type (11) and calcium-permeable AMPA-type glutamate receptors (11, 12). Its activation is a critical step in promoting many forms of LTP, including protein synthesis-independent early LTP induced by theta-burst stimulation that is thought to contribute to short term memory (13). It is also critical for protein-dependent late LTP and long term memory (14). In contrast, p38 MAP kinase has been most often associated with LTD, although conflicting results have been obtained (5, 15–17). However, p38 MAP kinase activity has been associated with theta-burst-induced early LTP that is associated with enhanced learning after exposure to an enriched environment (18).
In this study, we demonstrate that p38 MAP kinase activation is also involved in an age-dependent LTP mechanism, mediated by calcium-permeable AMPA receptors and RAS-GRF1, that contributes to the acquisition at ∼2 months of age of the ability of mice to distinguish closely related contexts.
EXPERIMENTAL PROCEDURES
Mice
ras-grf1−/− mice in a C57Bl6 background were described previously (5, 19). All procedures involving mice were in accordance with the animal welfare guidelines of Tufts University.
Extracellular Field Experiments
Mutant and control mice (1–6 months old) were anesthetized with halothane and decapitated. The transverse acute hippocampal slices (350 μm) were cut in ice-cold oxygenated sucrose-enhanced artificial cerebrospinal fluid (ACSF) containing 206 mm sucrose, 2 mm KCl, 2 mm MgSO4, 1.25 mm NaH2PO4, 1 mm CaCl2, 1 mm MgCl2, 26 mm NaHCO3, 10 mm d-glucose, pH 7.4, saturated with 95% O2 and 5% CO2 (pH 7.4), in which they were allowed to recover for at least 90 min before recording. Recordings were performed in the same solution at room temperature in a chamber submerged in ACSF.
To record field EPSPs (fEPSPs) in the CA1, CA3, and DG regions of the hippocampus, respectively, standard procedures were used. 1) For CA1, Schaffer collaterals were stimulated with a unipolar stimulating electrode (World Precision Instruments) placed in the lateral CA1 subfield. A borosilicate glass recording electrode filled with ACSF was positioned in the stratum radiatum of CA1. 2) For CA3, the mossy fiber CA3 pyramidal neuron responses were induced by the stimulation of mossy fiber axons with a unipolar stimulating electrode. A borosilicate glass recording electrode filled with ACSF was placed at least 500 μm from the stimulating electrodes along the trajectory of the mossy fiber pathway. d-APV (50 μm) was added in ACSF to prevent contamination with the NMDA receptor-dependent pathway converging on CA3 neurons. 3) For DG, stimulating and recording electrodes were placed in the medial aspect of the dentate molecular layer. 10 μm bicuculline methiodide was added in ACSF to prevent GABAA-mediated inhibition. Test stimuli were applied at low frequency (0.05 Hz) at a stimulus intensity that elicited an fEPSP amplitude that was 50% of maximum, and the test responses were recorded for 10 min before the experiment was begun to ensure stability of the response.
To induce LTP, we used three protocols in this study. 1) For high frequency stimulation (HFS)-induced LTP in the CA1 and CA3, two consecutive trains (1 s) of stimuli at 100 Hz separated by 20 s were applied to the CA1 and CA3 areas, respectively. 2) For theta-burst (TBS) stimulation in CA1, 15 bursts of four pulses at 100 Hz delivered at an interburst interval of 200 ms were applied to the CA1 area. 3) Four bursts of 50 pulses at 100 Hz, 30 s between bursts, were applied to the dentate gyrus. Traces were obtained by pClamp 9.2 and analyzed using the Clampfit 9.2. Data analysis was as follows. The fEPSP magnitude was measured using the initial fEPSP slope, and three consecutive slopes (1 min) were averaged and normalized to the mean value recorded 10 min before conditioning stimulus. Data were pooled across animals of the same treatment and genotype and are presented as mean ± S.E. Values expressed here represent 50-min time points after conditioning stimulus was initiated. The following statistical analysis was carried out. The same time window samples of the control and treatment were compared using paired, two-tailed Student's t test. An effect was considered significant if p < 0.05.
Hippocampal Brain Slice Preparation for Immunofluorescence
The brain was removed quickly and submerged in ice-cold oxygenated sucrose-replaced ACSF cutting solution (containing the following (in mm): 240 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 25 NaHCO3, 7 MgCl2, pH 7.4). After dissection, slices (350 μm, transverse) were incubated in ACSF that contained the following (in mm): 124 NaCl, 2.8 KCl, 1.5 MgSO4, 1.25 NaH2PO4, 2.5 CaCl2, 26 NaHCO3, 10 d-glucose saturated with 95% O2 and 5% CO2 (pH7.4), in which they were allowed to recover for at least 2 h before an experiment. Then a single slice was transferred to the recording chamber and submerged beneath a continuously perfusing the same ACSF.
A unipolar stimulating electrode was placed in the stratum radiatum, and HFS or TBS stimulation was delivered with an intensity of 140 μA. Slices were fixed in 4% paraformaldehyde 10 min after HFS stimulation overnight, which was then replaced with sucrose for 24 h, and were re-cut in a cryostat at 18 μm. The sections were blocked with 2% donkey serum in 0.3% Triton X-100 for 1 h at room temperature and incubated over two nights at 4 °C with anti-phospho-p38 antibody (anti-rabbit, 1:400; Cell Signaling). The sections were then incubated for 1 h at room temperature with Cy3-conjugated secondary antibody (1:300; Jackson ImmunoResearch Laboratories, West Grove, PA). The stained sections were examined with a Nikon (Tokyo, Japan) fluorescence microscope, and images were captured with a CCD spot camera and subsequently analyzed with ImageJ software (National Institutes of Health). For cell counting in the stratum pyramidal region of stimulated regions (200–250 μm from the location of the stimulating electrode), the three most representative and/or most complete sections from each slice were chosen, and labeled cells in a 20× optic field on each section were counted.
Viral Vectors
Ras-Grf1 microRNA adeno-associated virus was generated as follows. An AAV8 shRNA vector pFB-U6-R2-CMVmCherry expressing microRNA against GRF1 (sequence, TGCTGTTGACAGTGAGCGCGCTGATCAATATCTACAGAAATAGTGAAGCCACAGATGTATTTCTGTAGATATTGATCAGCTTGCCTACTGCCTCGAA) or a similar control random sequence, along with mCherry or GFP under the control of the U6 and CMV promoters, respectively, were generated by Virovek and were produced at working concentrations of ∼1.6 × 1013/ml. Adenovirus expressing Ras-Grf1 from the synapsin promoter was generated as described (20, 21). Briefly, the synapsin promoter and poly(A) regions were digested out of a plasmid provided by Dr. Colin Sumners (University of Florida) and then ligated into pShuttle (20). Then Ras-Grf1 cDNA was inserted in the resulting plasmid. pShSynGRF1 was digested with PmeI and then used to co-transform Escherichia coli strain BJ5183 along with pAdEasy-I. Colonies were screened for the presence of Ras-Grf1 gene and the pAdEasy-I backbone by colony PCR. The positive clones were tested for the correct orientation by restriction enzyme digestion. The plasmid was then digested with PacI and transfected into 293 cells. Then the culture supernatant containing viral particles was harvested. Cleared supernatant was used for subsequent serial infection of 293 cells. Resultant viral samples were subjected to adenovirus purification using Adenopure kit (Puresyn Inc.). The virus was concentrated by filtration, yielding a concentration greater than 1 × 1012 virus particles/ml.
