Background: OSM, a member of IL-6 family of cytokines, is involved in many inflammatory diseases.
Results: OSMRβ−/− mice exhibited phenotypic changes in ATMs to M1, increased proinflammatory cytokines in the adipose tissue, and systemic insulin resistance.
Conclusion: OSMRβ−/− mice exhibited adipose tissue inflammation and insulin resistance preceding obesity.
Significance: OSMRβ−/− mice constitute a unique mouse model of metabolic disorders.
Keywords: Adipose Tissue, Cytokine, Inflammation, Insulin Resistance, Macrophages, Obesity
Abstract
Oncostatin M (OSM), a member of the IL-6 family of cytokines, plays important roles in a variety of biological functions, including inflammatory responses. However, the roles of OSM in metabolic diseases are unknown. We herein analyzed the metabolic parameters of OSM receptor β subunit-deficient (OSMRβ−/−) mice under normal diet conditions. At 32 weeks of age, OSMRβ−/− mice exhibited mature-onset obesity, severer hepatic steatosis, and insulin resistance. Surprisingly, insulin resistance without obesity was observed in OSMRβ−/− mice at 16 weeks of age, suggesting that insulin resistance precedes obesity in OSMRβ−/− mice. Both OSM and OSMRβ were expressed strongly in the adipose tissue and little in some other metabolic organs, including the liver and skeletal muscle. In addition, OSMRβ is mainly expressed in the adipose tissue macrophages (ATMs) but not in adipocytes. In OSMRβ−/− mice, the ATMs were polarized to M1 phenotypes with the augmentation of adipose tissue inflammation. Treatment of OSMRβ−/− mice with an anti-inflammatory agent, sodium salicylate, improved insulin resistance. In addition, the stimulation of a macrophage cell line, RAW264.7, and peritoneal exudate macrophages with OSM resulted in the increased expression of M2 markers, IL-10, arginase-1, and CD206. Furthermore, treatment of C57BL/6J mice with OSM increased insulin sensitivity and polarized the phenotypes of ATMs to M2. Thus, OSM suppresses the development of insulin resistance at least in part through the polarization of the macrophage phenotypes to M2, and OSMRβ−/− mice provide a unique mouse model of metabolic diseases.
Introduction
Obesity is a major factor underlying the development of insulin resistance, which is associated with a number of metabolic disorders, including type 2 diabetes, hypertension, and hyperlipidemia (1). Several lines of evidence now converge on the notion that obesity causes low-grade chronic inflammation characterized by the recruitment of macrophages, T-cells, and neutrophils into the adipose tissue (2–5). Among such inflammatory cells, the increase in adipose tissue macrophages (ATMs)2 is associated with a further deterioration of adipose tissue inflammation and insulin sensitivity (6, 7). In contrast, a decrease in ATMs in obese mice correlates with the amelioration of adipose tissue inflammation and insulin resistance (8, 9). Therefore, ATMs play important roles in the development of the adipose tissue inflammation and insulin resistance associated with obesity.
Macrophages are a heterogeneous cell population and change their physiology in response to various microenvironmental signals. “Classically activated (M1)” macrophages are induced by two signals, IFN-γ and LPS or TNF (10). On the other hand, “alternatively activated (M2)” macrophages are induced by anti-inflammatory cytokines, such as IL-4 and IL-13 (11). In addition, M1 macrophages produce high levels of toxic intermediates (e.g. nitric oxide and reactive oxygen intermediates) via the activation of inducible nitric oxide synthase (iNOS) (12), whereas arginase production is increased in M2 macrophages (13).
It has recently been suggested that a high fat diet triggers the recruitment of M1 macrophages into the adipose tissue, whereas adipose tissue macrophages in lean animals exhibit an M2 phenotype (14). In obese mice, TNF-α, a potent proinflammatory cytokine, is produced by M1 ATMs (7, 15) and directly induces insulin resistance by inhibiting the insulin signaling and insulin-stimulated glucose transport, mainly in the skeletal muscle and white adipose tissue (16, 17). In contrast, M2 ATMs secrete an anti-inflammatory cytokine, IL-10 (15). The administration of IL-10 in diet-induced obese mice enhances the activation of insulin signaling and insulin-stimulated glucose uptake in the skeletal muscle (18). Thus, the balance between M1/M2 ATMs is important for maintaining the proper balance of pro-/anti-inflammatory cytokine production in the adipose tissue, and its imbalance can lead to the development of insulin resistance. However, the mechanisms underlying the determination of the ATM phenotypes are not fully understood.
Oncostatin M (OSM), a member of the IL-6 family of cytokines, exhibits a variety of biological effects depending on the target cells by binding to a heterodimeric membrane receptor comprising the OSM-specific β subunit (OSMRβ) and gp130 (19). OSM is synthesized by various inflammatory cells, such as activated T-cells, neutrophils, eosinophils, and macrophages (20, 21). In addition, the expression of OSMRβ is induced in human peripheral blood monocytes treated with LPS (22), suggesting that OSM plays an important role in monocyte/macrophage lineage cells during inflammation. However, the roles of OSM in ATMs and in metabolic disorders remain to be elucidated. In the present study we have addressed this question using OSMRβ-deficient (OSMRβ−/−) mice.
EXPERIMENTAL PROCEDURES
Animals
Male C57BL/6J mice (8 weeks old) were purchased from Nihon SLC (Hamamatsu, Japan). The generation of OSMRβ−/− mice has been described previously (23). OSMRβ+/+ wild-type (WT) and OSMRβ−/− littermates were obtained from our breeding colony using heterozygous (+/−) breeding pairs. Male and female WT and OSMRβ−/− mice from 8 to 32 weeks old were used in the present study. All mice were housed in specific pathogen-free facilities and under light (12 h light/dark cycle)-, temperature (22–25 °C)-, and humidity (50–60% relative humidity)-controlled conditions. Mice were allowed free access to food (MF; Oriental Yeast, Tokyo, Japan) and water.
Injection of OSM in C57BL/6J Mice
C57BL/6J mice were injected intraperitoneally with either vehicle or recombinant mouse OSM (12.5 ng/g body weight; R & D Systems, Minneapolis, MN) twice a day (10:00 and 18:00 h) for 1 week.
Injection of Sodium Salicylate in OSMRβ−/− Mice
OSMRβ−/− mice were injected intraperitoneally with either vehicle or sodium salicylate (120 μg/g body weight; Sigma) once a day (18:00 h) for 2 weeks.
Isolation of the Adipocyte Fraction and the Stromal Vascular Fraction (SVF)
The mice were deeply anesthetized with diethyl ether, and the epididymal adipose tissue were quickly removed. The adipose tissue was minced into fine pieces and digested with collagenase type 2 (Sigma) with PBS supplemented with 2% FCS at 37 °C for 20 min with high speed shaking. Next, the samples were passed through a 100-μm mesh (BD Biosciences) and fractioned by brief centrifugation (1200 rpm) at room temperature (RT) for 5 min. The floating cells were collected as the adipocyte fraction, and the pellets were collected as the SVF. The cells in the SVF were incubated with ammonium chloride buffer (PharmLyse; BD Biosciences) to lyse the erythrocytes.
Insulin Signaling Analysis
To evaluate insulin signaling, mice fasted for 24 h were intraperitoneally injected with human insulin (10 milliunits/g body weight). Ten minutes later epididymal fat, gastrocnemius muscle, and liver tissue were excised and frozen in liquid nitrogen. Tissue lysates were prepared as described below.
Preparation of Peritoneal Exudate Macrophages (PEMs)
The preparation of PEMs was performed as described previously with some modifications (24). Macrophages elicited in the 3 days after an intraperitoneal injection of 3 ml of thioglycollate medium (BD Biosciences) were harvested by flushing of the peritoneal cavity with Hanks' balanced salt solution (Invitrogen) with plastic syringes, suspended in DMEM (Invitrogen) with 10% FCS, and incubated on 35-mm plastic dishes for 2 h at a density of 1 × 106 cells/dish. Non-adherent cells were discarded, and the adherent cells were cultured at 37 °C for 3 days.
Treatment of PEMs with OSM
PEMs were starved in DMEM with 0.75% bovine serum albumin for 16 h before the stimulation. Then PEMs were treated with PBS or 100 ng/ml concentrations of recombinant mouse OSM and maintained for the appropriate periods.
Cell Culture
Cell culture was performed with some modifications as described previously (25). The mouse macrophage cell line, RAW 264.7, was grown in DMEM (Invitrogen) with 10% FCS, 100 units/ml of penicillin (Invitrogen), and 100 μg/ml of streptomycin (Invitrogen). All cells were grown at 37 °C in a humidified atmosphere of 5% CO2.
Treatment of LPS and OSM for RAW 264.7 Macrophages
RAW 264.7 macrophages were plated in 35-mm dishes at a density of 1 × 106 cells/dish and cultured in a standard medium for 24 h. The cells were then treated with 10 ng/ml lipopolysaccharide (Sigma) for 16 h and washed by a standard medium twice. Then the cells were treated with vehicle or 100 ng/ml recombinant mouse OSM and maintained for 24 h.
