Background: Knowledge regarding UDP-N-acetylglucosamine transporter (NGT; SLC35A3) is incomplete due to the lack of NGT-deficient model cell lines.
Results: The siRNA approach showed that NGT silencing reduces branching of complex N-glycans and keratan sulfate synthesis.
Conclusion: NGT function may be coupled to the specific glycosylation pathway(s) of particular macromolecules.
Significance: Our results add to the understanding of glycosylation, one of the basic posttranslational modifications.
Keywords: Glycosylation, Glycosyltransferases, Golgi, Keratan, Proteoglycan, UDP-N-Acetylglucosamine Transporter, Branched N-Glycans
Abstract
SLC35A3 is considered the main UDP-N-acetylglucosamine transporter (NGT) in mammals. Detailed analysis of NGT is restricted because mammalian mutant cells defective in this activity have not been isolated. Therefore, using the siRNA approach, we developed and characterized several NGT-deficient mammalian cell lines. CHO, CHO-Lec8, and HeLa cells deficient in NGT activity displayed a decrease in the amount of highly branched tri- and tetraantennary N-glycans, whereas monoantennary and diantennary ones remained unchanged or even were accumulated. Silencing the expression of NGT in Madin-Darby canine kidney II cells resulted in a dramatic decrease in the keratan sulfate content, whereas no changes in biosynthesis of heparan sulfate were observed. We also demonstrated for the first time close proximity between NGT and mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetylglucosaminyltransferase (Mgat5) in the Golgi membrane. We conclude that NGT may be important for the biosynthesis of highly branched, multiantennary complex N-glycans and keratan sulfate. We hypothesize that NGT may specifically supply β-1,3-N-acetylglucosaminyl-transferase 7 (β3GnT7), Mgat5, and possibly mannosyl (α-1,3-)-glycoprotein β-1,4-N-acetylglucosaminyltransferase (Mgat4) with UDP-GlcNAc.
Introduction
Glycosylation is one of the most frequent posttranslational modifications of macromolecules. The glycan moiety is synthesized and modified by glycosyltransferases acting in the lumen of the endoplasmic reticulum (ER)2 and Golgi apparatus. The substrates required by glycosyltransferases are sugars activated by the addition of a nucleoside mono- or diphosphate (UDP, GDP, or CMP). Nucleotide sugars are synthesized in the cytosol (1), except for CMP-sialic acid, which is synthesized in the nucleus (2), and transported by nucleotide sugar transporters (NSTs) into the ER and/or Golgi apparatus. NSTs are hydrophobic, multi-transmembrane proteins with a molecular mass of 30–45 kDa (3, 4). Several studies have demonstrated that NSTs function in the form of dimers or higher oligomers (5–11).
One of the best characterized NSTs is the UDP-Gal transporter (UGT; SLC35A2) (6, 12–16). Two splice variants of UGT (UGT1 and UGT2) have been identified in human tissues, the Chinese hamster ovary (CHO) and Madin-Darby canine kidney II (MDCK) cell lines (6, 12–14, 16). Detailed characterization of UGT was possible after mutant cell lines, such as MDCK cells resistant to Ricinus communis agglutinin (MDCK-RCAr) (17, 18), CHO-Lec8 cells (14, 19), and Had-1 cells (15, 20), had been generated. Nonsense mutations identified in the mutant cells cause inhibition of UGT production, resulting in macromolecules enriched in terminal N-acetylglucosamine (GlcNAc), deficient in terminal galactose (Gal) and sialic acid (17, 21, 22).
When compared with mammalian UGT, knowledge regarding mammalian UDP-N-acetylglucosamine transporter is limited. Within known UDP-GlcNAc transporters, the protein assigned as SLC35A3 (UDP-N-acetylglucosamine transporter (NGT)) is assumed to play a main role in glycosylation of macromolecules (23, 24), whereas the function of SLC35D2 (25, 26), SLC35B4 (27), and SLC35D1 (28) multi-specific transporters appears to be less important. Recently, it has been reported that a point mutation of the SLC35A3 gene causes complex vertebral malformation in animals, resulting from impaired UDP-GlcNAc transport into the Golgi vesicles (29).
We hypothesize that the role of NGT and UGT in glycosylation of macromolecules may be coupled and that both transporters may partially replace the function played by its partner. Firstly, both transporters are evolutionarily related (4, 10, 30–32). Secondly, we showed that overexpression of NGT in UGT-defective cells partially restores galactosylation (30) and that UGT-NGT chimeric transporter complemented the mutation defect (33). Finally, we recently demonstrated that NGT and UGT form complexes in the Golgi membrane (10).
Although NGT is considered the main UDP-GlcNAc transporter in mammals, its biological role awaits further attention. However, detailed analysis of this transporter is restricted because mammalian mutant cells defective in this activity have not been isolated. Therefore, using the siRNA approach, we developed and characterized several NGT-deficient mammalian cell lines.
EXPERIMENTAL PROCEDURES
Molecular Cloning of Hamster NGT and Canine β4GalT4
cDNA clones containing the complete coding regions for hamster NGT and canine β-1,4-galactosyltransferase 4 (β4GalT4) were generated and sequenced using degenerate primers designed based on known homologous mammalian sequences and the modified rapid amplification of cDNA ends technique as described previously (16).
Construction of NGT- and β4GalT4-targeting siRNA Plasmids
siRNA sequences targeting human NGT (NM_005660), canine NGT (NM_001003385.1), hamster NGT (FN825777.1), and canine β4GalT4 (AM989461.1) were selected using the InvivoGen siRNA WizardTM online tool. A pair of control sequences (scrambled siRNA) was also designed. Based on selected siRNA sequences, pairs of complementary (sense and antisense) oligonucleotides were designed (supplemental Table S1) using the above mentioned program. Complementary oligonucleotide pairs were PAGE-purified and annealed by incubation at the 50 μm concentration in 0.1 m NaCl at 80 °C (2 min) followed by slow (1 °C per min) cooling down to 35 °C. The resulting double-stranded DNA fragments were cloned into the psiRNA-DUO plasmid according to the manufacturer's instructions using a two-step procedure (InvivoGen). Briefly, the psiRNA-DUO plasmid was digested with Acc65I and HindIII restriction enzymes and ligated with the first insert. The resulting construct was transformed into Escherichia coli GT115 cells (InvivoGen), and positive colonies were selected using Fast-Media® Zeo X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (InvivoGen). The plasmid containing the first insert was subsequently digested with BbsI restriction enzyme and ligated with the second insert. The resulting construct was transformed into E. coli GT115 cells (InvivoGen), and positive colonies were selected using Fast-Media® Zeo 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid, cyclohexylammonium salt (X-gluc) (InvivoGen). The obtained shRNA expression plasmids used for the stable transfection of cells are listed in supplemental Table S2.