Stereotaxic Surgery
2-Month-old ras-grf1 knock-out mice or WT mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg)/xylazine (10 mg/kg). Once anesthetized, each mouse was placed in a stereotaxic frame (myNeuroLab, St. Louis, MO). A surgical incision was made along the midline of the head to expose the skull. Two holes were made in the skull overlying the dorsal hippocampus. Coordinates for CA1 injection or cannula implantation into the mice are: 2.0 mm posterior to bregma, ±1.9 mm lateral from the midline, 1.9 or 0.9 mm (cannula implantation) beneath the surface of the skull. Injections were performed with a 10-μl Hamilton syringe fitted with a custom made blunt-ended 30-gauge needle (Hamilton). Each injection consisted of 1 μl of adenovirus-expressing Ras-Grf1 or adeno-associated virus (AAV-8) expressing Ras-Grf1 shRNA with mCherry infused at a rate of 0.06 μl/min. An infusion pump controlling the plunger on the Hamilton syringe precisely regulated the rate of injection. The needle was then left in place for 8 min prior to withdrawal from the brain. Electrophysiological recordings or behavioral procedures began 7–11 days after stereotaxic injection of the viral vectors.
In some experiments, stainless steel double guide cannulas (21-gauge) were implanted bilaterally into the hemisphere. Mice were given at least 1 week to recover after cannula implantation. The 28-gauge injection cannula was 1 mm longer than the guide. For intrahippocampal infusion, BIRB0796 was prepared by dissolving in DMSO and was diluted in sterile saline to a concentration of 1.056 mg/ml in 10% DMSO. Vehicle-treated animals were infused with 90% saline and 10% DMSO. 1 μl/side of either drug or vehicle was injected in freely moving animals at a rate of 0.15 μl/min using a syringe infusion pump.
Behavior Assays
Mice were tested in contextual discrimination as described previously (7). In this task, the mice were trained to discriminate between two chambers, one in which they were shocked and another in which they were not. On day 1, the mice were pre-exposed to the two contexts for 10 min. On day 2, the mice were shocked in one context (paired); after 148 s, a 2-s shock (0.75 mA) was delivered, and the mice remained for another 30 s in the context. Additionally, the mice were exposed for 3 min to the other context (non-paired), during which time no shocks were delivered. On day 3, the mice were shocked again in the paired context and exposed to the non-paired context. On day 4, the amount of freezing was measured for 3 min in each of the two contexts. In some experiments, adeno-associated virus expressing GFP, control random shRNA, or shRNA against Ras-Grf1 was injected stereotaxically into the CA1 hippocampus 7–10 days before beginning the experiment. In other experiments, the CP-AMPA receptor selective blocker IEM-1460 (Tocris Bioscience, Ellisville, MI) was dissolved in filtered saline and administered via intraperitoneal injections. In another group of experiments, animals received an intraperitoneal injection of either saline or IEM-1460 each day of the experiments and 30 min later were trained in context A on day 1, day 2, and day 3, respectively. In another group of experiments, animals received an intrahippocampal infusion of vehicle, SB203580 (0.75 μg/side), or BIRB0796 (1.056 μg/side) each day of the experiments and 30 min later were trained in context A on day 1, day 2, and day 3, respectively. Animals received an intrahippocampal infusion of vehicle, SB203580, or BIRB0796 each day of the experiments and 30 min later were trained in context A on day 1, day 2, and day 3, respectively. Contextual fear conditioning was assessed by measuring the amount of freezing displayed by mice after they were placed back into the context where a single shock had been delivered 24 h previously. Mice were pretreated with shRNA against Ras-Grf1, BIRB0796, or IEM-1460 as described above. The results were tested with a one-way analysis of variance.
Histology
After the behavioral experiments, mice were killed by ketamine/xylazine overdose and intracardially perfused with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Brains were sliced coronally (40 μm). The section was mounted on gelatin-subbed slides and stained with crystal violet. The location of the site of infusion was evaluated and recorded for each animal.
RESULTS
RAS-GRF1 Begins to Contribute to HFS-induced LTP at 2 Months of Age in the CA1, but Not CA3 or Dentate Gyrus, Regions of the Hippocampus
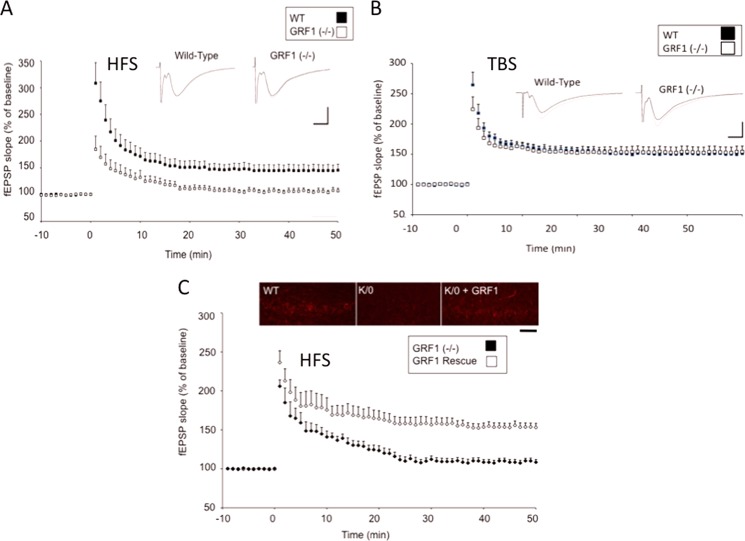
Using Ras-Grf knock-out mice, we previously showed that RAS-GRF1 is predominantly involved in mediating NMDAR activation of LTD, whereas RAS-GRF2 is predominantly involved in mediating NMDAR activation of LTP at the CA3/CA1 synapse beginning at approximately day 30 (early adolescence) in mice (5). However, because most behavior assays are performed with mice that are at least approaching late adolescence at 2 months of age, synaptic plasticity was retested in older Ras-Grf1 knock-out mice. To our surprise, 2-month-old Ras-Grf1 knock-out mice (as well as 6-month-old mice (data not shown)) displayed defective LTP-induced by HFS (two trains of 100 Hz of tetanus) (Fig. 1A) (WT, 161.76 ± 4.73% (n = 11 slices from six mice) versus 117.41 ± 2.54% (n = 12 slices from eight grf1−/− mice; p < 0.01)).