Flow Cytometry
The cells in the SVF were incubated with anti-CD16/CD32 antibodies (1:100, BD Biosciences) to block Fc binding at 4 °C for 20 min followed by incubation with fluorescently labeled primary antibodies or control IgG at 4 °C for 30 min. The FITC-conjugated anti-F4/80, FITC-conjugated rat IgG2a isotype controls, phycoerythrin (PE)-conjugated anti-CD11c, PE-conjugated Armenian hamster IgG2a isotype controls, FITC-conjugated anti-Gr-1, FITC-conjugated rat IgG2b isotype controls, PE-conjugated anti-CD11b, and PE-conjugated rat IgG2b isotype controls were purchased from eBiosciences (San Diego, CA). The PE- or Alexa Fluor 647-conjugated anti-CD206 and their isotype controls were purchased from AbD Serotec (Oxford, UK). To detect OSMRβ in the SVF and PEMs, cells were incubated with goat anti-OSMRβ antibodies (diluted at 1: 5, R&D Systems) or control goat IgG (Jackson ImmunoResearch, West Grove, PA) at 4 °C for 30 min. Then the cells were incubated with PE-conjugated donkey anti-goat IgG (diluted at 1: 20, R&D Systems). The stained cells were analyzed using the C6 flow cytometer (BD Biosciences) or the FACSCalibur flow cytometer (BD Biosciences). Dead cells were removed from the analysis using propidium iodide staining. The flow cytometry results were analyzed using the CFlow (BD Biosciences), the CellQuest software program (BD Biosciences), or FlowJo software suites (Tree Star, Ashland, OR). The events were first gated based on forward scatter versus propidium iodide to identify individual live cells. The plot of a forward versus side scatter was used as the second gate to gate out aggregates and debris. Next, the F4/80-positive cells were selected. Single color controls were used to set the compensation and gates.
Western Blot Analysis
Western blot analysis was performed with some modifications as described previously (25). Lysates were prepared by using radioimmune precipitation assay buffer (Upstate Biotechnology, Lake Placid, NY) containing protease inhibitor mixture (Upstate Biotechnology), 1 mm orthovanadate, 1 mm sodium fluoride, and 1 mm phenylmethylsulfonyl fluoride. The protein concentrations in the lysates were determined by using a BCA Protein Assay kit (Pierce). Twenty micrograms of protein from the samples were separated by SDS-PAGE and transferred to PVDF membranes (GE Healthcare). The blotted membranes were incubated with rat anti-CD206 antibody (diluted at 1:500, AbD Serotec), rabbit anti-CD163 antibody (diluted at 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-iNOS antibody (diluted at 1:500, Abcam, Cambridge, UK), mouse anti-arginase-1 antibody (BD Biosciences), rabbit anti-phosphorylated Akt antibody (diluted at 1:1000, Cell Signaling Technology, Beverly, MA), rabbit anti-Akt antibody (diluted at 1:1000, Cell Signaling Technology), rabbit anti-phosphorylated STAT3 antibody (diluted at 1:1000, Cell Signaling Technology), rabbit anti-STAT3 antibody (diluted at 1:1000, Cell Signaling Technology), rabbit anti-phosphorylated cAMP response element-binding protein (CREB) antibody (diluted at 1:1000, Cell Signaling Technology), and rabbit anti-CREB antibody (diluted at 1:1000, Cell Signaling Technology). Then the membranes were incubated with HRP-conjugated donkey anti-goat (diluted at 1:4,000, GE Healthcare), donkey anti-rat (diluted at 1:10,000, Jackson ImmunoResearch), donkey anti-rabbit (diluted at 1:20,000, GE Healthcare), or donkey anti-mouse (diluted at 1:20,000, GE Healthcare) IgG antibodies. Labeled proteins were detected with chemiluminescence using ECL detection reagent (GE Healthcare) according to the manufacturer's instructions. The membranes were exposed to hyperfilm ECL (GE Healthcare) for an appropriate period. The blotted membranes were stripped in 0.25 m glycine, pH 2.5, at RT for 10 min and incubated with rat anti-tubulin antibody (diluted at 1:500; Abcam) at 4 °C for 16 h followed by the incubation with HRP-conjugated donkey anti-rat antibody (diluted at 1:4000) at RT for 1 h.
Immunohistochemistry
Immunofluorescence staining was performed with some modifications as described previously (26, 27). Briefly, mice were deeply anesthetized with diethyl ether, and the epididymal fat pads were quickly removed. Then the fat pads were fixed with 1% paraformaldehyde in PBS at 4 °C for 1 h followed by the preincubation in 5% normal donkey serum at RT for 1 h. Then the fat pads were incubated with goat anti-OSM antibody (diluted at 1:400), goat anti-OSMRβ antibody (diluted at 1:400), rat anti-F4/80 antibody (diluted at 1:1000; AbD Serotec), and rabbit anti-caveolin-1 antibody (diluted at 1:400; BD Biosciences). The fat pads were incubated with Cy2-conjugated, Cy3-conjugated, or biotinylated secondary antibodies (diluted at 1:800; Jackson ImmunoResearch) at RT for 1 h. Then the fat pads were incubated with 7-amino-4-methylcoumarin-3-acetic acid-conjugated streptavidin (diluted at 1:500; Jackson ImmunoResearch) at RT for 30 min and mounted in the mounting media (90% glycerol and 10% PBS) on the chambered slide. Immunofluorescence images were acquired using a confocal laser scanning microscope (LSM700; Carl Zeiss, Tokyo, Japan).
To complete an immunohistochemical analysis in pancreas, mice were deeply anesthetized with diethyl ether and transcardially perfused with ice-cold 0.85% NaCl followed by ice-cold Zamboni's fixative (2% paraformaldehyde and 0.2% picric acid in 0.1 m PBS). Tissues were quickly removed, postfixed in the same fixative at 4 °C for 3 h, and cryoprotected in 20% sucrose in 0.1 m PBS. All specimens were frozen rapidly in cold n-hexane on dry ice and stored at −80 °C. Frozen sections were cut on a cryostat (6-μm thickness). The sections were preincubated in 5% normal donkey serum at RT for 1 h followed by the incubation with rabbit anti-insulin antibody (diluted at 1:400; Abcam). Then they were incubated with biotinylated donkey anti-rabbit IgG antibody (diluted at 1:800; Jackson ImmunoResearch) at RT for 1 h followed by incubation with HRP-conjugated streptavidin (DAKO, Carpinteria, CA) at RT for 30 min. Thereafter, the peroxidase reaction product was visualized with 0.05% diaminobenzidine tetrahydrochloride (Sigma) and 0.01% H2O2. After the reaction, the sections were counterstained with Eosin Y (Muto Pure Chemical, Tokyo, Japan). Images were acquired by using a BIOREVO BZ-9000 microscope (KEYENCE, Osaka, Japan). To evaluate the area of β-cell in pancreas, every 20th section was selected from a series of consecutive pancreatic sections (6 μm), and 12 sections per mouse were used for analysis. For each section the cells were considered to be positive for insulin if the cell bodies were stained brown. The area of β-cells and pancreas was measured by using Image J analysis software (Version 1.46r, Scion, Frederick, MD).
The following controls were performed: (i) incubation with protein A-purified goat or rabbit IgG instead of primary antibody; (ii) incubation without the primary antibody or without primary and secondary antibodies. None of the controls revealed any labeling (data not shown).
Measurement of Blood Glucose and Serum Insulin
These procedures were performed with some modifications as described previously (28). Mice were fasted for 4 h to remove the effects of food intake on glucose metabolism, and blood was taken from the tail vein at 18:00 h. In fasting experiments, mice were fasted for 24 h with free access to water. Then serum was immediately collected and stored at −20 °C. Blood glucose levels were measured by a glucose measurement device (Glucocard GT-1640, Arkray, Kyoto, Japan). The serum insulin concentrations were determined using kits from Morinaga (Tokyo, Japan).
Intraperitoneal Glucose Tolerance Test (ipGTT) and Insulin Tolerance Test (ITT)
For ipGTT, the mice were fasted for 16 h and received an intraperitoneal injection of d-glucose (1 g/kg body weight). The blood samples were collected from the tail vein before and at 15, 30, 60, and 120 min after the injection of d-glucose. For ITT, mice were fasted for 4 h and received an intraperitoneal injection of insulin (1 unit/kg body weight). The blood samples were collected from the tail vein before and at 15, 30, 60, and 120 min after the injection of insulin.
ELISA
Concentrations of serum TNF-α, IL-6, IL-10, adiponectin, and monocyte chemoattractant protein-1 (MCP-1) were measured by ELISA kits (R & D Systems) according to the manufacturer's instructions. The serum concentrations of leptin, serum amyloid A, and OSM were determined using ELISA kits from Morinaga, Invitrogen, and USCN Life Science (Wuhan, China), respectively.
Measurement of Lipid Content in the Serum and Liver
The serum levels of triglycerides, total cholesterol, and free fatty acids were measured at Nagahama Life Science Laboratory (Nagahama, Japan) using lipid assay kits (Triglyceride E-Test Wako, Total Cholesterol E-Test Wako, and NEFA C-Test Wako, Wako Pure Chemical Industries, Osaka, Japan) according to the manufacturer's instructions.
The contents of the triglycerides and total cholesterol in the liver were analyzed at Skylight Biotech (Akita, Japan). Lipids were extracted from the livers using the Folch method (29). Frozen liver tissues were homogenized, and triglycerides and total cholesterol were extracted from the homogenate with chloroform/methanol (2:1, v/v), dried, and resuspended in 2-propanol. The amounts of triglycerides and total cholesterol in the extract were measured using lipid assay kits (Cholestest TG and Cholestest CHO, Sekisui Medical, Tokyo, Japan).
Quantitative Real-time PCR
Quantitative real-time PCR was performed with some modifications as described previously (25). Briefly, total RNAs from PEMs were prepared using TRI reagent (Molecular Research Center, Cincinnati, OH). The cDNA from the total RNA was synthesized with TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). The following TaqMan Gene Expression Assays (Applied Biosystems) were used: TNF-α (Mm00443258_m1), IL-1β (Mm00434228_m1), IFN-γ (Mm00801778_m1), MCP-1 (Mm00441242_m1), C-C chemokine receptor 2 (CCR2) (Mm00438270_m1), toll-like receptor 4 (TLR4) (Mm00445273_m1), IL-6 (Mm00446190_m1), IL-10 (Mm00439616_m1), IL-13 (Mm00434204_m1), adiponectin (Mm00456425_m1), macrophage galactose-type C-type lectin (MGL) 1 (Mm00546124_m1), MGL2 (Mm00460844_m1), OSM (Mm01193966_m1), OSMRβ (Mm00495424_m1), and 18 S (Hs99999901_s1). Quantitative real-time PCR for each gene was performed using Rotor Gene Q (Qiagen, Hilden, Germany) and Rotor Gene Probe PCR kits (Qiagen). The PCR amplification protocol was 95 °C for 10 min and then 40 cycles of 95 °C for 10 s and 60 °C for 45 s. The relative abundance of transcripts was normalized by the expression of 18 S mRNA and analyzed using ΔΔCT method.