Construction of eGFP and mRFP Expression Plasmids
ORFs of human mannosyl (α-1,3-)-glycoprotein β-1,4-N-acetylglucosaminyltransferase, isozyme A (Mgat4A; NM_012214.2) and mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetylglucosaminyltransferase (Mgat5; NM_002410.3) with appropriate restriction sites at both ends were amplified using cDNA synthesized from total RNA isolated from HeLa cells and subsequently cloned into pTagGFP2-C vector (Evrogen). We assumed that among known Mgat4 isozymes (A, B, and C), Mgat4A is the main isozyme accounting for Mgat4 activity because the amount of the corresponding mRNA in HeLa cells was more abundant when compared with other isozymes (data not shown). Sequence encoding human NGT (NM_012243.2) with N-terminal mRFP fusion was subcloned from the expression plasmid previously constructed in pTagRFP-C vector (10) into pSELECT-zeo plasmid (InvivoGen), and the resulting construct was used instead of the initial one due to superior fluorescence properties of the resulting mRFP fusion protein. Briefly, insert encoding mRFP-NGT fusion protein was cut out from the parent vector (10) using NheI and BamHI restriction enzymes, and both 3′-ends and 5′-ends were blunted using mung bean nuclease (New England Biolabs). pSELECT-zeo plasmid (InvivoGen) was digested with BamHI restriction enzyme, blunt-ended as described above, and dephosphorylated using calf intestine alkaline phosphatase (Fermentas). ORF of human UDP-xylose/N-acetylglucosamine transporter (SLC35B4; NP_116215.1) with BglII and BamHI restriction sites at both ends was amplified using cDNA synthesized from total RNA isolated from HeLa cells and subsequently cloned into pTagGFP2-C vector (Evrogen). All ligations were performed using a rapid DNA ligation kit (Fermentas). Expression plasmid containing mRFP-UGT2 was constructed previously (10). Primers used for amplifications are listed in supplemental Table S3. The obtained eGFP and mRFP expression plasmids are listed in supplemental Table S2.
RT-PCR Evaluation of the NGT and β4GalT4 Silencing Efficiency
The efficiency of NGT and β4GalT4 silencing was determined at the mRNA level using a semiquantitative RT-PCR approach. For this purpose, total RNA was isolated from selected NGT- or β4GalT4-deficient clones as well as from cells transfected with control scrambled siRNA plasmid, and a fragment of NGT- or β4GalT4-encoding sequences was subsequently amplified using the Titan one tube RT-PCR kit according to the manufacturer's instructions (Roche Diagnostics). Fragments of either CMP-sialic acid (SLC35A1) or GDP-fucose (SLC35C1) transporter sequences were amplified in parallel as a reference. In addition, transcript levels of SLC35D1, SLC35D2, and SLC35B4 transporters were examined. Amplification products were separated in 0.8% agarose gel and visualized with ethidium bromide. Primer sequences and length of the amplified fragments are listed in supplemental Table S3.
Cell Maintenance and Transfection
MDCK, MDCK-RCAr, CHO, and CHO-Lec8 cells were grown as described previously (16). For the detection of keratan sulfate, MDCK and MDCK-RCAr cells were grown in a serum-free medium as reported previously (16). HeLa and A375 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 4 mm l-glutamine, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml. Cells were transfected with shRNA expression plasmids listed in supplemental Table S2. Stable transfectants were selected in complete medium containing 200 μg/ml (MDCK), 150 μg/ml (CHO and CHO-Lec8) or 100 μg/ml (HeLa) Zeocin (InvivoGen).
Immunoreactivity and Reactivity with Lectins
Cell lysates were subjected to SDS-PAGE using 10% polyacrylamide gels and transferred onto nitrocellulose membranes (Whatman) as described previously (16). Detection of keratan sulfate and reactivity of glycoproteins with lectins were performed as reported previously (16, 30). Heparan sulfate was detected using mouse anti-heparin/heparan sulfate antibody (1:5 000; Millipore; clone T320.11, catalog number MAB2040) followed by anti-mouse IgG antibody conjugated with HRP (1:10 000; Promega). Selected Golgi and ER proteins were detected with rabbit anti-GM130 and anti-calnexin antibodies (1:2 000; Abcam) followed by anti-rabbit IgG antibody conjugated with HRP (1:10 000; Sigma).
Isolation and Separation of Fluorescently Labeled N-Glycans and Matrix-assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) Analysis
N-Glycans were isolated as described previously (30). Briefly, cell lysates were diluted to 2 mg/ml using the lysis buffer, and 500-μl aliquots were precipitated overnight at −20 °C with an equal volume of cold acetone. After centrifugation at 10 000 × g for 10 min, precipitates were air-dried and resuspended in glycoprotein denaturation buffer (N-glycosidase F deglycosylation enzyme pack, New England Biolabs). Deglycosylation was performed using 1 μl of the enzyme (500 units) for 18 h at 37 °C in deglycosylation buffer. Released N-glycans were isolated, fluorescently labeled with 2-aminobenzamide, purified, and separated on a GlycoSep N column (Glyko) as described previously (30). In addition, enzymatic sequencing was performed to confirm particular structures and type of linkages in branched N-glycans (34). MALDI-TOF MS analysis was carried out in positive ion mode with Na+ excess as reported previously (30).
Subcellular Fractionation and Transport Assay
The Golgi fraction was isolated from mammalian cells, and UDP-Gal or UDP-GlcNAc transport into Golgi vesicles was subsequently determined as described previously (16, 30).
Confocal and Fluorescence Lifetime Imaging Microscopy (FLIM)
Confocal and FLIM microscopy was performed as described previously (10) except that A375 cells were used in some experiments and cells transiently transfected with the expression plasmids listed in supplemental Table S2 were seeded onto the 35-mm CELLviewTM glass bottom dishes (Greiner Bio-One) prior to imaging. Analysis of FLIM-FRET experiments was carried out as reported previously (10).
RESULTS
Molecular Cloning and Sequence Analysis of the Hamster NGT and Canine β4GalT4
In contrast to the human and canine NGT-encoding sequences, a hamster ortholog has not been identified so far. We therefore cloned its sequence using the rapid amplification of cDNA ends strategy (16), thus enabling silencing of NGT expression in CHO and CHO-Lec8 cells. The obtained ORF corresponded to a 326-amino acid protein sharing almost 95% identity with human NGT (data not shown). The sequence was deposited in the EMBL Nucleotide Sequence Database with accession number FN825777.1. A similar approach was applied for identification of the canine β4GalT4-encoding sequence, which resulted in the ORF corresponding to a 344-amino acid protein sharing 86% identity with human β4GalT4 (data not shown). The sequence was deposited in the EMBL Nucleotide Sequence Database with accession number AM989461.1.