FIGURE 1.
Ras-Grf1 knock-out mice display defective HFS-induced but not TBS-induced LTP in CA1 hippocampus of 2-month-old mice. A and B, hippocampus slices from 2-month-old wild-type (filled squares) or Ras-Grf1 knock-out mice (open squares) were stimulated with HFS (A) or TBS (B). The recorded fEPSP slope using field recordings is expressed as a percentage of base line ± S.E. For HFS: WT; n = 11 slices from six mice; grf1−/−; n = 12 slices from eight grf1−/− mice). For TBS: WT; n = 8 slices from five mice; grf1−/−; n = 9 slices from five mice. Insets, representative fEPSPs recorded. C, control GFP-expressing adenovirus (filled diamonds) or GRF1-expressing adenovirus (open diamonds) was injected into the CA1 hippocampus. 9–11 days later, HFS LTP was measured in isolated hippocampal slices. Data represent the mean ± S.E. (Ras-Grf1; n = 10 slices from five mice; GFP; n = 13 slices from nine mice). Inset, CA1 hippocampus was stained with anti-RAS-GRF1 antibodies (calibration: horizontal, 10 ms, vertical, 1 mV, scale bar, 50 μm).
Interestingly, LTP generated by TBS (15 bursts of four pulses at 100 Hz delivered at an interburst interval of 200 ms) was normal in 2-month-old RAS-GRF1 knock-out mice (Fig. 1B) (WT, 159.43 ± 2.77% (n = 8 slices from five mice) versus 157.80 ± 1.69% (n = 9 slices from five grf1−/− mice; p > 0.05)) as we showed previously for 1-month-old mice (5). 2-Month-old Ras-Grf2 knock-out mice show the opposite results, normal HFS LTP but defective TBS LTP (data not shown).
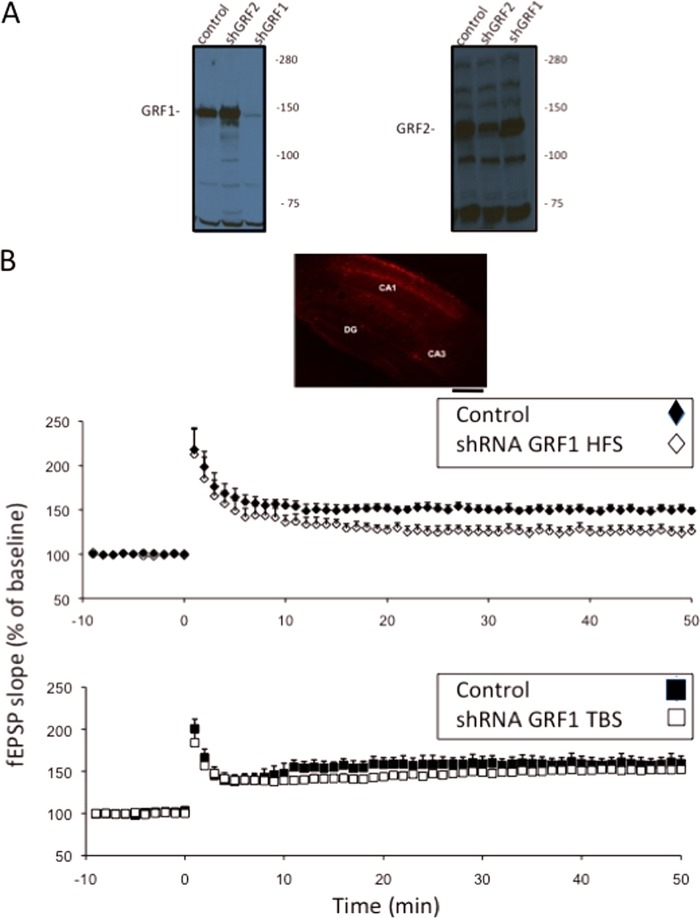
Confirming that this defect was not due to the indirect effects of the loss of the RAS-GRF1 in neighboring brain regions or during development, defective HFS LTP in Ras-Grf1 knock-out mice was restored to wild-type levels by re-expression of RAS-GRF1 in principle neurons of the CA1 hippocampus after injection of adenovirus-encoding Ras-Grf1 into the CA1 hippocampus of 2-month-old Ras-Grf1 knock-out mice (Fig. 1C) (Ras-Grf1, 165.40 ± 2.31% (n = 10 slices from five mice); GFP, 124.73 ± 3.16% (n = 13 slices from nine mice); p < 0.01). Moreover, RAS-GRF1 expression was suppressed acutely by stereotaxic injection of adeno-associated virus expressing shRNA against Ras-Grf1 and the red-cherry fluorophore into the CA1 region of the dorsal hippocampus. The shRNA sequence is specific because it blocks the expression of co-transfected Ras-Grf1, but not Ras-Grf2, in HEK 293 cells (Fig. 2A). As expected, HFS-induced LTP was suppressed when compared with infection with a control random shRNA-encoding virus (Fig. 2B, upper graph) (AAV control, 154.44 ± 1.75%; n = 8; AAV Ras-Grf1 shRNA, HFS, 132.91 ± 2.32%; n = 9; p < 0.01). The effect of virus infection was not as severe as that observed in the Ras-Grf1 knock-out mice presumably because not all pyramidal cells were infected and RAS-GRF1 loss was not complete. Importantly, theta-burst-induced LTP was not suppressed by Ras-Grf1 knockdown (Fig. 2B, lower graph) (AAV Ras-Grf1 shRNA, TBS, 147.34 ± 1.03%; n = 7, p > 0.05). Finally, infection of a similar virus expressing shRNA against Ras-Grf2 had the expected opposite effect, suppression of theta-burst LTP, but not HFS-induced LTP (data not shown).
FIGURE 2.
shRNA knockdown of Ras-Grf1 in the CA1 hippocampus of 2-month-old mice suppresses HFS-induced, but not TBS-induced, LTP. A, a mammalian expression vector expressing Ras-Grf1 or Ras-Grf2 was co-transfected with an empty control vector or a vector expressing shRNA against Ras-Grf2 or Ras-Grf1 into HEK 293 cells. 3 days later, lysates of cells were probed with antibodies that recognize RAS-GRF1 and RAS-GRF2. B, either control scrambled shRNA-expressing or Ras-Grf1 shRNA-expressing adeno-associated viruses co-expressing the red-cherry fluorophore were stereotaxically injected into the CA1 hippocampus. 9–11 days later, brain slices were isolated. HFS (top graph)- or TBS (lower graph)-induced LTP in the CA1 was then recorded (control AAV random shRNA (closed figures) n = 7; AAV Ras-Grf1 shRNA (open figures), n = 9). Data represent the mean ± S.E. Inset, a hippocampal slice from an infected mouse detecting red-cherry fluorescence. (scale bar, 250 μm).