Statistical Analysis
The results are shown as the means ± S.E. Statistically significant differences between groups were analyzed by Student's t test or an analysis of variance (ANOVA) followed by the post-hoc Bonferroni test. The criterion for statistical significance was p < 0.05.
RESULTS
Systemic Changes of Metabolic Parameters in OSMRβ−/− Mice
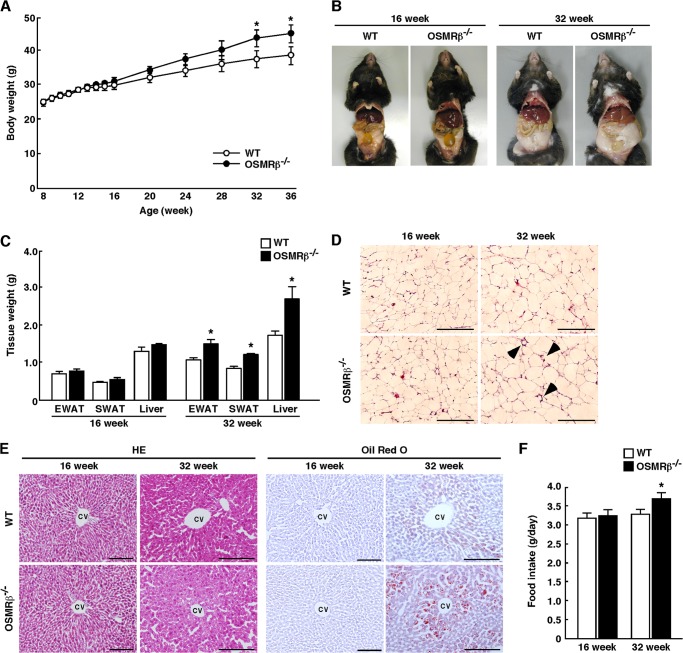
To assess the roles of OSMRβ in the metabolic diseases, we analyzed OSMRβ−/− mice under normal diet conditions. The macroscopic findings, body weights, and tissue weights (epididymal adipose tissue, subcutaneous adipose tissue, and liver) did not differ significantly between WT and OSMRβ−/− mice until 16 weeks of age (Fig. 1, A–C). However, OSMRβ−/− mice began to be heavier than WT mice at 20 weeks of age, and the significant increase in body weights compared with WT mice was observed in OSMRβ−/− mice at 32 weeks of age (Fig. 1A). At 32 weeks of age, the weights of the adipose tissue and liver in OSMRβ−/− mice were heavier than those in WT mice (Fig. 1, B and C). As shown in Fig. 1D, in the adipose tissue of OSMRβ−/− mice the adipocytes appeared to be larger than those in WT mice, and crown-like structures were also observed. In addition, lipid accumulation was greater in the livers of OSMRβ−/− mice compared with that in WT mice at 32 weeks of age (Fig. 1E). However, the adipose tissue and liver were histologically normal in OSMRβ−/− mice at 16 weeks of age (Fig. 1, D and E). Although there was no difference in the amount of food intake between WT and OSMRβ−/− mice at 16 weeks of age, OSMRβ−/− mice showed hyperphagia at 32 weeks of age (Fig. 1F).
FIGURE 1.
The characteristics of WT and OSMRβ−/− mice under normal diet conditions. A, shown are the body weights of WT and OSMRβ−/− mice from 8 to 36 weeks of age (n = 6–10). B, shown are representative images of WT and OSMRβ−/− mice at 16 and 32 weeks of age. C, shown are the tissue weights in WT and OSMRβ−/− mice at 16 and 32 weeks of age (n = 6–10). EWAT, epididymal white adipose tissue; SWAT, subcutaneous white adipose tissue. D, shown is a histological analysis with H&E (HE) staining in the EWAT in WT and OSMRβ−/− mice at 16 and 32 weeks of age. Arrowheads indicate crown-like structures. Scale bars = 200 μm. E, shown is a histological analysis with H&E staining and Oil Red O staining in the liver of WT and OSMRβ−/− mice at 16 and 32 weeks of age. CV, central vein. Scale bars = 100 μm. F, food intake in WT and OSMRβ−/− mice at 16 and 32 weeks of age (n = 6–10) is shown. The data represent the mean ± S.E. *, p < 0.05 WT versus OSMRβ−/− mice, ANOVA followed by the post-hoc Bonferroni test (A); Student's t test (C and F).
The serum concentration of leptin in OSMRβ−/− mice was higher than that in WT mice at 32 weeks of age, although it was not changed between WT and OSMRβ−/− mice at 16 weeks of age (Table 1). Both serum lipid levels (total cholesterol, triglyceride, and free fatty acid) and lipid contents in the liver (total cholesterol and triglyceride) were higher in OSMRβ−/− mice at 32 weeks of age (Table 1). Neither the serum lipid levels nor lipid contents in the liver were different between WT and OSMRβ−/− mice at 16 weeks of age (Table 1).
TABLE 1.
Various metabolic parameters in the serum and liver of WT and OSMR β−/− mice at 16 and 32 weeks of age (n = 6–8)
The data represent the mean ± S.E.
| Parameters (units) | WT (16 weeks) | OSMRβ−/− (16 weeks) | WT (32 weeks) | OSMRβ−/− (32 weeks) |
|---|---|---|---|---|
| Serum concentrations | ||||
| Total cholesterol (mg/dl) | 94.2 ± 8.1 | 90.2 ± 3.1 | 102.7 ± 4.6 | 141.3 ± 0.7a |
| Triglyceride (mg/dl) | 107.0 ± 10.0 | 102.7 ± 13.1 | 87.7 ± 8.3 | 102.0 ± 5.8a |
| Free fatty acid (mmol/liter) | 1.40 ± 0.16 | 1.51 ± 0.07 | 1.90 ± 0.02 | 1.98 ± 0.01a |
| Leptin (ng/ml) | 5.97 ± 0.39 | 7.32 ± 1.63 | 12.6 ± 2.8 | 18.8 ± 2.5a |
| Serum amyloid A (ng/ml) | 8.84 ± 1.74 | 14.24 ± 1.93a | 14.9 ± 1.4 | 30.6 ± 6.4a |
| TNF-α (pg/ml) | 3.61 ± 0.24 | 4.67 ± 0.45a | 4.22 ± 0.62 | 5.69 ± 0.85a |
| IL-6 (pg/ml) | 0.27 ± 0.05 | 0.31 ± 0.08 | 0.51 ± 0.06 | 0.68 ± 0.06a |
| MCP-1 (pg/ml) | 20.1 ± 0.6 | 24.3 ± 2.1a | 46.9 ± 7.6 | 84.2 ± 11.2a |
| IL-10 (pg/ml) | 7.04 ± 0.57 | 5.55 ± 0.43a | 10.6 ± 0.6 | 9.08 ± 0.61a |
| Adiponectin (μg/ml) | 25.1 ± 2.1 | 22.2 ± 0.8a | 15.3 ± 0.7 | 13.2 ± 0.5a |
| OSM (pg/ml) | 64.7 ± 8.1 | 60.92 ± 7.7 | 46.9 ± 9.7 | 73.5 ± 11.8a |
| Glucose (fed) (mg/dl) | 135.3 ± 12.7 | 152.0 ± 9.1 | 162.0 ± 16.3 | 198.3 ± 18.1a |
| Insulin (fed) (ng/ml) | 0.66 ± 0.05 | 5.41 ± 1.60a | 2.10 ± 0.11 | 9.98 ± 0.60b |
| Glucose (fasted) (mg/dl) | 68.7 ± 2.6 | 64.0 ± 3.1 | 80.0 ± 4.2 | 89.7 ± 2.9a |
| Insulin (fasted) (ng/ml) | 0.31 ± 0.09 | 2.21 ± 0.71a | 0.96 ± 0.33 | 5.63 ± 0.21b |
| Liver concentrations | ||||
| Total cholesterol (mg/g) | 2.03 ± 0.10 | 2.23 ± 0.06 | 2.10 ± 0.03 | 3.12 ± 0.20a |
| Triglyceride (mg/g) | 9.82 ± 3.21 | 13.6 ± 2.9 | 15.2 ± 0.4 | 105.4 ± 18.4b |
a p < 0.05 WT versus OSMRβ−/− mice; Student's t test.
b p < 0.01 WT versus OSMRβ−/− mice; Student's t test.
To investigate the systemic inflammation in OSMRβ−/− mice, we analyzed the serum levels of some inflammatory markers. The serum concentrations of serum amyloid A, TNF-α, IL-6, and MCP-1 were higher, whereas the concentrations of IL-10 and adiponectin in the serum were lower in OSMRβ−/− mice than in WT mice at 32 weeks of age (Table 1). The changes in those inflammatory markers, except for IL-6, were already observed at 16 weeks of age (Table 1). Serum concentration of IL-6 also tended to increase but not significantly in OSMRβ−/− mice at 16 weeks of age (Table 1).
At 32 weeks of age, both blood glucose and serum insulin levels in fed and fasted conditions were higher in OSMRβ−/− mice compared with those in WT mice (Table 1). At 16 weeks of age, there were no differences in blood glucose levels between WT and OSMRβ−/− mice, whereas serum insulin levels were higher in OSMRβ−/− mice in both fed and fasted conditions (Table 1).