Construction of Mammalian NGT- and β4GalT4-deficient Cells
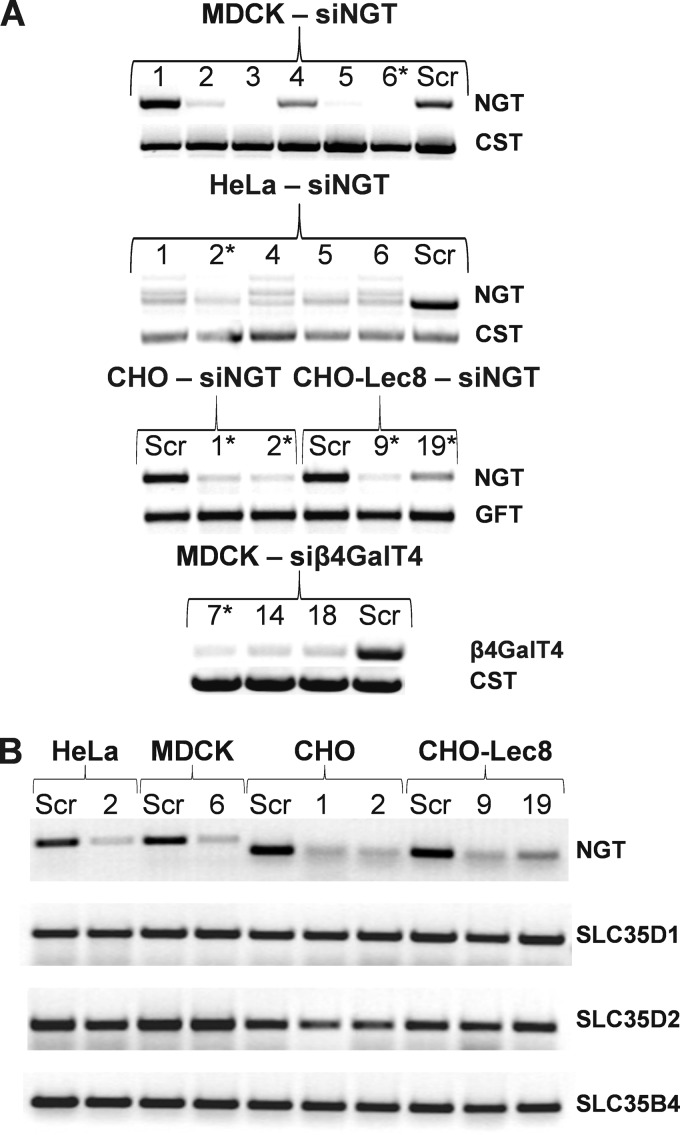
To produce shRNA sequences targeting NGT- or β4GalT4-specific mRNAs, the psiRNA-DUO plasmid was used, which allowed generation of two different siRNAs in parallel, thus increasing inhibition of a desired target. Preliminary data showed that after stable transfection, production of transcripts encoding NGT or β4GalT4 in several clones was significantly decreased (Fig. 1A). In contrast, no significant changes in transcript levels were observed in the case of SLC35D1, SLC35D2, and SLC35B4 transporters (Fig. 1B). In preliminary experiments, several clones were examined, and those subjected to further analyses, from which results are shown in this study, are marked with asterisks.
FIGURE 1.
Analysis of nucleotide sugar transporters and β-1,4-galactosyltransferase 4 expression in NGT-deficient and β4GalT4-deficient mammalian cells. A and B, total RNA was isolated from several stable transfectants, and the RT-PCR reaction was performed using primers designed to NGT and β4GalT4 (A) or NGT and SLC35D1, SLC35D2, and SLC35B4 (B). Sequences encoding fragments of either CMP-sialic acid (CST) or GDP-fucose (GFT) transporters were amplified in parallel as a reference. RT-PCR products were separated in 0.8% agarose gels and visualized with ethidium bromide. Representative data are shown, and clones subjected to further analyses (A), from which results are shown in this study, are indicated with asterisks. B, numbers indicate NGT-deficient clones. CHO, wild-type Chinese hamster ovary cells; CHO-Lec8, CHO cells lacking functional UDP-Gal transporter; HeLa, human cervical carcinoma cell line; siNGT, cells deficient in NGT production; siβ4GalT4, cells deficient in β-1,4-galactosyltransferase 4 production; Scr, scrambled siRNA.
NGT Silencing Reduces Keratan Sulfate Biosynthesis in Mammalian Cells
MDCK-RCAr cells are completely devoid of keratan sulfate due to a mutation in the UGT-encoding gene. As shown in Fig. 2A, NGT silencing also caused a dramatic decrease in keratan sulfate production in MDCK cells. As expected, significantly decreased production of keratan sulfate was also observed in the β4GalT4-deficient cells. In contrast, no changes in heparan sulfate synthesis were observed after NGT silencing (Fig. 2E), as demonstrated by heparan sulfate proteoglycan analysis. Similar results were demonstrated in CHO, CHO-Lec8, and HeLa cells (data not shown).
FIGURE 2.
Analysis of keratan sulfate and heparan sulfate synthesis in NGT-deficient mammalian cells. Proteins present in cell lysates derived from MDCK wild-type cells or cells deficient in NGT (MDCK-siNGT), cells deficient in β4GalT4 (MDCK-siβ4GalT4), or cells deficient in UGT (MDCK-RCAr) were separated by SDS-PAGE (20 μg) and transferred onto nitrocellulose membranes. A and E, keratan sulfate (A) and heparan sulfate proteoglycans (E) were subsequently visualized with specific antibodies followed by incubation with HRP-conjugated secondary antibody. B, C, and D, to demonstrate equal loading, proteins were stained with Coomassie Brilliant Blue G-250 (D) or selected Golgi (GM130; B), and ER (calnexin; C) marker proteins were detected with specific antibodies. Representative data out of three sets with a similar pattern are shown. MDCK-RCAr, Madin-Darby canine kidney II cells resistant to R. communis agglutinin lacking functional UDP-Gal transporter; siβ4GalT4, cells deficient in β4GalT4 production; siNGT, cells deficient in NGT production.