The dentate gyrus and CA3 regions of the hippocampus have been implicated in distinct aspects of hippocampus-mediated learning and memory including pattern separation required for distinguishing closely related contexts (3). Thus, the role of RAS-GRF1 in LTP in these regions was also tested. In contrast to the CA1, HFS-induced LTP was normal in both of these regions of the hippocampus from 2-month-old Ras-Grf1 knock-out mice (LTP, DG, WT, 154.68 ± 1.22% (n = 9 slices from six mice) versus 154.88 ± 0.64% (n = 10 slices from six grf1−/− mice; p > 0.05); CA3, WT, 148.70 ± 1.51% (n = 8 slices from five mice) versus 147.48 ± 1.63% (n = 9 slices from five grf1−/− mice; p > 0.05)). Overall, these findings show that RAS-GRF1 plays an age-, stimulation-, and synapse-dependent role in promoting LTP induction in the hippocampus.
RAS-GRF1 Induces LTP in the CA1 of 2-Month-old Mice through Activation of p38 MAP Kinase
One of the known properties of RAS-GRF proteins is their ability to activate the RAS/ERK MAP kinase pathway, which can promote LTP. However, we showed previously that RAS-GRF1 is a weak inducer of NMDA-mediated LTP in the CA1 when compared with RAS-GRF2 in 1-month-old mice, at least in part, because it is less effective than RAS-GRF2 in activating ERK MAP kinase (5). Nevertheless, it was possible that RAS-GRF1 acquires the ability to promote LTP through ERK MAP kinase by 2 months of age. To test this possibility, hippocampal slices from 2-month-old wild-type mice were pre-exposed to U0126, an inhibitor of ERK activation, or buffer only for 30 min before LTP induction. Fig. 3A shows that as demonstrated previously (13, 22), HFS-induced LTP was not blocked by ERK inhibition (control, 161.86 ± 4.73% (n = 11); U0126, 154.34 ± 2.79% (n = 9); p > 0.05), although HFS-induced LTP was associated with an increase in ERK activity in CA1 neurons that was blocked by pretreatment with U0126 (data not shown). In contrast, TBS-induced LTP in 2-month-old mice, which is mediated by RAS-GRF2/ERK signaling (see Ref. 5), was suppressed by treatment with U0126 (Fig. 3B) (control, 159.43 ± 2.77% (n = 8); U0126, 133.46 ± 1.50% ( n = 7); p < 0.01).
FIGURE 3.
HFS-induced LTP, but not TBS-induced LTP, in the CA1 hippocampus of 2-month-old mice is dependent upon p38. A and B, hippocampal slices were preincubated with either vehicle (filled squares) or MAP kinase inhibitor U0126 (20 μm) (open squares). C, hippocampal slices were preincubated with vehicle (closed squares), p38 MAP kinase inhibitors SB203580 (10 μm) (open squares), or BIRB0796 (10 μm) (open diamonds) and tested for HFS LTP (A and C) or TBS LTP (B and D), n > 6. Data represent the mean ± S.E.
RAS-GRF1 can also mediate p38 activation by NMDA receptors potentially through its RAC-activating DH domain or through interaction with JIP2, a p38 scaffold that binds to the N-terminal region of RAS-GRF1 (5, 23). Thus, we pretreated hippocampus slices from 2-month-old mice with the p38 inhibitor, SB203580 (10 μm), and found that HFS-induced LTP was suppressed (Fig. 3C) (control, 161.86 ± 4.73% (n = 11); SB203580, 126.95 ± 1.45%; (n = 8; p < 0.01)). Inhibition of p38 with SB20380 was quite specific because as expected it did not block TBS LTP in 2-month-old mice nor HFS LTP in 1-month-old animals. Neither of these forms of LTP is affected in Ras-Grf1 knock-out mice (data not shown). Nevertheless, because the SB203580 inhibitor is not totally specific for p38, we tested another p38 inhibitor, BIRB0796. It also suppressed HFS LTP (Fig. 3C) (BIRB0796, 136.87 ± 1.47%; n = 8;p < 0.01) but not TBS LTP (Fig. 3D) in 2-month-old mice. Specific inhibition by both of these molecules is considered strong proof that p38 MAP kinase is involved (24).
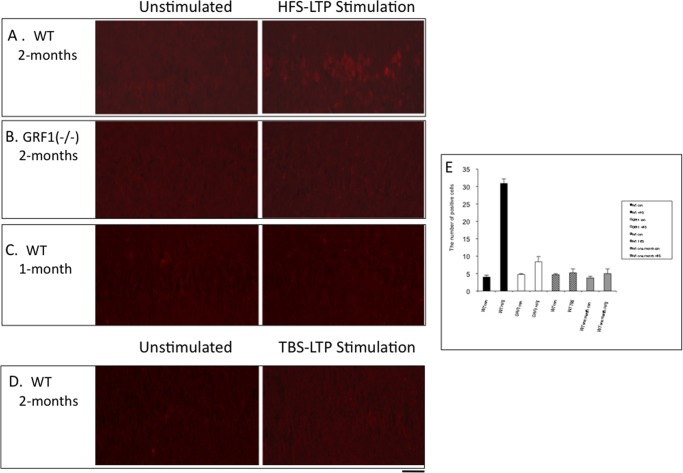
To gain additional support for the idea that this form of LTP is mediated by p38 MAP kinase, HFS LTP was induced in hippocampal slices from 2-month-old mice and p38 activation was measured by immunofluorescence using antibodies specific for its active form. HFS activated p38 in principal cells of the CA1 hippocampus of control mice (Fig. 4A) but not in hippocampal slices from Ras-Grf1 knock-out mice (Fig. 4B). Moreover, LTP induced by HFS treatment did not activate p38 in 1-month-old mice in which Ras-Grf1 does not yet have the capacity to promote LTP (Fig. 4C). Finally, theta-burst LTP, which is not blocked by p38 inhibitors in 2-month-old mice, did not activate p38 (Fig. 4D) (for quantification of data, see Fig. 4E). Together these data strongly support the idea that HFS-induced LTP that begins at 2 month of age depends upon RAS-GRF1-mediated activation of p38 MAP kinase.
FIGURE 4.
HFS-induced, but not TBS-induced, LTP in 2-month-old mice activates p38 MAP kinase activity in principal neurons of the CA1 hippocampus. A–D, hippocampal slices from 2-month-old (A, B, and D) or 1-month-old (C) WT orRas-Grf1 knock-out mice were stimulated with HFS LTP induction (A–C) or TBS LTP induction. The tissues were fixed 15 min later and stained with anti-phospho-p38 MAP kinase antibody. E, stained cells/visual field were quantified in each experiment. Data represent the average of three fields ± S.E. (scale bar, 50 μm).