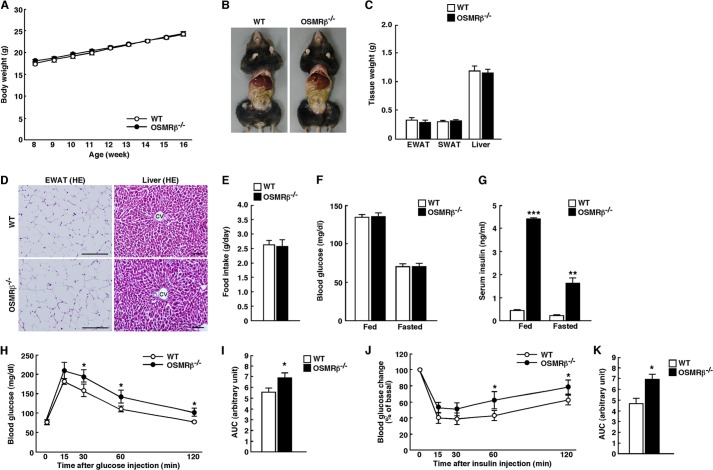
Similar to the male mice, neither female WT nor female OSMRβ−/− mice showed any differences in body weight, tissue weights, or food intake (see Fig. 3, A–E). The blood glucose levels did not differ between WT and OSMRβ−/− mice; however, the serum insulin levels were increased in OSMRβ−/− mice in fed and fasted conditions at 16 weeks of age (see Fig. 3, F and G). These results suggest that OSMRβ−/− mice exhibit systemic inflammation and disturbance of glucose metabolism preceding obesity. We next analyzed glucose and insulin levels in more detail.
FIGURE 3.
The characteristics of female WT and OSMRβ−/− mice under normal diet conditions. A, shown are the body weights of female WT and OSMRβ−/− mice from 8 to 16 weeks of age (n = 6). B, shown are representative images of female WT and OSMRβ−/− mice at 16 weeks of age. C, shown are the tissue weights in female WT and OSMRβ−/− mice at 16 weeks of age (n = 6). EWAT, epididymal white adipose tissue; SWAT, subcutaneous white adipose tissue. D, shown is a histological analysis with H&E (HE) staining in the epididymal white adipose tissue and liver in female WT and OSMRβ−/− mice at 16 weeks of age. CV, central vein. Scale bars = 200 μm (epididymal white adipose tissue); 100 μm (liver). E, shown is food intake in female WT and OSMRβ−/− mice at 16 weeks of age (n = 6). F and G, shown are blood glucose (F) and serum insulin (G) levels in female WT and OSMRβ−/− mice at 16 weeks of age under fed and fasted conditions. H–K, shown are the results of the ipGTTs (H) and ITTs (J) in female WT and OSMRβ−/− mice at 16 weeks of age (n = 6). The areas under the curves (AUC) for blood glucose in the ipGTTs (I) and ITTs (K) are shown. The data represent the mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.005 WT versus OSMRβ−/− mice, ANOVA followed by the post-hoc Bonferroni test (A, H, and J); Student's t test (C, E, F, G, I, and K).
OSMRβ−/− Mice Develop Glucose Intolerance and Insulin Resistance
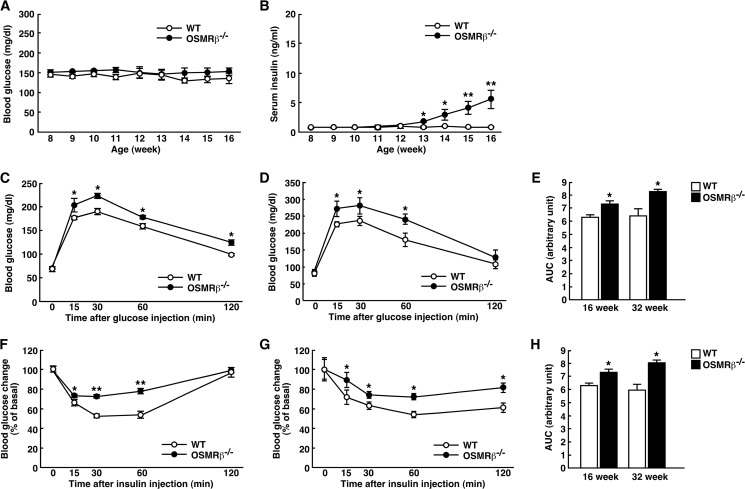
To examine the effects of OSMRβ deficiency on glucose metabolism, we measured the blood glucose and serum insulin levels once a week for 8 weeks in WT and OSMRβ−/− mice under normal diet conditions. As shown in Fig. 2A, there were no significant changes in the blood glucose levels between WT and OSMRβ−/− mice until 16 weeks of age. However, the serum insulin levels began to increase in OSMRβ−/− mice compared with those observed in WT mice starting from 13 weeks of age (Fig. 2B). In addition, ipGTTs and ITTs revealed that OSMRβ−/− mice displayed glucose intolerance and insulin resistance at both 16 and 32 weeks of age (Fig. 2, C–H). Consistent with the data in the male mice, the female OSMRβ−/− mice at 16 weeks of age exhibited glucose intolerance and insulin resistance, as measured with ipGTTs and ITTs (Fig. 3, H–K).
FIGURE 2.
Glucose intolerance and insulin resistance in OSMRβ−/− mice under normal diet conditions. A and B, shown are blood glucose (A) and serum insulin (B) levels in WT and OSMRβ−/− mice from 8 to 16 weeks of age (n = 6). C–H, shown are the results of the ipGTTs (C and D) and ITTs (F and G) in WT and OSMRβ−/− mice at 16 (C and F) and 32 (D and G) weeks of age (n = 6). The areas under the curves (AUC) for blood glucose in the ipGTTs (E) and ITTs (H) are shown. The data represent the mean ± S.E. *, p < 0.05; **, p < 0.01 WT versus OSMRβ−/− mice, ANOVA followed by the post-hoc Bonferroni test (A–D, F, and G); Student's t test (E and H).
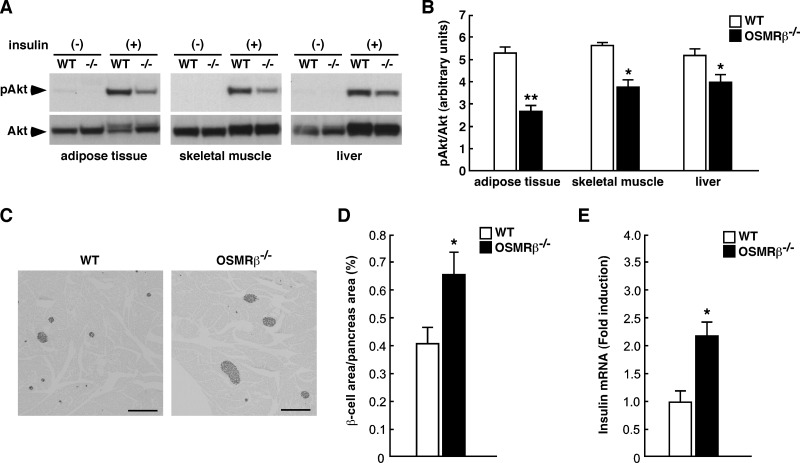
To investigate the tissue-specific insulin resistance in OSMRβ−/− mice at 16 weeks of age, we analyzed insulin-stimulated Akt phosphorylation in the adipose tissue, skeletal muscle, and liver. Insulin-stimulated Akt phosphorylation was decreased in the adipose tissue, skeletal muscle, and liver in OSMRβ−/− mice compared with that observed in WT mice (Fig. 4, A and B). Histological examination of pancreas revealed that the percentages of insulin-positive areas (β-cells) among total areas of the pancreas were higher in OSMRβ−/− mice compared with those in WT mice at 16 weeks of age (Fig. 4, C and D), suggesting that OSMRβ−/− mice exhibit hyperplasia of β-cells in the pancreas. In addition, the expression of insulin mRNA was increased in the pancreas of OSMRβ−/− mice compared with that in WT mice at 16 weeks of age (Fig. 4E).
FIGURE 4.
Reduced insulin signaling and pancreatic β-cell hyperplasia in OSMRβ−/− mice at 16 weeks of age under normal diet conditions. A, shown is insulin-stimulated Akt phosphorylation in the adipose tissue, skeletal muscle, and liver of WT and OSMRβ−/− mice. B, shown is quantitative analysis of phosphorylation of Akt in the adipose tissue, skeletal muscle, and liver of WT and OSMRβ−/− mice (n = 6). C–E, shown are hyperplasia of β-cells and insulin production in the pancreas of WT and OSMRβ−/− mice. C, shown is immunohistochemistry for insulin (black) in the pancreas of WT and OSMRβ−/− mice. Scale bars = 500 μm. D, shown is quantitative analysis of the area of β-cells in the total area of the pancreas. E, shown is the mRNA expression of insulin in the pancreas of WT and OSMRβ−/− mice. The data represent the mean ± S.E. *, p < 0.05; **, p < 0.01 WT versus OSMRβ−/− mice, Student's t test (B, D, and E).