NGT Silencing Reduces N-Glycan Branching in Mammalian Cells
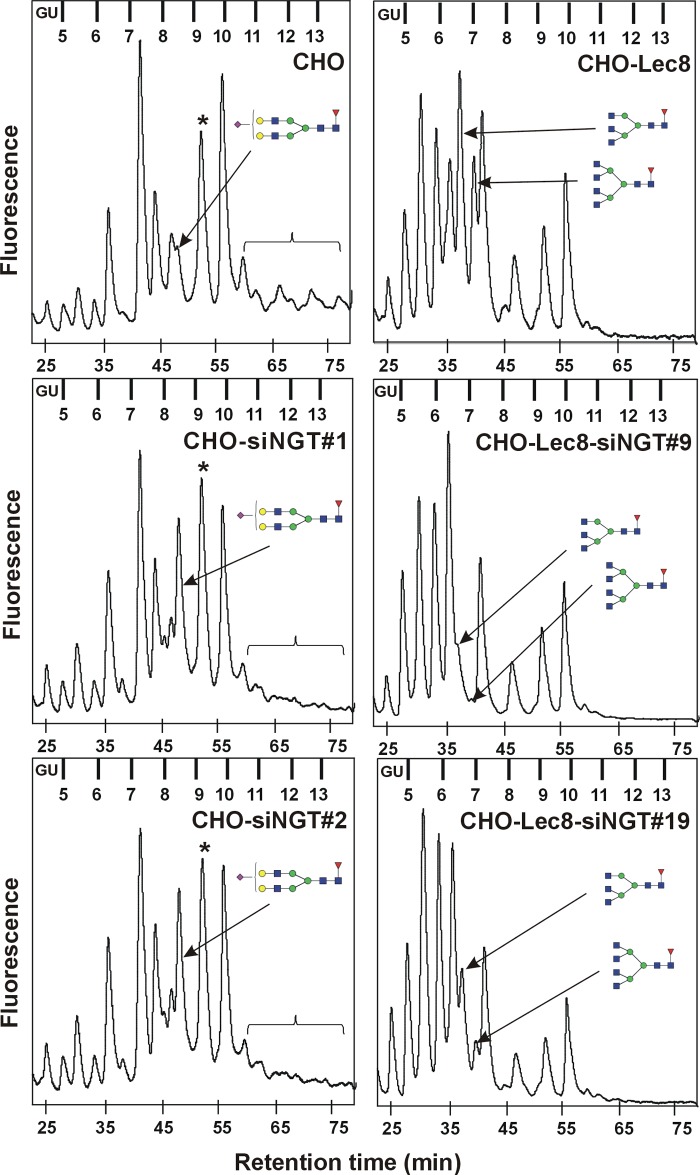
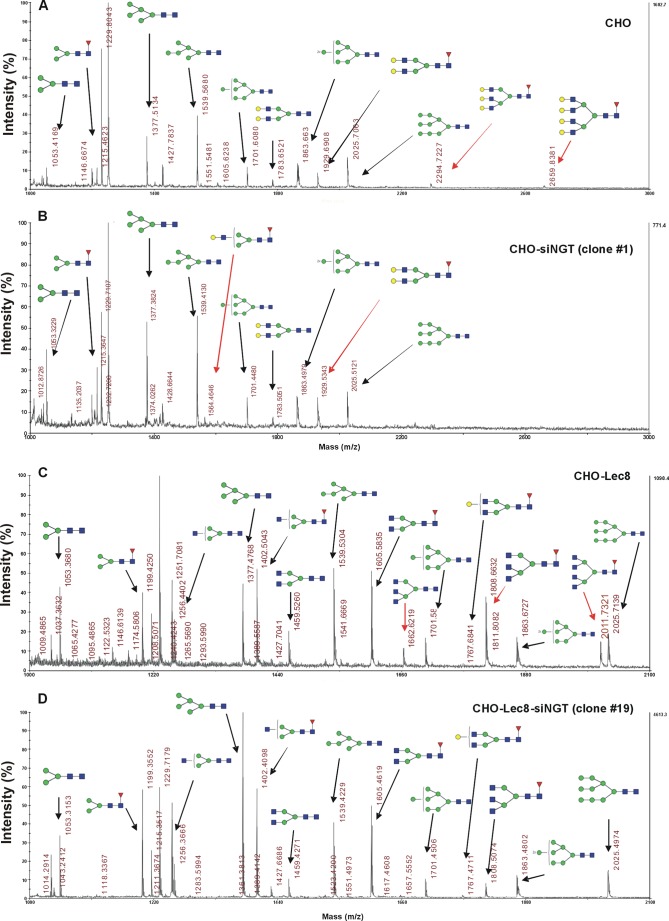
Because all complex N-glycans synthesized in the Golgi apparatus contain GlcNAc, it was reasonable to assume that NGT silencing would alter this type of oligosaccharides. Therefore, we performed a detailed, structural analysis of N-glycan structures using chromatographic separation of fluorescently labeled oligosaccharides released from cellular glycoproteins. Surprisingly, in all examined cells, a reduction in the relative amount of highly branched tri- and tetraantennary complex N-glycans was mainly observed. As shown in Fig. 3, in the case of CHO wild-type cells, a decrease in tri- and tetraantennary oligosaccharide structures with accompanying accumulation of diantennary N-glycans was shown in analyzed oligosaccharide profiles obtained after separation on a GlycoSepN column. Differences in specific oligosaccharide structures were more visible after MALDI-TOF MS identification (the most notable differences are marked in Fig. 4, A and B, with red arrows). The NGT silencing effect was even more profound in CHO-Lec8 mutant cells because they are significantly deficient in terminal Gal and sialic acid residues, thus allowing better observation of N-glycans terminated with GlcNAc. Differences in N-glycan profiles were mostly expressed in a decrease in tri- and tetraantennary oligosaccharides (Fig. 3), comprising a considerable part of the total pool of N-glycans in nonsilenced cells. These results were confirmed in detail by MALDI-TOF MS analysis (the most notable differences are marked in Fig. 4, C and D, with red arrows). In HeLa cells, a similar effect as in CHO wild-type cells was observed, although at a lower intensity (Fig. 5 and data not shown). Analysis of several clones demonstrated that this effect may be explained by lower efficiency of NGT silencing (Fig. 1).
FIGURE 3.
N-Glycan profiles of NGT-deficient mammalian cells. N-Glycans were enzymatically released from glycoproteins produced by CHO (left panel) or CHO-Lec8 (right panel) cells, fluorescently labeled with 2-aminobenzamide, purified, and separated on the GlycoSep N column using HPLC (30). Arrows indicate glycan fractions, comprising oligosaccharides demonstrating the most profound differences. Changes in levels of sialylated tri- and tetraantennary oligosaccharides are shown with brackets. Asterisks indicate peaks comprising increased levels of disialylated and diantennary fucosylated oligosaccharides after NGT silencing. These peaks also comprise high mannose oligosaccharides, the levels of which are not influenced by NGT silencing, as examined by detailed structural analysis. Blue squares, GlcNAc; green circles, Man; yellow circles, Gal; red triangles, fucose; purple rhombs, Sia. For glycans indicated in the left panel: retention time = 48.4 min, glucose units (GU) = 8.46. For glycans indicated in the right panel: retention time = 36.5 min, GU = 6.43 and retention time = 39.0 min, GU = 6.83, top and bottom, respectively. Results for two selected clones are shown. CHO, wild-type Chinese hamster ovary cells; CHO-Lec8, CHO cells lacking functional UDP-Gal transporter; siNGT, cells deficient in NGT production.
FIGURE 4.