CP-AMPARs Mediate HFS-induced LTP in the CA1 but Not CA3 or Dentate Gyrus
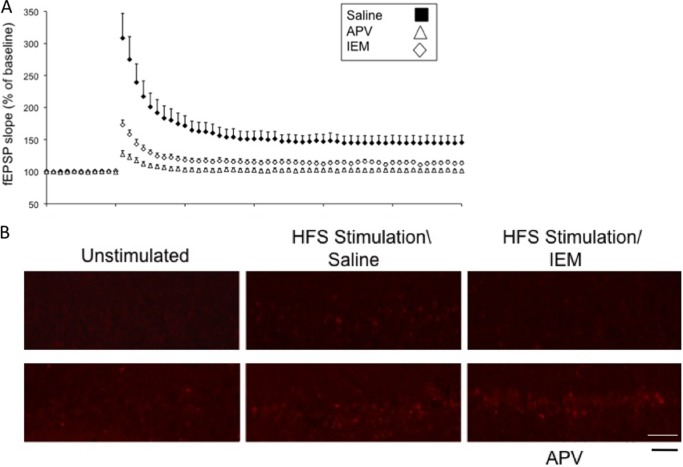
Both the NMDA-type and the calcium-permeable, AMPA-type glutamate receptor (CP-AMPAR)-type of glutamate receptors are known to bind to RAS-GRF1 (12, 25), and both permeate calcium, an activator of RAS-GRF proteins. Thus, to determine which receptor type mediates HFS induction of LTP by RAS-GRF1 in the CA1 hippocampus of 2-month-old mice, hippocampal slices were pretreated with inhibitors of either NMDARs (APV) or CP-AMPARs (IEM-1460). Either inhibitor blocked HFS LTP (Fig. 5A) (control, 161.86 ± 4.73% (n = 11); IEM-1460, 118.15 ± 1.61% (n = 8); p < 0.01; control, 161.86 ± 4.73% (n = 11); APV, 104.12 ± 0.71% (n = 7); p < 0.01), but only the CP-AMPAR inhibitor blocked HFS-induced p38 activation (Fig. 5B) These findings imply that CP-AMPAR/Ras-Grf1/p38 signaling adds an additional component to LTP induction that complements that involving NMDARs.
FIGURE 5.
HFS-induced LTP is blocked by inhibitors of either NMDARs or CP-AMPARs, but only inhibitors of CP-AMPARs block HFS-induced p38 activation. HFS LTP was induced in brain slices from WT mice in the presence of saline, NMDA receptor inhibitor, APV, (100 μm), or the CP-AMPA receptor inhibitor IEM-1460 (IEM, 30 μm). A and B, fEPSP slopes were then measured in some slices (A) (data represent the mean ± S.E. of >6 slices), and others were stained with phospho-p38 MAP kinase antibodies (B). Data are representative of three fields (scale bar, 50 μm).
Moreover, the CP-AMPAR inhibitor did not block LTP induction in the dentate gyrus or CA3 regions of the hippocampus from 2-month-old mice or the CA1 region from 1-month-old mice, situations in which RAS-GRF1 and p38 do not contribute to LTP (DG, control, 154.68 ± 1.22% (n = 9) versus IEM-1460, 155.51 ± 1.09% (n = 8); p > 0.05; CA3, control, 148.69 ± 1.51% (n = 8) versus IEM-1460, 153.81 ± 1.64% (n = 8); p > 0.05 and CA1 (1-month-old), control, 161.10 ± 3.32% (n = 8) versus 157.82 ± 2.49% (n = 9); p > 0.05). Also, the p38 inhibitor has already been shown not to block LTP in the dentate gyrus (26). Together, the results described above define a novel CP-AMPAR/RAS-GRF1/p38 MAP kinase signaling cascade that promotes LTP specifically in the CA1 only after mice reach ∼2 months of age.
Contextual Discrimination Is Mediated by RAS-GRF1 in the CA1 Hippocampus, p38 MAP Kinase, and CP-AMPARs
We previously showed that 2-month-old Ras-Grf1 knock-out mice fail to display contextual fear discrimination but have little if any defect in contextual fear conditioning (7). Thus, when 2-month-old Ras-Grf1 knock-out mice were exposed to foot shock in a specific environment and then placed back in that environment 24 h later, they recognized the context and became immobile (froze), expecting a shock with a frequency that was similar to control mice treated similarly. However, Ras-Grf1 knock-out mice failed to detect similar contexts. In particular, mice were trained to discriminate two closely related contexts, only one in which they received foot shocks. Freezing was then assessed in the non-shock environment 2 days later. Fig. 6A shows that wild-type mice recognized that the related environment was different from the one in which they received a foot shock and froze less than when placed in the shock context. Consistent with our previous results, Ras-Grf1 knock-out mice froze equally in the non-shock-related context as they did in the shock context (Fig. 6A) (WT (n = 7), froze 31.35 ± 5.90% of the testing time in the paired context versus 18.25 ± 6.06% in the non-paired context, F(1,12) = 5.39, p < 0.05; Ras-Grf1 KO (n = 8), froze 27.09 ± 5.93% of the testing time in the paired context versus 25 ± 6.03% in the non-paired context, F(1,14) = 0.06, p = 0.81).
FIGURE 6.
Contextual discrimination is blocked by expression of Ras-Grf1 shRNA in the CA1 hippocampus, p38 inhibitor infusion into the CA1 hippocampus, or injection of a CP-AMPAR inhibitor. Mice were exposed to a contextual discrimination paradigm as described (7). The amount of freezing was measured when mice were placed in the cage where they were shocked (paired, filled bar) and in the related cage in which they were not (non-paired, open bar). A, WT mice were compared with grf1−/− mice. B, adeno-associated virus expressing GFP (or random shRNA with similar results) or an shRNA against Ras-Grf1 was stereotaxically injected into the dorsal CA1 hippocampus of mice 8–10 days before beginning the experiment. C, saline or the p38 MAP kinase inhibitor BIRB0796 (Birb, 1 μl of 1.056 mg/ml) was infused through a cannula into the hippocampus of mice 30 min before each exposure to fear conditioning equipment. D, saline or the p38 MAP kinase inhibitor SB203580 (1 μl of 0.75 mg/ml) was infused through a cannula into the hippocampus of mice 30 min before each exposure to fear conditioning equipment. E, saline or the CP-AMPAR inhibitor, IEM-1460 (IEM, 8 mg/kg), was injected intraperitoneally into mice 30 min before each exposure to the fear conditioning equipment. F, WT mice at 1 month of age were used. Data represent the mean ± S.E. of at least nine animals each.