Adipose Tissue Inflammation and Phenotypes of ATMs in OSMRβ−/− Mice
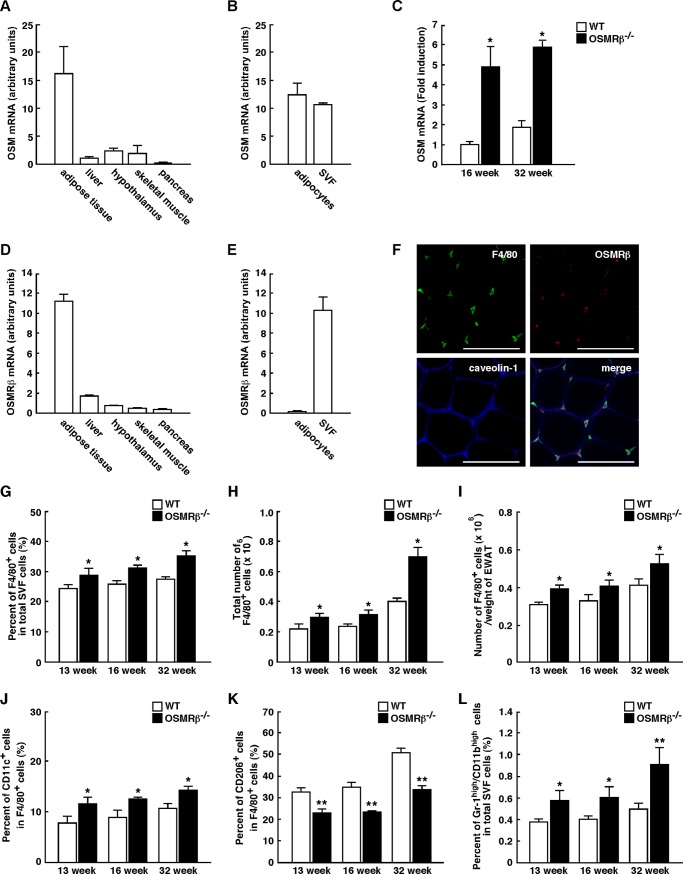
It is well established that obesity-induced adipose tissue inflammation is important for the development of insulin resistance (5). Both OSM and OSMRβ were expressed strongly in the adipose tissue and little in the liver, hypothalamus, skeletal muscle, and pancreas at 16 weeks of age (Fig. 5, A and D). In the adipose tissue, the expression of OSM was observed in both SVF and adipocyte fractions (Fig. 5B). In contrast, OSMRβ was mainly expressed in the SVF, whereas the expression of OSMRβ was rarely detected in the adipocyte fraction (Fig. 5E).
FIGURE 5.
Phenotypes of ATMs in WT and OSMRβ−/− mice under normal diet conditions. A–F, shown is expression of OSM and OSMRβ in the adipose tissue. A, shown is the mRNA expression of OSM in various tissues of C57BL/6J mice (n = 6). B, shown is mRNA expression of OSM in the SVF and adipocyte fraction in the adipose tissue of C57BL/6J mice (n = 6). C, shown is mRNA expression of OSM in the adipose tissue of WT and OSMRβ−/− mice at 16 and 32 weeks of age (n = 6). D, shown is mRNA expression of OSMRβ in various tissues of C57BL/6J mice (n = 6). E, shown is mRNA expression of OSMRβ in the SVF and adipocyte fraction in the adipose tissue of C57BL/6J mice (n = 6). F, shown is immunofluorescence staining for OSMRβ (red) with F4/80 (green) and caveolin-1 (blue) in the adipose tissue of C57BL/6J mice. Scale bars = 100 μm. G–I, shown are the percentages (G) and total numbers (H) of F4/80-positive cells among the total numbers of cells in the SVF of the epididymal fat pads in WT and OSMRβ−/− mice at 13, 16, and 32 weeks of age (n = 4–6). The total numbers of macrophages were normalized by the weights of the epididymal fat pads (I). J and K, shown are the percentages of CD11c-positive (J) and CD206-positive (K) cells in the F4/80-positive cells of WT and OSMRβ−/− mice at 13, 16, and 32 weeks of age (n = 4–6). L, shown are the percentages of neutrophils (Gr-1high/CD11bhigh cells) in the total cells in the SVF of WT and OSMRβ−/− mice at 13, 16, and 32 weeks of age (n = 4–6). The data represent the mean ± S.E. *, p < 0.05; **, p < 0.01 WT versus OSMRβ−/− mice, Student's t test.
Serum concentration of OSM was not changed between WT and OSMRβ−/− mice at 16 weeks of age (Table 1). However, the expression of OSM mRNA in the adipose tissue was increased in OSMRβ−/− mice compared with that in WT mice (Fig. 5C). At 32 weeks of age, both serum concentration of OSM and the expression of OSM mRNA in the adipose tissue were higher in OSMRβ−/− mice compared with those in WT mice (Fig. 5C and Table 1).
In addition, double-immunofluorescence staining revealed that OSMRβ was exclusively expressed in F4/80-positive macrophages in the adipose tissue (Fig. 5F). Therefore, OSM appears to act primarily on macrophages in the adipose tissue.
Next we examined the phenotypes of ATMs in OSMRβ−/− mice under normal diet conditions. The flow cytometric analyses of the SVF showed that the percentages and total numbers of F4/80-positive cells were increased in OSMRβ−/− mice compared with those observed in WT mice at 13, 16, and 32 weeks of age (Fig. 5, G–I). To discriminate between M1 and M2 ATMs with flow cytometry, we used antibodies against CD11c and CD206 as markers of M1 and M2 ATMs, respectively (15). The percentages of CD11c-positive M1 ATMs among the total numbers of ATMs were higher in OSMRβ−/− mice than in WT mice (Fig. 5J). In contrast, the percentages of CD206-positive M2 ATMs among the total numbers of ATMs were lower in OSMRβ−/− mice than in WT mice (Fig. 5K). In addition, the percentages of neutrophils, which highly expressed both Gr-1 and CD11b, among total SVF cells were higher in OSMRβ−/− mice than in WT mice (Fig. 5L). Such changes in the phenotypes of ATMs were also observed at 13 weeks of age when serum insulin levels just started to rise (Fig. 5, J–L). These results indicate that OSMRβ−/− mice exhibit phenotypic changes in ATMs to M1 at 13, 16, and 32 weeks of age.
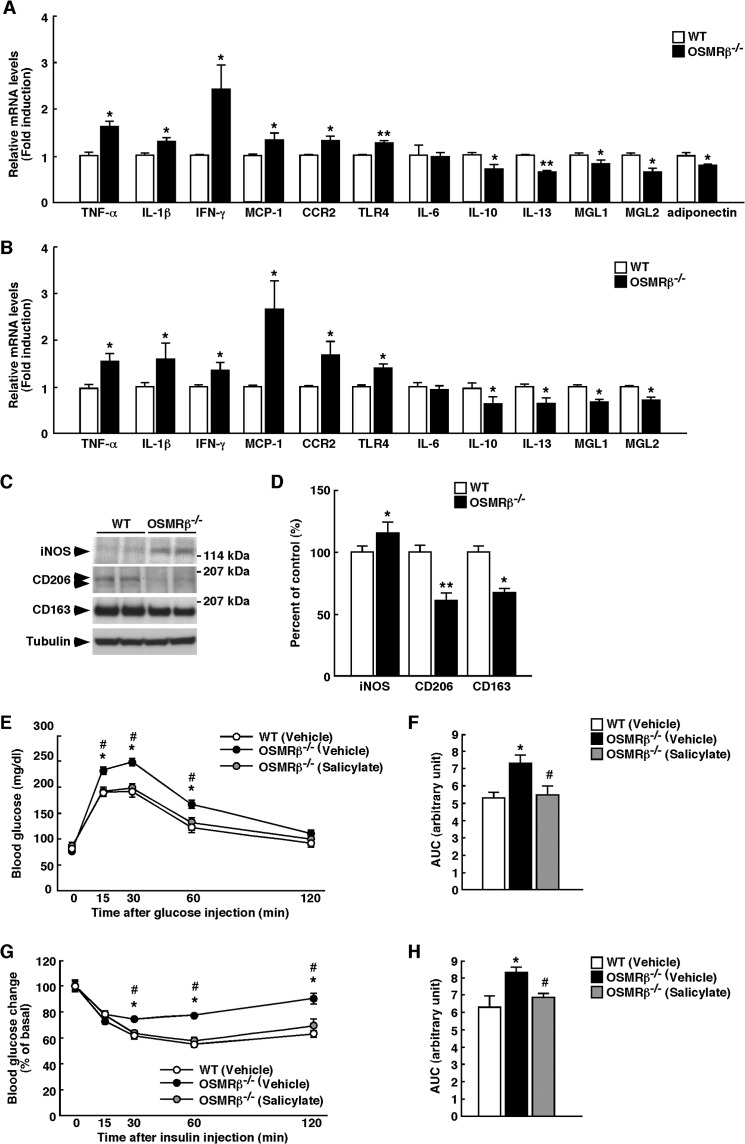
To evaluate the adipose tissue inflammation in OSMRβ−/− mice at 16 weeks of age, we examined the expression levels of various pro- and anti-inflammatory markers. The gene expressions of proinflammatory markers, including TNF-α, IL-1β, IFN-γ, MCP-1, CCR2, and TLR4, were more abundant in the adipose tissue and SVF of OSMRβ−/− mice than in the adipose tissue and SVF of WT mice (Fig. 6, A and B). In contrast, the gene expressions of anti-inflammatory markers, including IL-10, IL-13, MGL1, and MGL2, in the adipose tissue and SVF of OSMRβ−/− mice were lower than those observed in the adipose tissue and SVF of WT mice (Fig. 6, A and B). The gene expression of adiponectin in the adipose tissue also decreased in OSMRβ−/− mice (Fig. 6A). There were no differences in the IL-6 gene expression levels in the adipose tissue and SVF between WT and OSMRβ−/− mice (Fig. 6, A and B). Although iNOS was expressed more abundantly in the adipose tissue of OSMRβ−/− mice than in the adipose tissue of WT mice, the expression levels of CD206 and CD163 were lower in OSMRβ−/− mice (Fig. 6, C and D). These results indicate that OSMRβ−/− mice exhibit adipose tissue inflammation under normal diet conditions.
FIGURE 6.