Structure analysis of N-glycans from NGT-deficient mammalian cells. A–D, 2-aminobenzamide-labeled N-glycans derived from CHO (A and B) or CHO-Lec8 (C and D) cells were subjected to MALDI-TOF MS analysis carried out in positive ion mode with Na+ excess. Glycans were identified by comparing experimental data expressed in GU resulting from separation on a GlycoSep N column with data deposited in GlycoBase 3.0 followed by comparing these data with molecular weights of respective structures, experimentally determined by MALDI-TOF MS. N-Glycan composition was subsequently estimated using the GlycoMod tool (30). Representative data out of 2–4 independent measurements with a similar tendency are shown. Blue squares, GlcNAc; green circles, Man; yellow circles, Gal; red triangles, fucose. Black arrows, respective peaks; red arrows, peaks corresponding to glycans demonstrating the most profound differences. CHO, wild-type Chinese hamster ovary cells; CHO-Lec8, CHO cells lacking functional UDP-Gal transporter; siNGT, cells deficient in NGT production.
FIGURE 5.

N-Glycan profiles of NGT-deficient HeLa cells. N-Glycans were enzymatically released from glycoproteins produced by HeLa cells, fluorescently labeled with 2-aminobenzamide, purified, and separated on the GlycoSep N column using HPLC (30). Arrows indicate glycan fractions, comprising oligosaccharides, demonstrating the most profound differences. Changes in levels of sialylated tri- and tetraantennary oligosaccharides are shown with brackets. Blue squares, GlcNAc; green circles, Man; yellow circles, Gal; red triangles, fucose; purple rhombs, Sia. For glycan indicated in the figure: retention time = 48.4 min, GU = 8.46. HeLa, human cervical carcinoma cell line; siNGT, cells deficient in NGT production.
NGT Silencing Reduces Both UDP-GlcNAc and UDP-Gal Transport in Mammalian Cells
Because UDP-GlcNAc is considered the main NGT substrate, we isolated the Golgi fraction from NGT-deficient CHO and CHO-Lec8 cells and measured UDP-GlcNAc transport across the Golgi membrane. UDP-GlcNAc transport activity was decreased in NGT-deficient cells when compared with the wild-type cells (Fig. 6), but the effect was not as dramatic as we expected. In the CHO-Lec8 mutant cells defective in UDP-Gal transport and deficient in NGT synthesis, no significant difference was observed when compared with mutant CHO-Lec8 cells. Our previous data showed that NGT is also involved in UDP-Gal delivery to the Golgi apparatus (10, 30). In accordance with those data, here we demonstrated that in NGT-deficient CHO cells, UDP-Gal transport was severely diminished (Fig. 6). This effect was not profound in CHO-Lec8 cells. UDP-Gal transport into Golgi vesicles of previously developed CHO-Lec8 cells stably overexpressing UGT1 (16) was measured as an additional reference.
FIGURE 6.
Nucleotide sugar transport assay. A and B, transport of UDP-GlcNAc (A) and UDP-Gal (B) into mammalian Golgi vesicles. Data are shown as mean ± S.D. from 3–5 independent experiments performed in duplicate. CHO, wild-type Chinese hamster ovary cells; CHO-siNGT, wild-type NGT-deficient cells; CHO-Lec8, mutant cells defective in UDP-Gal transport activity; CHO-Lec8-siNGT, NGT-deficient mutant cells defective in UDP-Gal transport activity; CHO-Lec8+UGT, mutant cells defective in UDP-Gal transport activity overexpressing UGT.
FLIM-FRET Analysis Demonstrates Interaction between NGT and Mgat5
Because NGT silencing resulted in a decrease in the amount of tri- and tetraantennary complex N-glycans, we attempted to investigate putative interactions between NGT and transferases mediating biosynthesis of these structures using the FLIM-FRET approach. For this purpose, A375 cells were transiently transfected with plasmids enabling expression of NGT and Mgat4A or Mgat5 in fusion with mRFP and eGFP, respectively (supplemental Table S2). However, overexpression of Mgat4A in fusion with eGFP resulted in protein mislocalization (data not shown) so that FLIM-FRET experiments could not be conducted. In contrast, both NGT and Mgat5 localized properly to the Golgi apparatus when overexpressed in fusion with respective fluorescent proteins (Fig. 7, A, C, and D). In the FLIM-FRET approach, a reduction in the fluorescence lifetime of the donor fluorophore in the presence of the acceptor fluorophore is indicative of the interaction between analyzed fusion proteins (fluorescence lifetime of the donor fluorophore in the absence of acceptor fluorophore is considered as a reference). We demonstrated that in the absence of an acceptor fluorophore, the mean lifetime of eGFP-tagged Mgat5 was 2.63 ± 0.03 ns (n = 19; Fig. 7, B and K), whereas coexpression with mRFP-tagged NGT reduced it significantly to 2.22 ± 0.18 ns (n = 24; Fig. 7, E and K). A reduction in the fluorescence lifetime was best described by means of a biexponential model, allowing for the distinguishing of two lifetime components: a longer one (2.56 ± 0.1 ns), corresponding to that of the control and resulting from the presence of a noninteracting donor fraction, and a shorter one (1.06 ± 0.19 ns), reflecting donor fraction involved in the energy transfer (differential contribution of this fraction accounts for a slightly higher S.D. that accompanies the mean lifetime value of the donor fluorophore in the presence of the acceptor fluorophore). These data strongly demonstrate very close proximity of NGT and Mgat5 in the Golgi membrane of living cells. One may assume that extensive overexpression of two transmembrane proteins may trigger their nonspecific aggregation. To prove that close proximity of Mgat5 and NGT in the Golgi membrane does not result from such artificial aggregation, we employed a negative control comprising two multi-transmembrane fusion proteins, namely eGFP-B4 (human UDP-xylose/N-acetylglucosamine transporter) and mRFP-UGT2 (human UDP-galactose transporter, splice variant 2). As we expected, both fusion proteins were properly overexpressed in the ER membrane of UGT-deficient MDCK-RCAr cells and displayed significant colocalization (Fig. 7, H and I). In this case, however, we did not observe any reduction in the mean lifetime of the donor fluorophore (i.e. eGFP-B4) in the presence of the acceptor fluorophore (i.e. mRFP-UGT2; Fig. 7, G, J, and L). This observation allowed us to conclude that the interaction between Mgat5 and NGT might occur.
FIGURE 7.
In vivo FLIM-FRET analysis of interaction between Mgat5 and NGT. A–E, confocal intensity-resolved (A, C, and D) and time-resolved (B and E) imaging of eGFP-Mgat5 interaction with mRFP-NGT (C, D, and E) in A375 cells in comparison with cells expressing eGFP-Mgat5 only (A and B). B and E show typical pseudocolored FLIM data representing average donor lifetimes across images. Red-to-blue color changes reflect shortening of the fluorescence lifetime. The rainbow scale bar placed next to time-resolved images (B and E) represents fluorescence lifetime range between 2.3 (blue) and 2.9 ns (red). F–J, confocal intensity-resolved (F, H and I) and time-resolved (G and J) imaging of a control combination comprising noninteracting eGFP-B4 and mRFP-UGT2 (C, D and E) in MDCK-RCAr cells in comparison with cells expressing eGFP-B4 only (F and G). The rainbow scale bar placed next to time-resolved images (G and J) represents fluorescence lifetime range between 2.5 (blue) and 3.5 ns (red). K and L, mean GFP lifetime values in the absence and in the presence of the acceptor are also presented. Data are shown as mean ± S.D. from several measurements of the indicated cell number. Statistically significant (Student's t test, p < 0.001) reduction of GFP lifetime upon coexpression of fluorophore-tagged Mgat5 and NGT was demonstrated by comparing with eGFP-Mgat5 alone (K). No difference in GFP lifetime was found when fluorophore-tagged B4 and UGT2 were coexpressed (L). Bar = 20 μm. τ, fluorescence lifetime; B4, UDP-xylose/N-acetylglucosamine transporter; UGT2, UDP-galactose transporter (splice variant 2); A375, human melanoma cell line; MDCK-RCAr, Madin-Darby canine kidney II cells resistant to R. communis agglutinin.