Because these mice lacked RAS-GRF1 in all tissues, we tested its role in the CA1 hippocampus by assaying the consequence of knocking down RAS-GRF1 expression specifically in the CA1 as described in the legend for Fig. 2. In particular, adeno-associated viruses expressing shRNA against Ras-Grf1 or control viruses were injected bilaterally into the CA1 hippocampus of 2-month-old mice. 7–9 days later, mice were compared in contextual discrimination assays. Fig. 6B shows that control, but not Ras-Grf1 shRNA, virus-treated mice displayed contextual discrimination (AAV control (n = 8), froze 55.13 ± 5.56% of the testing time in the paired context versus 40.08 ± 4.37% in the non-paired context, F(1,14) = 4.53, p < 0.05; AAV Ras-Grf1 shRNA (n = 8), froze 57.54 ± 6.25% of the testing time in the paired context versus 56.56 ± 3.49% in the non-paired context, F(1,14) = 0.02, p = 0.89).
To test the role of p38 MAP kinase in contextual discrimination, the p38 MAP kinase inhibitors, SB203580 or BIRB0796, or vehicle were infused through a cannula placed above the CA1 region 30 min before mice were habituated to the two related contexts. Mice were then used in contextual discrimination assays. Although vehicle-infused mice retained the ability to distinguish related contexts, BIRB0796- or SB203580-infused mice did not (Fig. 6, C and D) (vehicle (n = 11), froze 53.01 ± 2.86% of the testing time in the paired context versus 36.94 ± 4.81% in the non-paired context, F(1,20) = 8.24, p < 0.01; BIRB0796 (n = 9), froze 59.03 ± 5.79% of the testing time in the paired context versus 62.37 ± 5.77% in the non-paired context, F(1,16) = 0.17, p = 0.69); SB203580 (n = 9), froze 38.29 ± 9.89% in paired versus 33.75 ± 6.45% in “unpaired” context, F(1,16) = 0.52, p = 0.48). Importantly, like Ras-Grf1 shRNA knockdown mice, both sets of p38 inhibitor-infused mice displayed normal contextual fear conditioning (data not shown).
Finally, to test the role of CP-AMPARs in contextual discrimination, the CP-AMPAR inhibitor, IEM-1460, or saline was introduced via intraperitoneal injection 30 min before mice were habituated to the related contexts. Like mice with suppressed GRF1 expression and inhibited p38 MAP kinase in the CA1, these mice also did not recognize a difference in the non-shock context and froze just as often when placed in the non-shock context as when they were placed in the shock environment (Fig. 6E) (saline(n = 10), froze 47.48 ± 4.10% of the testing time in the paired context versus 31.19 ± 5.36% in the non-paired context, F(1,20) = 5.83, p < 0.05; IEM-1460 (n = 6), froze 42.52 ± 12.98% of the testing time in the paired context versus 44.48 ± 10.05% in the non-paired context, F(1,10) = 0.01, p = 0.91) but did display normal contextual fear conditioning (data not shown).
Because we did not detect a contribution of CP-AMPARs, GRF1, or p38 MAP kinase in HFS-induced LTP in the CA1 hippocampus of 1-month-old mice, we tested them for the ability to distinguish closely related contexts. Fig. 6F shows that, in contrast to 2-month-old mice, 1-month-old mice do not yet have the capacity to distinguish related contexts.
Overall, these findings implicate the appearance of a CP-AMPAR/RAS-GRF1/p38 MAP kinase LTP pathway in the acquisition of the ability to distinguish closely related contexts during early adolescent development in mice.
DISCUSSION
This study uses electrophysiology, genetics, immunohistochemistry, and behavioral analysis to reveal that the postnatal emergence of the ability of mice to distinguish closely related contexts at ∼2 months of age involves the appearance of a newly discovered signaling pathway involving CP-AMPARs, RAS-GRF1, and p38 MAP kinase that contributes to LTP in the CA3/CA1 synapse of the hippocampus. One of the most striking findings of this work is the detection of a role for p38 MAP kinase, rather than ERK MAP kinase, in promoting LTP because p38 has mainly been implicated in promoting LTD (5, 15–17) or inhibiting LTP (27, 28). For example, LTD induced through metabotropic glutamate receptors in the CA1 (15, 29) and dentate gyrus of the hippocampus functions through p38. Evidence suggests p38 also mediates NMDA receptor-induced LTD, although the degree of inhibition of this form of LTD by p38 inhibitors has ranged from complete (16), to partial (5) to none (17). Some of this discrepancy may arise from the fact that these studies used varied methods to detect LTD, different rodents, and/or animals of different ages.
One example where p38 has been implicated in LTP induction mediated by NMDA receptors is via theta-burst stimulation of the CA1 after exposure of adolescent mice to an enriched environment (18). In that case, a cAMP/p38 MAP kinase signaling pathway emerges after enrichment that compliments the existing ERK MAP kinase pathway regulated by RAS-GRF2. Apparently, p38 MAP kinase can have multiple functions in cells, a concept consistent with the fact that kinases exist in distinct signaling complexes containing different scaffold proteins that can target them to specific subsets of their many substrates in cells (30). Of the ∼100 p38 putative substrates identified (31), most are involved in gene transcription or translation regulation, which occurs at a time scale too slow to contribute to HFS LTP. However, some tyrosine kinase receptors, cytoplasmic kinases, and proteins involved in endocytosis also have been proposed to be substrates and thus could potentially influence synaptic plasticity. Which p38 substrates are involved here remains to be determined. This form of LTP appears to be a postsynaptic process because no change in paired pulse facilitation was observed, indicating a lack of presynaptic involvement (data not shown).
Another interesting aspect of this CP-AMPAR/RAS-GRF1/p38 MAP kinase pathway is that it contributes to HFS-induced, but not TBS-induced, early LTP. HFS usually involves one train of 100 stimuli at 100 Hz, whereas theta-burst stimulation is typically four 100-Hz pulses with 200-ms interburst intervals, a protocol designed to match endogenous theta waves (32). Although both of these types of tetanization produce similar magnitudes of early LTP when applied to the Schaffer collateral innervating synapses in the CA1 hippocampus, differences in their mechanisms have been noted. For example, although HFS is often described as a more intense form of stimulation, theta-burst actually leads to more calcium influx (33). In addition, theta-burst stimulation leads to significant inhibitory postsynaptic potentials soon after tetanus, whereas HFS stimulation produces a distinct NMDA-mediated suppression of these inhibitory responses (33). Which, if any, of these differences contribute to the specificity in evoking the CP-AMPAR/RAS-GRF1/p38 pathway at 2 months of age remains to be determined. Finally, although theta-burst LTP is considered a more “physiological” stimulus, our finding that the appearance of contextual discrimination at ∼2 months of age correlates with the appearance of HFS LTP mediated by RAS-GRF1 and p38, rather than theta-burst LTP mediated by ERK MAP kinase that has been present since birth, suggests that HFS LTP contributes to physiological events in the hippocampus.