Contribution of inflammatory status on insulin resistance in OSMRβ−/− mice at 16 weeks of age. A and B, shown are the expressions of proinflammatory markers (TNF-α, IL-1β, IFN-γ, MCP-1, CCR2, TLR4, and IL-6) and anti-inflammatory markers (IL-10, IL-13, MGL1, MGL2, and adiponectin) in the adipose tissue (A) and SVF (B) of WT and OSMRβ−/− mice (n = 6). C, shown is Western blot analysis of markers of macrophage phenotypes (iNOS, CD206, and CD163) in the adipose tissue of WT and OSMRβ−/− mice. The apparent molecular masses are indicated on the right. D, shown is quantitative analysis of the protein expression of iNOS, CD206, and CD163 (n = 6). E–H, shown are the effects of sodium salicylate on glucose intolerance and insulin resistance of OSMRβ−/− mice. OSMRβ−/− mice were injected intraperitoneally with either vehicle or sodium salicylate (120 μg/g body weight) once a day for 2 weeks. E–H, shown are the results of the ipGTTs (E) and ITTs (G) in OSMRβ−/− mice injected with sodium salicylate. The areas under the curves (AUC) for blood glucose in the ipGTTs (F) and ITTs (H) are shown. The data represent the mean ± S.E. *, p < 0.05; **, p < 0.01 WT versus OSMRβ−/− mice; #, p < 0.05 OSMRβ−/− (Vehicle) versus OSMRβ−/− (Salicylate) mice, ANOVA followed by the post-hoc Bonferroni test (E and G); Student's t test (A, B, D, F, and H).
To address the question of whether insulin resistance in OSMRβ−/− mice resulted from inflammation in the adipose tissue, we treated OSMRβ−/− mice with an anti-inflammatory agent, sodium salicylate. Both glucose intolerance and insulin resistance in OSMRβ−/− mice were improved when the mice were treated with sodium salicylate (Fig. 6, E–H). These data suggest that inflammatory status in the adipose tissue is responsible for systemic insulin resistance of OSMRβ−/− mice.
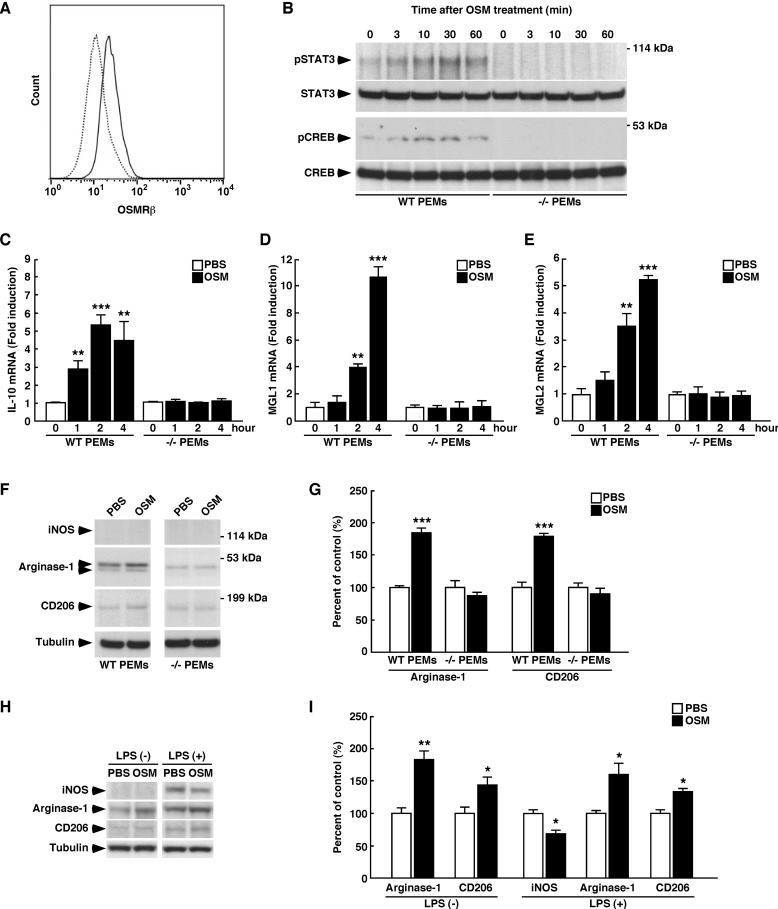
OSM Polarizes Macrophages to the M2 Phenotype
To investigate the effects of OSM on macrophage polarization, the expressions of several macrophage markers were determined in PEMs obtained from WT and OSMRβ−/− mice. The expression of OSMRβ was observed in F4/80-positive cells in PEMs obtained from WT mice using flow cytometry (Fig. 7A). In addition, OSM activated STAT3 and CREB in PEMs obtained from WT mice but not in PEMs obtained from OSMRβ−/− mice (Fig. 7B). Strikingly, the expressions of IL-10, MGL1, and MGL2 were markedly increased by OSM in PEMs obtained from WT mice (Fig. 7, C–E). In addition, OSM significantly increased the expression levels of arginase-1 and CD206 in PEMs obtained from WT mice (Fig. 7, F and G). The effects of OSM on the expressions of IL-10, MGL1, MGL2, arginase-1, and CD206 were completely abolished in PEMs obtained from OSMRβ−/− mice (Fig. 7, C–G). The expression of iNOS, a marker of M1 macrophages, was not observed in PEMs regardless of whether the PEMs were treated with OSM (Fig. 7F). In addition to that observed in PEMs, OSM induced the expressions of arginase-1 and CD206 in RAW264.7 cells, a mouse macrophage cell line (Fig. 7, H and I). OSM also increased the expressions of arginase-1 and CD206 and decreased the expression of iNOS in LPS-stimulated RAW264.7 cells (Fig. 7, H and I). Therefore, OSM can polarize the phenotypes of macrophages to M2.
FIGURE 7.
The functional roles of OSM in macrophage. A, shown is the expression of OSMRβ in PEMs obtained from WT mice. PEMs obtained from WT mice were stained with OSMRβ (solid line) or its control (dotted line) and analyzed by flow cytometry. B, shown is the activation of STAT3 and CREB by OSM in PEMs obtained from WT and OSMRβ−/− mice. Western blot analysis of pSTAT3 and pCREB was performed in OSM-treated PEMs. The apparent molecular masses are indicated on the right. C–E, shown is the induction of IL-10 (C), MGL1 (D), and MGL2 (E) expression by OSM in PEMs. Quantitative real-time PCR was performed using mRNA prepared from OSM-treated PEMs obtained from WT and OSMRβ−/− mice. F, shown are Western blot analyses of markers of macrophage phenotypes (iNOS, arginase-1, and CD206) in the OSM-treated PEMs obtained from WT and OSMRβ−/− mice. The apparent molecular masses are indicated on the right. G, shown is quantitative analysis of the protein expression of arginase-1 and CD206. H and I, shown are the roles of OSM in RAW264.7 macrophages. H, shown is a Western blot analysis of markers of macrophage phenotypes (iNOS, arginase-1, and CD206) in non-stimulated or LPS-stimulated RAW264.7 macrophages. I, shown is a quantitative analysis of the protein expressions of iNOS, arginase-1, and CD206. The data represent the mean ± S.E. of three independent experiments. The data are expressed as percentages of control values (white bars). *, p < 0.05; **, p < 0.01; ***, p < 0.005 versus control, ANOVA followed by the post-hoc Bonferroni test (C–E); Student's t test (G and I).
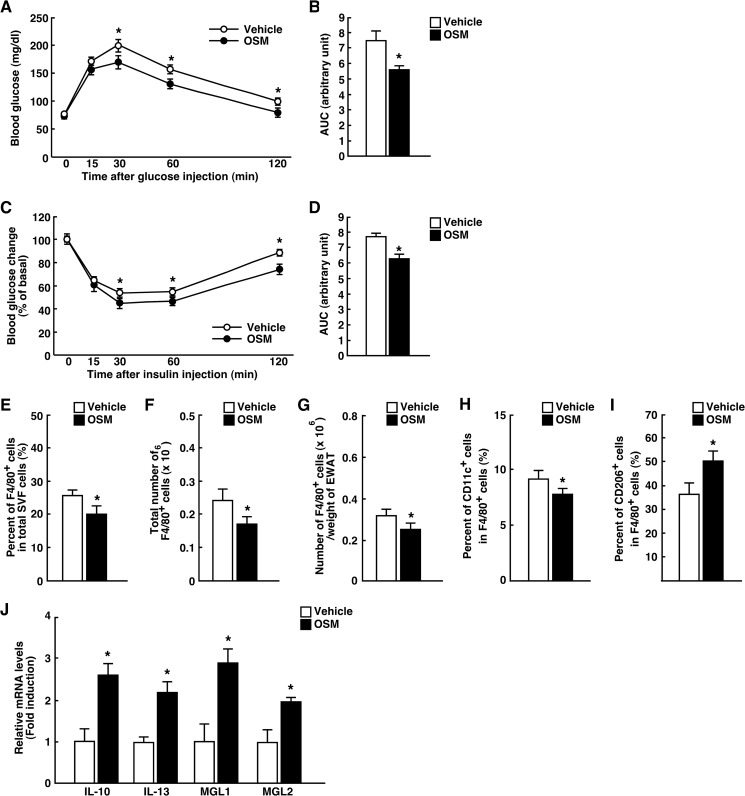
OSM Polarizes ATMs to M2 Phenotype and Increases Insulin Sensitivity in Vivo
To investigate the effects of OSM on insulin sensitivity and phenotypic changes of ATMs, we treated C57BL/6J mice with OSM for 1 week. Both glucose tolerance and insulin sensitivity in C57BL/6J mice were increased when the mice were treated with OSM (Fig. 8, A–D). In addition, both percentages and total numbers of F4/80-positive cells were reduced in mice treated with OSM (Fig. 8, E–G). The treatment with OSM increased the percentage of M2 ATMs but decreased the percentage of M1 ATMs in the adipose tissue (Fig. 8, H and I). In addition, OSM increased the expression of IL-10, IL-13, MGL1, and MGL2 in the adipose tissue (Fig. 8J). These data suggest that OSM can change the phenotypes of ATMs to M2 and increase insulin sensitivity in vivo.
