Abstract
Background
A number of studies evaluated the association of intracellular adhesion molecule-1 (ICAM-1) K469E (rs5498, A/G) gene polymorphism with diabetic microvascular complications (DMI) including diabetic nephropathy (DN) and diabetic retinopathy (DR) in different populations. However, the results of individual studies remain conflicting.
Methods
A comprehensive search was conducted to identify all eligible studies of the above-mentioned associations. The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were assessed using the fixed or random effect model.
Results
Seven studies involving 3411 subjects were included. Overall, the meta-analysis showed a significant association of the A allele with increased risk of DMI susceptibility in a recessive model (OR = 1.37, 95% CI 1.04–1.80, P = 0.02). In the subgroup analysis stratified by ethnicity, significant association was found in Asians but not in Caucasians (OR = 1.78, 95% CI 1.13–2.81, P = 0.01; OR = 1.10, 95% CI 0.79–1.54, P = 0.58, respectively). Moreover, it showed a significant association between the A allele and risk of DN in a recessive model (OR = 1.25, 95% CI 1.02–1.55, P = 0.04).
Conclusions
This meta-analysis suggested that the K469E polymorphism in ICAM-1 gene might affect individual susceptibility to DMI and showed a discrepancy in different ethnicities. Further investigations are needed to validate the association.
Introduction
There has been a rising epidemic of diabetes mellitus throughout the world in recent years and an alarming increase in the rate of mortality and morbidity due to coexisting dyslipidemia, atherosclerosis and cardiovascular disease [1]. It has been known that diabetic patients often develop macrovascular and/or microvascular complications. Although long-term complications of diabetes develop gradually, they can eventually be disabling or even life threatening. Diabetic microvascular complications (DMI), mainly including diabetic nephropathy (DN) and diabetic retinopathy (DR), have become major causes of chronic kidney disease and blindness [2]. Moreover, diabetic microvascular complication patients have been shown to have higher mortality among diabetic subjects [3], [4]. Both DN and DR are wide ranging and are due at least in part to chronic elevation of blood glucose levels, which leads to damage of smallest blood vessels. The pathophysiology of DN and DR are more or less similar, which commence with increase in vascular permeability. Despite the exact mechanism of DMI has not so far been clearly described, many risk factors, like the duration of diabetes, degree of glycemic control and age of the patient, are identified in causation of DMI [5], [6]. Genetic susceptibility has also been suggested as one of the reasons proven in previous studies [7], [8], [9].
Intercellular adhesion molecule-1 (ICAM-1), a cell surface glycoprotein, is a member of the immunoglobulin superfamily of adhesion molecules [10], [11]. It mediates adhesion of circulating leukocytes to the activated endothelium, which is one of the earliest events in the pathogenesis of inflammation and atherosclerosis [12]. Many lines of evidence, ranging from in vitro experiments and pathological examinations to epidemiological studies, have shown that inflammation is a cardinal pathogenic mechanism in diabetic microvascular diseases [13]. Although the precise role of inflammation in the development of diabetic microvascular diseases is still unclear, it is likely that inflammation can accelerate atherosclerosis in patients with diabetes [14]. Several studies have documented an increase in expression of ICAM-1 in animal models of diabetic nephropathy and diabetic retinopathy [15], [16], [17]. The single nucleotide G to A polymorphism in the sixth exon of the ICAM-1 gene (K469E, rs5498) results in an amino acid substitution from glutamic acid (E) to lysine (K) in immunoglobulin-like domain 5 of the ICAM-1 protein. It has been shown to influence the binding of ICAM-1 on endothelial cells and lymphocyte function-associated antigen-1 (LFA-1) and Mac-1 on leucocytes, mediating leukocytosis and its migration in an inflammatory environment [18], [19]. Recent genome wide association study has demonstrated a strong correlation between K469E polymorphism and sICAM-1 levels [20]. This polymorphism has been reported to be involved in inflammatory related diseases including DMI [21], [22], [23].
Although several studies investigated the association between ICAM-1 gene K469E polymorphism and DMI, their results remain inconsistent [23], [24], [25], [26], [27], [28], [29]. Considering a single study may lack the power to provide dependable conclusion, we carried out a meta-analysis to evaluate the precise effect of K469E polymorphism in ICAM-1 gene on risk of DMI including DN and DR. This was, to our knowledge, the first meta-analysis of the association between ICAM-1 gene K469E polymorphism and DMI susceptibility.
Materials and Methods
Publication Search
The electronic databases PubMed, Embase and Web of Science (ISI) were searched using the following terms: “ICAM-1″, “diabetic retinopathy” or “diabetic nephropathy”, “mutation” or “single nucleotide polymorphism (SNP)” or “variant”. Both type 1 and type 2 diabetic patients were enrolled in the study. Additional studies not captured by our database searches were identified through reviewing the reference lists of retrieved articles. The included studies were published ranging from 2002 to 2012 (last research was updated on January, 2013).
Inclusion Criteria
Two investigators reviewed all identified articles independently to determine whether an individual study was eligible for inclusion in our meta-analysis. The inclusion criteria were as follows: (1) case-control study published as an original study to evaluate the association between K469E polymorphism in ICAM-1 gene and risk of DN and/or DR; (2) available genotype distribution of cases and controls that can provide sufficient data for calculation; (3) published in English.
Data Extraction
All data were collected according to a standard technological process. Studies that were repeated, did not satisfy the inclusion criteria, and provided little information or insufficient data were excluded. Table 1 lists the characteristics of the extracted data, including the name of the first author, publication year, ethnicity of the study population, number in case and control groups, genotype distributions, mean age, female sex percentage in case and control groups. We verified accuracy of data by comparing collection forms from two independent investigators. Any discrepancy between the two investigators was resolved by the third investigator.