Furthermore, our finding that a new signaling cascade contributes to HFS-induced LTP only after 2 months of age during late adolescence highlights how the biochemical underpinnings of LTP change with age along with the changing role of synaptic plasticity. For example, until ∼1 month of age, the hippocampus does not contribute to learning and memory so that synaptic plasticity at this time is likely involved in promoting the final maturation of this brain region (1, 34). Soon after birth, cAMP is a predominant inducer of LTP in the CA1 hippocampus, and then at ∼2 weeks of age, Ca2+/calmodulin-dependent protein kinase (CaMKII) activity takes over (35). At 1 month of age, when contextual fear learning is first enabled, RAS-GRF2 begins to contribute to LTP in the CA1 along with Ca2+/calmodulin-dependent protein kinase (5). The present study shows that at 2 months of age, more refined learning required for discriminating closely related contexts becomes possible due, at least in part, to the appearance of an additional signaling pathway that contributes to LTP, involving CP-AMPAR, RAS-GRF1, and p38 MAP kinase.
These findings also highlight how RAS-GRF1 plays distinct roles in different regions of the brain. Previous studies showed that RAS-GRF1 contributes to TBS-induced LTP in the basal amygdala (36) but not the CA1 hippocampus (5). Here we show that RAS-GRF1 contributes to HFS-induced LTP in the CA1, but not the CA3 or dentate gyrus of the hippocampus. Moreover, GRF1 mediates dopamine signaling through ERK activation in the striatum (37), whereas it is a poor mediator of NMDA receptor activation of ERK in the CA1 (5). In addition, RAS-GRF1 affects visual recognition memory and both LTP and LTD in the perirhinal cortex via regulation of ERK (38). Finally, RAS-GRF1 mediates CP-AMPAR activation of ERK in the cortex at 1 month of age (12), but p38 activation starting at 2 months of age in the CA1, but not CA3 or dentate gyrus, regions of the hippocampus. The biochemical underpinnings of these differences have not yet been revealed. However, it most likely involves discretionary use of its known interactions with a plethora of scaffold proteins that target the protein to distinct effector proteins in cells (23, 39, 40).
CP-AMPARs consist of homodimers of GluA1 subunits that are permeable to calcium, making them likely mediators of synaptic plasticity (41). Their role in synaptic plasticity in inhibitory interneurons has been well established; however, their role in CA1 pyramidal neurons has been controversial. Plant et al. (42) first demonstrated that the early phase of LTP in these neurons involves signaling through CP-AMPARs at synapses, but this was quickly refuted (43). However, additional studies have reaffirmed that this class of receptors represents a significant fraction (∼8–10%) of the AMPA receptor population in these cells (44). They appear to contribute specifically to the early phase of LTP in an age-dependent manner (45). Increased CP-AMPAR content in mice increases the magnitude of HFS LTP in the CA1, and their inhibition blocks NMDA-independent learning, where animals have a prior experience on a similar behavioral task (46). Here, we expand these findings by showing that CP-AMPARs begin to function through RAS-GRF1 to regulate p38 at ∼2 months of age in mice to contribute to contextual discrimination.
The present study also reveals that the contribution of CP-AMPARs, RAS-GRF1, and p38 to HFS LTP is limited to the CA1 region as inhibition of any of these proteins in the CA3 or dentate gyrus had no effect on this form of synaptic plasticity. Most studies have pinpointed specific roles for the dentate gyrus and CA3 regions in pattern separation, an important component of contextual discrimination (47). In particular, the DG and CA3 have been proposed to play contrasting roles in memory processing, with the dentate gyrus functioning as a pattern separator splitting the signal input from the entorhinal cortex into distinct parts and encoding stimuli as novel. The CA3 contributes to pattern completion that matches incoming stimuli from entorhinal cortex with previous familiar experiences. The CA1 generates the output of the hippocampus and therefore the end point of its processing properties. The CA1 has been proposed to be a mismatch detector (48) because it receives both direct input about current events from entorhinal cortex and stored information from the CA3. However, evidence suggests that this region also adds a temporal context to events (49), another key aspect of pattern separation involved in contextual discrimination studied here, where contexts are experienced temporally and retrieval of stored information during testing is required. Which of these aspects of contextual discrimination involve synaptic plasticity provided by the CP-AMPAR/RAS-GRF1/p38/LTP pathway is presently under investigation.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 MH083324 (to L. A. F.) and Grant P30 NS047243 through the Tufts Center for Neuroscience Research.
L. A. Feig, unpublished observations.
- NMDAR
- NMDA receptor
- CP-AMPAR
- calcium-permeable 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid receptor
- RAS-GRF1
- RAS guanine nucleotide-releasing factor 1
- RAS-GRF2
- RAS guanine nucleotide-releasing factor 2
- LTP
- long term potentiation
- LTD
- long term depression
- ACSF
- artificial cerebrospinal fluid
- fEPSP
- field excitatory postsynaptic potential
- DG
- dentate gyrus
- TBS
- theta-burst
- HFS
- high frequency stimulation
- DMSO
- dimethyl sulfoxide
- AAV
- adeno-associated virus
- APV
- dl-2-amino-5-phosphonopentanoic acid.
REFERENCES
- 1. Dumas T. C. (2005) Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus 15, 562–578 [DOI] [PubMed] [Google Scholar]
- 2. Yassa M. A., Lacy J. W., Stark S. M., Albert M. S., Gallagher M., Stark C. E. (2011) Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21, 968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langston R. F., Stevenson C. H., Wilson C. L., Saunders I., Wood E. R. (2010) The role of hippocampal subregions in memory for stimulus associations. Behav. Brain Res. 215, 275–291 [DOI] [PubMed] [Google Scholar]
- 4. Feig L. A. (2011) Regulation of neuronal function by Ras-GRF exchange factors. Genes Cancer 2, 306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li S., Tian X., Hartley D. M., Feig L. A. (2006) Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J. Neurosci. 26, 1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin S. X., Feig L. A. (2010) Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS One 5, e11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giese K. P., Friedman E., Telliez J. B., Fedorov N. B., Wines M., Feig L. A., Silva A. J. (2001) Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1). Neuropharmacology 41, 791–800 [DOI] [PubMed] [Google Scholar]
- 8. d'Isa R., Clapcote S. J., Voikar V., Wolfer D. P., Giese K. P., Brambilla R., Fasano S. (2011) Mice lacking Ras-GRF1 show contextual fear conditioning but not spatial memory impairments: Convergent evidence from two independently generated mouse mutant lines. Front Behav. Neurosci. 5, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sweatt J. D. (2001) The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 76, 1–10 [DOI] [PubMed] [Google Scholar]
- 10. Klesse L. J., Parada L. F. (1999) Trks: signal transduction and intracellular pathways. Microsc. Res. Tech. 45, 210–216 [DOI] [PubMed] [Google Scholar]