FIGURE 8.
Effects of OSM on phenotypes of ATMs and insulin sensitivity. C57BL/6J mice were injected intraperitoneally with either vehicle or recombinant mouse OSM (12.5 ng/g body weight) twice a day for 1 week. A–D, shown are the results of the ipGTTs (A) and ITTs (C) in C57BL/6J mice injected with OSM (n = 4). The areas under the curves (AUC) for blood glucose in the ipGTTs (B) and ITTs (D) are shown. E–G, shown are the percentages (E) and total numbers (F) of F4/80-positive cells among the total numbers of cells in the SVF of the epididymal fat pads in C57BL/6J mice injected with OSM (n = 4). The total numbers of macrophages were normalized by the weights of the epididymal fat pads (G). H and I, shown are the percentages of CD11c-positive (H) and CD206-positive (I) cells in the F4/80-positive cells of C57BL/6J mice injected with OSM (n = 4). J, shown are the expressions of anti-inflammatory markers (IL-10, IL-13, MGL1, and MGL2) in the adipose tissue of C57BL/6J mice injected with OSM (n = 4). The data represent the mean ± S.E. *, p < 0.05 vehicle versus OSM, ANOVA followed by the post-hoc Bonferroni test (A and C); Student's t test (B, D, and E–J).
DISCUSSION
OSM belongs to the IL-6 family of cytokines, including IL-6, IL-11, leukemia inhibitory factor, ciliary neurotrophic factor, and cardiotrophin-1 (30) and exhibits a variety of physiological functions, including the development of neurons and hepatocytes, hematopoiesis, and the modulation of inflammatory responses (21, 31–33). Although some members in this family, IL-6, ciliary neurotrophic factor, and cardiotrophin-1, are known to be associated with the development of obesity and insulin resistance (34–36), the role of OSM in these metabolic disturbances remains unclear. In this paper we have addressed this question using OSMRβ−/− mice. OSMRβ−/− mice exhibited obesity and insulin resistance at 32 weeks of age. Interestingly, insulin resistance preceding obesity was already observed in OSMRβ−/− mice at 16 weeks of age.
It is well established that the balance between pro- and anti-inflammatory cytokines secreted from the adipose tissue is important for systemic insulin sensitivity. Proinflammatory cytokines, including TNF-α, IL-1β, and IFN-γ, promote the development of insulin resistance (16, 17, 37–40), whereas an anti-inflammatory cytokine, IL-10, improves obesity-induced insulin resistance (18). In the adipose tissue, these pro- and anti-inflammatory cytokines are produced by M1 and M2 macrophages, respectively (15). In the present study we found that the percentage of M1 macrophages and the expression of proinflammatory cytokines were increased in the adipose tissue of OSMRβ−/− mice compared with WT mice. In contrast, the percentage of M2 macrophages and the expression of IL-10 were reduced in the adipose tissue of OSMRβ−/− mice. Treatment of OSMRβ−/− mice with sodium salicylate improved their insulin resistance, suggesting that systemic inflammation is important for the development of insulin resistance in OSMRβ−/− mice. In addition, OSM was shown to directly polarize the phenotype of PEMs and RAW264.7 cells to M2. Furthermore, insulin sensitivity and the percentage of M2 ATMs were increased by the treatment with OSM in vivo. These findings suggest that OSM plays an important role in the regulation of energy homeostasis by insulin at least in part through the regulation of M1/M2 balance. In addition, Gr-1high/CD11bhigh cells, which are considered to be activated neutrophils, increased in the adipose tissue of OSMRβ−/− mice. The increase of Gr-1high/CD11bhigh cells may contribute to drive or sustain the adipose tissue inflammation in OSMRβ−/− mice.
As OSM is known to inhibit the differentiation of preadipocytes to mature adipocytes in vitro (41), our initial hypothesis was that OSM might reduce adiposity. However, there were no differences in the weight of adipose tissue between WT and OSMRβ−/− mice under normal diet conditions. Most of the OSMRβ-positive cells were F4/80-positive macrophages, and few Dlk-1-positive preadipocytes were found in the adipose tissue. Furthermore, the phenotypes of the ATMs were polarized from M2 to M1 in OSMRβ−/− mice fed a normal diet. These results suggest that macrophages play a more important role in the regulation of energy metabolism by OSM than do preadipocytes under normal diet conditions.
IL-6 potentially acts as a proinflammatory cytokine and induces hepatic insulin resistance in rodents (42, 43). However, chronic treatment of mice with IL-6 does not affect the insulin signaling in the skeletal muscle (42). In addition, IL-6-deficient mice showed systemic insulin resistance (34, 44). Therefore, the role of IL-6 in the development of systemic insulin resistance is controversial. In the present study, the IL-6 levels did not change in the adipose tissue of OSMRβ−/− mice compared with those observed in the controls at 16 weeks of age when systemic insulin resistance developed in OSMRβ−/− mice. By contrast, some proinflammatory cytokines (TNF-α, IL-1β, and IFN-γ), known to contribute to the development of insulin resistance (16, 17, 37–40), was significantly increased in the adipose tissue of OSMRβ−/− mice. Therefore, IL-6 may function in the development of adipose tissue inflammation and insulin resistance in a manner distinct from that exhibited by other proinflammatory cytokines, including TNF-α, IL-1β, and IFN-γ. These observations suggest that our mouse model of metabolic syndrome with different expression patterns of proinflammatory cytokines may help in understanding functional differences between IL-6 and other proinflammatory cytokines in adipose tissue inflammation and insulin resistance.
The food intake is regulated by the hypothalamus and other associated regions of the brain, including the mesolimbic region and the brain stem (45). It has been reported that OSMRβ is expressed in a hypothalamic neuronal cell line, Gnv-4 cells (46). This finding raises the possibility that OSM regulates food intake through the hypothalamus. However, we reported previously that OSMRβ is expressed only in the astrocytes of the olfactory bulb, the epithelial cells of the choroid plexus, and meningeal cells and not in the brain regions associated with food intake in normal adult mice (47). In addition, there were no significant differences in the food intake between WT and OSMRβ−/− mice fed a normal diet at 16 weeks of age, suggesting that OSM signaling is unlikely to regulate food intake under the normal conditions.
It has been most widely accepted that obesity is a major risk factor for the development of insulin resistance (1), which is followed by hyperinsulinemia, the exhaustion of pancreatic β cells, and then the development of type 2 diabetes. However, there are also some reports inconsistent with this obesity-induced model of the pathogenesis of type 2 diabetes in human; that is, insulin resistance without obesity or hyperinsulinemia preceding obesity (48, 49). Thus, the relationship between obesity, insulin resistance, and type 2 diabetes remains unclear yet. In addition, there are few reports in the mouse model that insulin resistance occurs preceding obesity. Although insulin resistance without obesity is observed in the mice deficient in insulin or insulin signaling genes, including Akt2, these mice do not exhibit systemic inflammation (50, 51). As systemic inflammation, hyperinsulinemia, and insulin resistance preceded obesity in OSMRβ−/− mice, OSMRβ−/− mice constitute a unique mouse model of metabolic diseases and may help to clarify a novel relationship among systemic inflammation, hyperinsulinemia, insulin resistance, and obesity.
This work was supported in part by a Research Grant on Priority Areas from Wakayama Medical University and the 2012 Wakayama Medical Award for Young Researchers.
- ATM
- adipose tissue macrophage
- ANOVA
- analysis of variance
- CREB
- cAMP response element-binding protein
- iNOS
- nitric oxide synthase
- ipGTT
- intraperitoneal glucose tolerance test
- ITT
- insulin tolerance test
- MCP
- monocyte chemoattractant protein
- MGL
- macrophage galactose-type C-type lectin
- OSM
- oncostatin M
- OSMRβ
- OSM-specific β subunit
- OSMRβ−/−
- OSMRβ-deficient
- PE
- phycoerythrin
- PEM
- peritoneal exudate macrophage
- RT
- room temperature
- SVF
- stromal vascular fraction.