DISCUSSION
It has been reported that missense mutation (G to T transversion) of evolutionarily conserved valine at position 180 to phenylalanine in the SLC35A3 gene is responsible for a congenital disorder identified in cattle (29). This defect resulted in glycosylation changes of glycoproteins derived from calf cardiac and muscle tissues and probably in defective glycosaminoglycan synthesis, causing severe malformations in animals. In addition, the wild-type gene, but not the mutated one, complemented the Kluyveromyces lactis mutant deficient in UDP-GlcNAc transport. However, no detailed phenotypic analysis of those cells has been performed and, to date, mammalian mutant cell lines defective in NGT activity have not been isolated. Therefore, we developed model studies using wild-type (CHO, MDCK, and HeLa) and mutant mammalian cell lines defective in UGT activity (CHO-Lec8) in which expression of endogenous NGT was significantly decreased. For this purpose, the siRNA approach was successfully employed, resulting in several NGT-deficient mammalian cell lines, which were subsequently analyzed in terms of synthesized N-glycan structures and keratan sulfate production.
Detailed structural analysis of N-glycans was carried out using CHO- and HeLa-derived NGT-deficient cells. It is worth noting that most commonly used lectins did not clearly discriminate between NGT-deficient and wild-type cells (data not shown), which may explain why mutant cell lines lacking NGT activity have not been isolated so far. GlcNAc-containing N-glycans of complex type are synthesized in the Golgi apparatus by the subsequent action of respective mannoside GlcNAc transferases (Mgats). Mono- and diantennary glycans are formed by the actions of mannosyl (α-1,3-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase (Mgat1) and mannosyl (α-1,6-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase (Mgat2), whereas further branching is performed by Mgat4 and Mgat5, resulting in the respective formation of tri- and tetraantennary structures (Fig. 8). Here we demonstrated that NGT silencing caused a decrease in the amount of tri- and tetraantennary N-glycans, whereas diantennary ones remained mainly unaffected or even were accumulated. Based on these findings, we hypothesize that NGT might supply UDP-GlcNAc as a substrate for Golgi-resident glycosyltransferases generating branching of diantennary oligosaccharides, namely Mgat4 and Mgat5. It is possible that UDP-GlcNAc may be delivered as a substrate for Mgat1 and Mgat2 by another transporter because we did not observe decreased activity of these transferases, which was demonstrated by similar mono- and diantennary N-glycan structures produced after silencing of NGT. However, this hypothesis should be viewed with caution, and several reasons should be taken into consideration. One cannot exclude the possibility that differences between activity of Mgat1/Mgat2 and Mgat4/Mgat5 may result from different affinities for UDP-GlcNAc. For example, Mgat1 and Mgat2 were reported to display lower Km values for UDP-GlcNAc than Mgat4 and Mgat5, which are both believed to be limited by concentrations of this nucleotide sugar (35). It should be noted, however, that after significant silencing of NGT (inhibition of transcript production higher than 90%), one may expect a decrease in the number of mono- or diantennary structures containing GlcNAc. Instead we observe a similar amount or even accumulation of such structures in NGT-deficient CHO wild-type and HeLa cells, most likely due to inhibition of further branching. It is also possible that higher structural complexity of N-glycans might affect the rate of biosynthesis, with higher complexity structures requiring higher concentrations of the donor.
FIGURE 8.

Schematic diagram showing selected pathways of N-glycosylation in the Golgi apparatus in mammalian cells. Blue squares, GlcNAc; green circles, Man; red triangle, fucose; Fut8, fucosyltransferase 8, (α-1,6)-fucosyltransferase; NGT, UDP-N-acetylglucosamine transporter (SLC35A3) delivering UDP-GlcNAc.
In this study, we demonstrated for the first time that NGT and Mgat5 are in close proximity in the Golgi membrane, which might significantly facilitate direct supplementation of the latter with UDP-GlcNAc. Unlike in the case of interaction between NGT and UGT (10), we were not able to confirm NGT and Mgat5 association using coimmunoprecipitation analysis. This effect may be caused by technical difficulties in finding proper conditions allowing both the presence of two membrane proteins in solution and the preservation of their native interactions. One may also suspect weaker interaction between NGT and Mgat5 when compared with the two nucleotide sugar transporter molecules because transferases contain only one transmembrane domain that could mediate NGT binding, which may be insufficient for maintaining an intact protein complex during coimmunoprecipitation. To date, putative interactions between NSTs and glycosyltransferases have been suggested, but the only confirmed data have been presented for the interaction between UGT and UDP-galactose:ceramide galactosyltransferase in the ER (36). Here we demonstrate for the first time the close proximity of UDP-GlcNAc transporter with mannoside GlcNAc transferase in the Golgi membrane of living cells.
NGT silencing in the wild-type CHO cells resulted in slightly decreased UDP-GlcNAc transport to the Golgi vesicles, whereas in the mutant CHO-Lec8 cells, a slight increase of the transporting activity was demonstrated. In both cases, observed differences were different from what we expected and were not profound. Determination of transport activity performed in this study is based on analysis of tritium-labeled monosugars, which are transported by nucleotide sugar transporters into the Golgi apparatus and subsequently incorporated into glycoconjugates. Our structural studies (Fig. 4) demonstrated that NGT silencing result in a decreased amount of multiantennary oligosaccharides and accumulation of mono- and diantennary oligosaccharides, which may, at least in part, explain this phenomenon. We suspect that this compensation may be caused by the activity of another nucleotide sugar transporter(s) of UDP-GlcNAc, which delivers the substrate to mono- and diantennary oligosaccharides. Surprisingly, in NGT-deficient CHO wild-type cells, we observed a significant reduction in UDP-Gal transport. This effect was not profound in CHO-Lec8 cells because they do not possess UGT activity, and the observed phenomenon, at least in part, may explain our hypothesis that NGT may play a role in UDP-Gal transport and NGT might be required for the efficient delivery of both UDP-GlcNAc and UDP-Gal to the Golgi apparatus. Recently, we demonstrated that UGT-NGT chimeric transporter restores galactosylation defect in UGT-deficient cells (33). We also showed that UGT and NGT are able to form heterologous complexes in the Golgi membrane (10). NGT silencing would disrupt such functional complexes, thus impairing delivery of both nucleotide sugars to the Golgi apparatus. This hypothesis is now being confirmed by our detailed studies, which are underway.