- 11. Xia Z., Dudek H., Miranti C. K., Greenberg M. E. (1996) Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tian X., Feig L. A. (2006) Age-dependent participation of Ras-GRF proteins in coupling calcium-permeable AMPA glutamate receptors to Ras/Erk signaling in cortical neurons. J. Biol. Chem. 281, 7578–7582 [DOI] [PubMed] [Google Scholar]
- 13. Selcher J. C., Weeber E. J., Christian J., Nekrasova T., Landreth G. E., Sweatt J. D. (2003) A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem. 10, 26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams J. P., Roberson E. D., English J. D., Selcher J. C., Sweatt J. D. (2000) MAPK regulation of gene expression in the central nervous system. Acta. Neurobiol. Exp. (Wars) 60, 377–394 [DOI] [PubMed] [Google Scholar]
- 15. Bolshakov V. Y., Carboni L., Cobb M. H., Siegelbaum S. A., Belardetti F. (2000) Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat. Neurosci. 3, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 16. Zhu J. J., Qin Y., Zhao M., Van Aelst L., Malinow R. (2002) Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455 [DOI] [PubMed] [Google Scholar]
- 17. Peineau S., Nicolas C. S., Bortolotto Z. A., Bhat R. V., Ryves W. J., Harwood A. J., Dournaud P., Fitzjohn S. M., Collingridge G. L. (2009) A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol. Brain 2, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li S., Tian X., Hartley D. M., Feig L. A. (2006) The environment versus genetics in controlling the contribution of MAP kinases to synaptic plasticity. Curr. Biol. 16, 2303–2313 [DOI] [PubMed] [Google Scholar]
- 19. Tian X., Gotoh T., Tsuji K., Lo E. H., Huang S., Feig L. A. (2004) Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk, and CREB. EMBO J. 23, 1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He T. C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 95, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cashman S. M., Sadowski S. L., Morris D. J., Frederick J., Kumar-Singh R. (2002) Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors—implications for gene therapy. Mol. Ther. 6, 813–823 [DOI] [PubMed] [Google Scholar]
- 22. Kelleher R. J., 3rd, Govindarajan A., Jung H. Y., Kang H., Tonegawa S. (2004) Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116, 467–479 [DOI] [PubMed] [Google Scholar]
- 23. Buchsbaum R. J., Connolly B. A., Feig L. A. (2002) Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol. Cell. Biol. 22, 4073–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krapivinsky G., Krapivinsky L., Manasian Y., Ivanov A., Tyzio R., Pellegrino C., Ben-Ari Y., Clapham D. E., Medina I. (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40, 775–784 [DOI] [PubMed] [Google Scholar]
- 26. Saleshando G., O'Connor J. J. (2000) SB203580, the p38 mitogen-activated protein kinase inhibitor blocks the inhibitory effect of β-amyloid on long-term potentiation in the rat hippocampus. Neurosci. Lett. 288, 119–122 [DOI] [PubMed] [Google Scholar]
- 27. Izumi Y., Tokuda K., Zorumski C. F. (2008) Long-term potentiation inhibition by low-level N-methyl-d-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase. Hippocampus 18, 258–265 [DOI] [PubMed] [Google Scholar]
- 28. Butler M. P., O'Connor J. J., Moynagh P. N. (2004) Dissection of tumor-necrosis factor-α inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early—but not late—phase LTP. Neuroscience 124, 319–326 [DOI] [PubMed] [Google Scholar]
- 29. Rush A. M., Wu J., Rowan M. J., Anwyl R. (2002) Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J. Neurosci. 22, 6121–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown M. D., Sacks D. B. (2009) Protein scaffolds in MAP kinase signalling. Cell Signal 21, 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trempolec N., Dave-Coll N., Nebreda A. R. (2013) SnapShot: p38 MAPK substrates. Cell 152, 924–924 [DOI] [PubMed] [Google Scholar]
- 32. Albensi B. C., Oliver D. R., Toupin J., Odero G. (2007) Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp. Neurol. 204, 1–13 [DOI] [PubMed] [Google Scholar]
- 33. Perez Y., Chapman C. A., Woodhall G., Robitaille R., Lacaille J. C. (1999) Differential induction of long-lasting potentiation of inhibitory postsynaptic potentials by theta patterned stimulation versus 100-Hz tetanization in hippocampal pyramidal cells in vitro. Neuroscience 90, 747–757 [DOI] [PubMed] [Google Scholar]
- 34. Rudy J. W., Morledge P. (1994) Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 108, 227–234 [DOI] [PubMed] [Google Scholar]
- 35. Malenka R. C., Bear M. F. (2004) LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 [DOI] [PubMed] [Google Scholar]
- 36. Brambilla R., Gnesutta N., Minichiello L., White G., Roylance A. J., Herron C. E., Ramsey M., Wolfer D. P., Cestari V., Rossi-Arnaud C., Grant S. G., Chapman P. F., Lipp H. P., Sturani E., Klein R. (1997) A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature 390, 281–286 [DOI] [PubMed] [Google Scholar]
- 37. Fasano S., D'Antoni A., Orban P. C., Valjent E., Putignano E., Vara H., Pizzorusso T., Giustetto M., Yoon B., Soloway P., Maldonado R., Caboche J., Brambilla R. (2009) Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol. Psychiatry 66, 758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silingardi D., Angelucci A., De Pasquale R., Borsotti M., Squitieri G., Brambilla R., Putignano E., Pizzorusso T., Berardi N. (2011) ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex. Front Behav. Neurosci. 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buchsbaum R. J., Connolly B. A., Feig L. A. (2003) Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J. Biol. Chem. 278, 18833–18841 [DOI] [PubMed] [Google Scholar]
- 40. Connolly B. A., Rice J., Feig L. A., Buchsbaum R. J. (2005) Tiam1-IRSp53 complex formation directs specificity of Rac-mediated actin cytoskeleton regulation. Mol. Cell. Biol. 25, 4602–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Man H. Y. (2011) GluA2-lacking, calcium-permeable AMPA receptors—inducers of plasticity? Curr. Opin. Neurobiol. 21, 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Plant K., Pelkey K. A., Bortolotto Z. A., Morita D., Terashima A., McBain C. J., Collingridge G. L., Isaac J. T. (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 9, 602–604 [DOI] [PubMed] [Google Scholar]
- 43. Adesnik H., Nicoll R. A. (2007) Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J. Neurosci. 27, 4598–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rozov A., Sprengel R., Seeburg P. H. (2012) GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses: evidence from gene-targeted mice. Front Mol. Neurosci. 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu Y., Allen M., Halt A. R., Weisenhaus M., Dallapiazza R. F., Hall D. D., Usachev Y. M., McKnight G. S., Hell J. W. (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 26, 4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiltgen B. J., Royle G. A., Gray E. E., Abdipranoto A., Thangthaeng N., Jacobs N., Saab F., Tonegawa S., Heinemann S. F., O'Dell T. J., Fanselow M. S., Vissel B. (2010) A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS One 5, e12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yassa M. A., Stark C. E. (2011) Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duncan K., Ketz N., Inati S. J., Davachi L. (2012) Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus 22, 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kesner R. P., Hunsaker M. R. (2010) The temporal attributes of episodic memory. Behav. Brain Res. 215, 299–309 [DOI] [PubMed] [Google Scholar]