REFERENCES
- 1. Després J. P., Lemieux I. (2006) Abdominal obesity and metabolic syndrome. Nature 444, 881–887 [DOI] [PubMed] [Google Scholar]
- 2. Elgazar-Carmon V., Rudich A., Hadad N., Levy R. (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 3. Talukdar S., Oh da Y., Bandyopadhyay G., Li D., Xu J., McNelis J., Lu M., Li P., Yan Q., Zhu Y., Ofrecio J., Lin M., Brenner M. B., Olefsky J. M. (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumeng C. N., Maillard I., Saltiel A. R. (2009) T-ing up inflammation in fat. Nat. Med. 15, 846–847 [DOI] [PubMed] [Google Scholar]
- 5. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirasaka K., Kohno S., Goto J., Furochi H., Mawatari K., Harada N., Hosaka T., Nakaya Y., Ishidoh K., Obata T., Ebina Y., Gu H., Takeda S., Kishi K., Nikawa T. (2007) Deficiency of Cbl-b gene enhances infiltration and activation of macrophages in adipose tissue and causes peripheral insulin resistance in mice. Diabetes 56, 2511–2522 [DOI] [PubMed] [Google Scholar]
- 7. Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. (2006) MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lesniewski L. A., Hosch S. E., Neels J. G., de Luca C., Pashmforoush M., Lumeng C. N., Chiang S. H., Scadeng M., Saltiel A. R., Olefsky J. M. (2007) Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat. Med. 13, 455–462 [DOI] [PubMed] [Google Scholar]
- 9. Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton T. A. (2002) in The Macrophage (Bourke B., Lewis C. eds) pp. 73–102, Oxford University Press, Oxford [Google Scholar]
- 11. Gordon S. (2003) Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 12. Modolell M., Corraliza I. M., Link F., Soler G., Eichmann K. (1995) Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 25, 1101–1104 [DOI] [PubMed] [Google Scholar]
- 13. Munder M., Eichmann K., Modolell M. (1998) Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance. Competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 160, 5347–5354 [PubMed] [Google Scholar]
- 14. Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y., Tsuneyama K., Nagai Y., Takatsu K., Urakaze M., Kobayashi M., Tobe K. (2009) Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58, 2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Alvaro C., Teruel T., Hernandez R., Lorenzo M. (2004) Tumor necrosis factor-α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279, 17070–17078 [DOI] [PubMed] [Google Scholar]
- 17. Nguyen M. T., Satoh H., Favelyukis S., Babendure J. L., Imamura T., Sbodio J. I., Zalevsky J., Dahiyat B. I., Chi N. W., Olefsky J. M. (2005) JNK and tumor necrosis factor-α mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 280, 35361–35371 [DOI] [PubMed] [Google Scholar]
- 18. Hong E. G., Ko H. J., Cho Y. R., Kim H. J., Ma Z., Yu T. Y., Friedline R. H., Kurt-Jones E., Finberg R., Fischer M. A., Granger E. L., Norbury C. C., Hauschka S. D., Philbrick W. M., Lee C. G., Elias J. A., Kim J. K. (2009) Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 58, 2525–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka M., Hara T., Copeland N. G., Gilbert D. J., Jenkins N. A., Miyajima A. (1999) Reconstitution of the functional mouse oncostatin M (OSM) receptor. Molecular cloning of the mouse OSM receptor β subunit. Blood 93, 804–815 [PubMed] [Google Scholar]
- 20. Tamura S., Morikawa Y., Miyajima A., Senba E. (2002) Expression of oncostatin M in hematopoietic organs. Dev. Dyn. 225, 327–331 [DOI] [PubMed] [Google Scholar]
- 21. Wallace P. M., MacMaster J. F., Rouleau K. A., Brown T. J., Loy J. K., Donaldson K. L., Wahl A. F. (1999) Regulation of inflammatory responses by oncostatin M. J. Immunol. 162, 5547–5555 [PubMed] [Google Scholar]
- 22. Dillon S. R., Sprecher C., Hammond A., Bilsborough J., Rosenfeld-Franklin M., Presnell S. R., Haugen H. S., Maurer M., Harder B., Johnston J., Bort S., Mudri S., Kuijper J. L., Bukowski T., Shea P., Dong D. L., Dasovich M., Grant F. J., Lockwood L., Levin S. D., LeCiel C., Waggie K., Day H., Topouzis S., Kramer J., Kuestner R., Chen Z., Foster D., Parrish-Novak J., Gross J. A. (2004) Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 5, 752–760 [DOI] [PubMed] [Google Scholar]
- 23. Tanaka M., Hirabayashi Y., Sekiguchi T., Inoue T., Katsuki M., Miyajima A. (2003) Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood 102, 3154–3162 [DOI] [PubMed] [Google Scholar]
- 24. Morikawa Y., Furotani M., Matsuura N., Kakudo K. (1993) The role of antigen-presenting cells in the regulation of delayed-type hypersensitivity. II. Epidermal Langerhans' cells and peritoneal exudate macrophages. Cell. Immunol. 152, 200–210 [DOI] [PubMed] [Google Scholar]
- 25. Komori T., Doi A., Furuta H., Wakao H., Nakao N., Nakazato M., Nanjo K., Senba E., Morikawa Y. (2010) Regulation of ghrelin signaling by a leptin-induced gene, negative regulatory element-binding protein, in the hypothalamic neurons. J. Biol. Chem. 285, 37884–37894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Komori T., Gyobu H., Ueno H., Kitamura T., Senba E., Morikawa Y. (2008) Expression of kin of irregular chiasm-like 3/mKirre in proprioceptive neurons of the dorsal root ganglia and its interaction with nephrin in muscle spindles. J. Comp. Neurol. 511, 92–108 [DOI] [PubMed] [Google Scholar]
- 27. Lumeng C. N., DelProposto J. B., Westcott D. J., Saltiel A. R. (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komori T., Morikawa Y., Tamura S., Doi A., Nanjo K., Senba E. (2005) Subcellular localization of glucose transporter 4 in the hypothalamic arcuate nucleus of ob/ob mice under basal conditions. Brain Res. 1049, 34–42 [DOI] [PubMed] [Google Scholar]
- 29. Folch J., Lees M., Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 30. Taga T., Kishimoto T. (1997) Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15, 797–819 [DOI] [PubMed] [Google Scholar]
- 31. Kamiya A., Kinoshita T., Ito Y., Matsui T., Morikawa Y., Senba E., Nakashima K., Taga T., Yoshida K., Kishimoto T., Miyajima A. (1999) Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 18, 2127–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morikawa Y., Tamura S., Minehata K., Donovan P. J., Miyajima A., Senba E. (2004) Essential function of oncostatin m in nociceptive neurons of dorsal root ganglia. J. Neurosci. 24, 1941–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mukouyama Y., Hara T., Xu M., Tamura K., Donovan P. J., Kim H., Kogo H., Tsuji K., Nakahata T., Miyajima A. (1998) In vitro expansion of murine multipotential hematopoietic progenitors from the embryonic aorta-gonad-mesonephros region. Immunity 8, 105–114 [DOI] [PubMed] [Google Scholar]
- 34. Wallenius V., Wallenius K., Ahrén B., Rudling M., Carlsten H., Dickson S. L., Ohlsson C., Jansson J. O. (2002) Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79 [DOI] [PubMed] [Google Scholar]
- 35. Watt M. J., Dzamko N., Thomas W. G., Rose-John S., Ernst M., Carling D., Kemp B. E., Febbraio M. A., Steinberg G. R. (2006) CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat. Med. 12, 541–548 [DOI] [PubMed] [Google Scholar]
- 36. Moreno-Aliaga M. J., Pérez-Echarri N., Marcos-Gómez B., Larequi E., Gil-Bea F. J., Viollet B., Gimenez I., Martínez J. A., Prieto J., Bustos M. (2011) Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. 14, 242–253 [DOI] [PubMed] [Google Scholar]
- 37. Stienstra R., Joosten L. A., Koenen T., van Tits B., van Diepen J. A., van den Berg S. A., Rensen P. C., Voshol P. J., Fantuzzi G., Hijmans A., Kersten S., Müller M., van den Berg W. B., van Rooijen N., Wabitsch M., Kullberg B. J., van der Meer J. W., Kanneganti T., Tack C. J., Netea M. G. (2010) The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12, 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J. F. (2007) Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148, 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Rourke R. W., White A. E., Metcalf M. D., Winters B. R., Diggs B. S., Zhu X., Marks D. L. (2012) Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice. Metabolism 61, 1152–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grzelkowska-Kowalczyk K., Wieteska-Skrzeczyńska W. (2010) Treatment with IFN-γ prevents insulin-dependent PKB, p70S6k phosphorylation and protein synthesis in mouse C2C12 myogenic cells. Cell Biol. Int. 34, 117–124 [DOI] [PubMed] [Google Scholar]
- 41. Miyaoka Y., Tanaka M., Naiki T., Miyajima A. (2006) Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J. Biol. Chem. 281, 37913–37920 [DOI] [PubMed] [Google Scholar]
- 42. Klover P. J., Zimmers T. A., Koniaris L. G., Mooney R. A. (2003) Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 52, 2784–2789 [DOI] [PubMed] [Google Scholar]
- 43. Senn J. J., Klover P. J., Nowak I. A., Zimmers T. A., Koniaris L. G., Furlanetto R. W., Mooney R. A. (2003) Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J. Biol. Chem. 278, 13740–13746 [DOI] [PubMed] [Google Scholar]
- 44. Matthews V. B., Allen T. L., Risis S., Chan M. H., Henstridge D. C., Watson N., Zaffino L. A., Babb J. R., Boon J., Meikle P. J., Jowett J. B., Watt M. J., Jansson J. O., Bruce C. R., Febbraio M. A. (2010) Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53, 2431–2441 [DOI] [PubMed] [Google Scholar]
- 45. Coll A. P., Farooqi I. S., O'Rahilly S. (2007) The hormonal control of food intake. Cell 129, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Igaz P., Salvi R., Rey J. P., Glauser M., Pralong F. P., Gaillard R. C. (2006) Effects of cytokines on gonadotropin-releasing hormone (GnRH) gene expression in primary hypothalamic neurons and in GnRH neurons immortalized conditionally. Endocrinology 147, 1037–1043 [DOI] [PubMed] [Google Scholar]
- 47. Tamura S., Morikawa Y., Tanaka M., Miyajima A., Senba E. (2002) Developmental expression pattern of oncostatin M receptor β in mice. Mech. Dev. 115, 127–131 [DOI] [PubMed] [Google Scholar]
- 48. Odeleye O. E., de Courten M., Pettitt D. J., Ravussin E. (1997) Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes 46, 1341–1345 [DOI] [PubMed] [Google Scholar]
- 49. Ishikawa M., Pruneda M. L., Adams-Huet B., Raskin P. (1998) Obesity-independent hyperinsulinemia in nondiabetic first-degree relatives of individuals with type 2 diabetes. Diabetes 47, 788–792 [DOI] [PubMed] [Google Scholar]
- 50. Mehran A. E., Templeman N. M., Brigidi G. S., Lim G. E., Chu K. Y., Hu X., Botezelli J. D., Asadi A., Hoffman B. G., Kieffer T. J., Bamji S. X., Clee S. M., Johnson J. D. (2012) Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16, 723–737 [DOI] [PubMed] [Google Scholar]
- 51. Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]