Silencing NGT activity did not completely abolish attachment of GlcNAc to synthesized oligosaccharides. Based on our data, we cannot exclude the possibility of existence of another NST, which delivers UDP-GlcNAc as a substrate mostly for glycosyltransferases active at early stages of N-glycan synthesis in the Golgi apparatus, resulting in mono- and diantennary structures (Fig. 8). It is highly unlikely that this role may be played by known transporters. When compared with SLC35A3, which is ubiquitously expressed, SLC35D1, SLC35D2, and SLC35B4 are less common and are rather tissue-specific (25–28). Another issue is their differential subcellular localization. Immunofluorescence microscopic analysis of human SLC35A3 overexpressed in CHO (24) and MDCK-RCAr (37) cells and human SLC35D2 overexpressed in CHO cells (25) demonstrated their localization in the Golgi apparatus. On the other hand, analysis of SLC35D1 showed its ER localization (28). Although SLC35B4 has been shown to be located in the Golgi apparatus (27), recently we demonstrated its ER localization (37). Therefore, another UDP-GlcNAc transporter, not yet identified, should be taken into consideration.
NGT function is not only limited to N-glycan synthesis but is also crucial for keratan sulfate synthesis. MDCK cells are not the best model for oligosaccharide studies because they produce mostly N-glycans of high mannose type (30). However, in contrast to CHO cells, which do not produce keratan sulfate, MDCK cells synthesize significant amounts of this glycosaminoglycan (16, 18), which is mainly composed of repeating disaccharide units containing Gal and GlcNAc, sulfated to a various degree. Elongation of the keratan sulfate molecule is performed by the sequential action of β4GalT4 and β-1,3-N-acetylglucosaminyltransferase 7 (β3GnT7) (38). We showed that NGT-deficient MDCK cells exhibit a dramatic decrease in keratan sulfate production when compared with the wild-type cells, suggesting high dependence of β3GnT7 activity on NGT function. Apart from GlcNAc, Gal is another main constituent of keratan sulfate chains. A reduction of UDP-Gal delivery to the Golgi vesicles in NGT-deficient cells may therefore additionally contribute to a decrease in keratan sulfate production. In contrast to keratan sulfate, it seems that NGT does not participate in heparan sulfate synthesis because comparable levels of this compound present in heparan sulfate proteoglycans were detected before and after NGT silencing, even in the mutant CHO-Lec8 cell line after NGT silencing, deficient in both UGT and NGT. It is likely that other transporters, such as SLC35D2 (25), may participate in heparan sulfate synthesis.
The results gained in this study add to the understanding of glycosylation, one of the basic posttranslational modifications, which affects the majority of macromolecules. Our data suggest that NGT function might be coupled to the specific glycosylation pathway(s) of particular macromolecules. We also hypothesize that interaction between NSTs and glycosyltransferases might constitute a general mechanism utilized in the glycosylation process.
Supplementary Material
This work was supported by Grant 2011/03/B/NZ1/02084 from the National Science Center (NCN), Krakow, Poland.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) FN825777.1 and AM989461.1.

This article contains supplemental Tables S1–S3.
- ER
- endoplasmic reticulum
- NST
- nucleotide sugar transporter
- UGT
- UDP-galactose transporter
- NGT
- UDP-N-acetylglucosamine transporter
- MDCK
- Madin-Darby canine kidney II
- eGFP
- enhanced GFP
- mRFP
- monomeric red fluorescent protein
- FLIM
- fluorescence lifetime imaging microscopy
- β4GalT4
- β-1,4-galactosyltransferase 4
- Mgat
- mannoside GlcNAc transferase
- Mgat1
- mannosyl (α-1,3-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase
- Mgat2
- mannosyl (α-1,6-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase
- Mgat4
- mannosyl (α-1,3-)-glycoprotein β-1,4-N-acetylglucosaminyltransferase
- Mgat5
- mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetylglucosaminyltransferase
- GU
- glucose units.
REFERENCES
- 1. Coates S. W., Gurney T., Jr., Sommers L. W., Yeh M., Hirschberg C. B. (1980) Subcellular localization of sugar nucleotide synthetases. J. Biol. Chem. 255, 9225–9229 [PubMed] [Google Scholar]
- 2. Münster A. K., Eckhardt M., Potvin B., Mühlenhoff M., Stanley P., Gerardy-Schahn R. (1998) Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: a nuclear protein with evolutionarily conserved structural motifs. Proc. Natl. Acad. Sci. U.S.A. 95, 9140–9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerardy-Schahn R., Oelmann S., Bakker H. (2001) Nucleotide sugar transporters: Biological and functional aspects. Biochimie 83, 775–782 [DOI] [PubMed] [Google Scholar]
- 4. Caffaro C. E., Hirschberg C. B. (2006) Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc. Chem. Res. 39, 805–812 [DOI] [PubMed] [Google Scholar]
- 5. Eckhardt M., Gotza B., Gerardy-Schahn R. (1999) Membrane topology of the mammalian CMP-sialic acid transporter. J. Biol. Chem. 274, 8779–8787 [DOI] [PubMed] [Google Scholar]
- 6. Olczak M., Guillen E. (2006) Characterization of a mutation and an alternative splicing of UDP-galactose transporter in MDCK-RCAr mutant cell line. Biochim. Biophys. Acta 1763, 82–92 [DOI] [PubMed] [Google Scholar]
- 7. Puglielli L., Hirschberg C. B. (1999) Reconstitution, identification, and purification of the rat liver Golgi membrane GDP-fucose transporter. J. Biol. Chem. 274, 35596–35600 [DOI] [PubMed] [Google Scholar]
- 8. Puglielli L., Mandon E. C., Rancour D. M., Menon A. K., Hirschberg C. B. (1999) Identification and purification of the rat liver Golgi membrane UDP-N-acetylgalactosamine transporter. J. Biol. Chem. 274, 4474–4479 [DOI] [PubMed] [Google Scholar]
- 9. Gao X. D., Dean N. (2000) Distinct protein domains of the yeast Golgi GDP-mannose transporter mediate oligomer assembly and export from the endoplasmic reticulum. J. Biol. Chem. 275, 17718–17727 [DOI] [PubMed] [Google Scholar]
- 10. Maszczak-Seneczko D., Sosicka P., Majkowski M., Olczak T., Olczak M. (2012) UDP-N-acetylglucosamine transporter and UDP-galactose transporter form heterologous complexes in the Golgi membrane. FEBS Lett. 586, 4082–4087 [DOI] [PubMed] [Google Scholar]
- 11. Hong K., Ma D., Beverley S. M., Turco S. J. (2000) The Leishmania GDP-mannose transporter is an autonomous, multi-specific, hexameric complex of LPG2 subunits. Biochemistry 39, 2013–2022 [DOI] [PubMed] [Google Scholar]
- 12. Ishida N., Miura N., Yoshioka S., Kawakita M. (1996) Molecular cloning and characterization of a novel isoform of the human UDP-galactose transporter, and of related complementary DNAs belonging to the nucleotide-sugar transporter gene family. J. Biochem. 120, 1074–1078 [DOI] [PubMed] [Google Scholar]
- 13. Miura N., Ishida N., Hoshino M., Yamauchi M., Hara T., Ayusawa D., Kawakita M. (1996) Human UDP-galactose translocator: molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J. Biochem. 120, 236–241 [DOI] [PubMed] [Google Scholar]
- 14. Oelmann S., Stanley P., Gerardy-Schahn R. (2001) Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 276, 26291–26300 [DOI] [PubMed] [Google Scholar]
- 15. Yoshioka S., Sun-Wada G. H., Ishida N., Kawakita M. (1997) Expression of the human UDP-galactose transporter in the Golgi membranes of murine Had-1 cells that lack the endogenous transporter. J. Biochem. 122, 691–695 [DOI] [PubMed] [Google Scholar]
- 16. Maszczak-Seneczko D., Olczak T., Wunderlich L., Olczak M. (2011) Comparative analysis of involvement of UGT1 and UGT2 splice variants of UDP-galactose transporter in glycosylation of macromolecules in MDCK and CHO cell lines. Glycoconj. J. 28, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brändli A. W., Hansson G. C., Rodriguez-Boulan E., Simons K. (1988) A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J. Biol. Chem. 263, 16283–16290 [PubMed] [Google Scholar]
- 18. Toma L., Pinhal M. A., Dietrich C. P., Nader H. B., Hirschberg C. B. (1996) Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J. Biol. Chem. 271, 3897–3901 [DOI] [PubMed] [Google Scholar]
- 19. Stanley P. (1983) Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic. Cell Genet. 9, 593–608 [DOI] [PubMed] [Google Scholar]
- 20. Hara T., Endo T., Furukawa K., Kawakita M., Kobata A. (1989) Elucidation of the phenotypic change on the surface of Had-1 cell, a mutant cell line of mouse FM3A carcinoma cells selected by resistance to Newcastle Disease virus infection. J. Biochem. 106, 236–247 [DOI] [PubMed] [Google Scholar]
- 21. Ishida N., Yoshioka S., Iida M., Sudo K., Miura N., Aoki K., Kawakita M. (1999) Indispensability of transmembrane domains of Golgi UDP-galactose transporter as revealed by analysis of genetic defects in UDP-galactose transporter-deficient murine Had-1 mutant cell lines and construction of deletion mutants. J. Biochem. 126, 1107–1117 [DOI] [PubMed] [Google Scholar]
- 22. Briles E. B., Li E., Kornfeld S. (1977) Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J. Biol. Chem. 252, 1107–1116 [PubMed] [Google Scholar]
- 23. Guillen E., Abeijon C., Hirschberg C. B. (1998) Mammalian Golgi apparatus UDP-N-acetylglucosamine transporter: molecular cloning by phenotypic correction of a yeast mutant. Proc. Natl. Acad. Sci. U.S.A. 95, 7888–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishida N., Yoshioka S., Chiba Y., Takeuchi M., Kawakita M. (1999) Molecular cloning and functional expression of the human Golgi UDP-N-acetylglucosamine transporter. J. Biochem. 126, 68–77 [DOI] [PubMed] [Google Scholar]
- 25. Ishida N., Kuba T., Aoki K., Miyatake S., Kawakita M., Sanai Y. (2005) Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics 85, 106–116 [DOI] [PubMed] [Google Scholar]
- 26. Suda T., Kamiyama S., Suzuki M., Kikuchi N., Nakayama K., Narimatsu H., Jigami Y., Aoki T., Nishihara S. (2004) Molecular cloning and characterization of a human multisubstrate specific nucleotide-sugar transporter homologous to Drosophila fringe connection. J. Biol. Chem. 279, 26469–26474 [DOI] [PubMed] [Google Scholar]
- 27. Ashikov A., Routier F., Fuhlrott J., Helmus Y., Wild M., Gerardy-Schahn R., Bakker H. (2005) The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J. Biol. Chem. 280, 27230–27235 [DOI] [PubMed] [Google Scholar]
- 28. Muraoka M., Kawakita M., Ishida N. (2001) Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 495, 87–93 [DOI] [PubMed] [Google Scholar]
- 29. Thomsen B., Horn P., Panitz F., Bendixen E., Petersen A. H., Holm L. E., Nielsen V. H., Agerholm J. S., Arnbjerg J., Bendixen C. (2006) A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation. Genome Res. 16, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maszczak-Seneczko D., Olczak T., Jakimowicz P., Olczak M. (2011) Overexpression of UDP-GlcNAc transporter partially corrects galactosylation defect caused by UDP-Gal transporter mutation. FEBS Lett. 585, 3090–3094 [DOI] [PubMed] [Google Scholar]
- 31. Martinez-Duncker I., Mollicone R., Codogno P., Oriol R. (2003) The nucleotide-sugar transporter family: a phylogenetic approach. Biochimie 85, 245–260 [DOI] [PubMed] [Google Scholar]
- 32. Liu L., Xu Y. X., Hirschberg C. B. (2010) The role of nucleotide sugar transporters in development of eukaryotes. Semin. Cell Dev. Biol. 21, 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olczak M., Maszczak-Seneczko D., Sosicka P., Jakimowicz P., Olczak T. (2013) UDP-Gal/UDP-GlcNAc chimeric transporter complements mutation defect in mammalian cells deficient in UDP-Gal transporter. Biochem. Biophys. Res. Commun. 434, 473–478 [DOI] [PubMed] [Google Scholar]
- 34. Olczak M., Watorek W. (2002) Structural analysis of N-glycans from human neutrophil azurocidin. Biochem. Biophys. Res. Commun. 293, 213–219 [DOI] [PubMed] [Google Scholar]
- 35. Lau K. S., Dennis J. W. (2008) N-Glycans in cancer progression. Glycobiology 18, 750–760 [DOI] [PubMed] [Google Scholar]
- 36. Sprong H., Degroote S., Nilsson T., Kawakita M., Ishida N., van der Sluijs P., van Meer G. (2003) Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol. Biol. Cell 14, 3482–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maszczak-Seneczko D., Olczak T., Olczak M. (2011) Subcellular localization of UDP-GlcNAc, UDP-Gal, and SLC35B4 transporters. Acta Biochim. Pol. 58, 413–419 [PubMed] [Google Scholar]
- 38. Kitayama K., Hayashida Y., Nishida K., Akama T. O. (2007) Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J. Biol. Chem. 282, 30085–30096 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.