Table 1. Study Characteristics of genotypes in DMI cases and controls in the analysis of ICAM-1 gene K469E polymorphism.
| Author | Year | Population | Case type | Genotypes (AA/AG/GG) | Mean age(Case/Control) | |||
| Case | Control | |||||||
| Number (%) | Ph* | Number (%) | Ph* | |||||
| Kamiuchi et al. | 2002 | Japanese | DR | 34/35/12 (42/43/15) | 0.55 | 10/30/10 (20/60/20) | 0.16 | 64.3/64.1 |
| Liu et al. | 2006 | Chinese | DR | 81/40/11 (61/30/9) | 0.07 | 16/15/9 (40/38/22) | 0.15 | – |
| Ma et al. | 2006 | Swedish | DN | 54/117/25 (27/60/13) | <0.05 | 50/156/28 (21/67/12) | <0.05 | 46/44 |
| Ma et al. | 2008 | Swedish | DN | 210/311/141 (32/47/21) | 0.20 | 171/326/121 (28/53/19) | 0.12 | 44/40 |
| Petrovic et al. | 2008 | Caucasian | DR | 47/96/52 (24/49/27) | 0.84 | 44/77/22 (31/54/15) | 0.22 | 65/67 |
| Balasubbu et al. | 2010 | Indian | DR | 103/162/80 (30/47/23) | 0.29 | 99/174/86 (28/48/24) | 0.58 | 57/59 |
| Vinita et al. | 2012 | Indian | DR | 60/92/47 (30/46/24) | 0.31 | 29/84/44 (18/54/28) | 0.32 | 58.8/64.3 |
Abbreviations and definitions: DR, diabetic retinopathy; DN, diabetic nephropathy.
Exact p value for HWE test.
Statistical Analysis
The relationship between K469E (A/G) polymorphism and DMI including DR and DN was compared using odds ratios (ORs) and their corresponding 95% confidence intervals (95% CIs). The Z test was used to determine the pooled OR with the significance level set at P<0.05. Dominant, recessive and additive genetic models were all used in our meta-analysis. The I2 of Higgins and Thompson was used to test the heterogeneity between the studies [30]. In the presence of substantial heterogeneity (I2>50%) [31], the random-effects model (REM) by the DerSimonian and Laird method was adopted as the pooling method. Otherwise, the pooled OR was estimated using the fixed-effects model (FEM).
An exact Chi-square test was used to assess the Hardy–Weinberg equilibrium (HWE) with the significance level set at P<0.05. The potential publication bias was evaluated by Egger’s test and visual inspection of funnel plots. All statistical analyses were performed using the STATA version 11.0 software (Stata Corporation, College Station, TX, USA).
Results
Studies and Populations
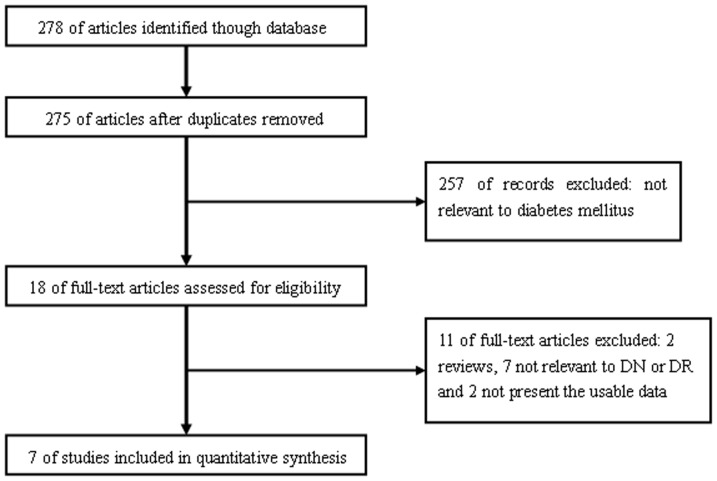
Figure 1 outlines our study selection process. Briefly, a total of 278 articles were identified after an initial search. After reading the abstracts, we removed 3 duplications and 257 articles not relevant to diabetes. After reading full texts of the remaining 18 articles, 11 were then excluded. Finally, a total of 7 case-control studies in 7 articles were identified met our inclusion criteria [23], [24], [25], [26], [27], [28], [29], including 1810 DMI patients and 1601 diabetic controls. The general characteristics of each study, genotype numbers, and HWE examination results are presented in Table 1.
Figure 1. The flow chart of literature search and study selection.
Quantitative Data Synthesis
All studies
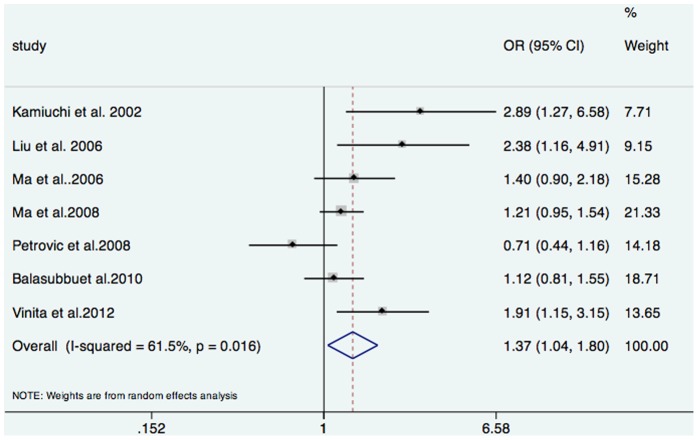
As shown in Figure 2, a significant association between the ICAM-1 K469E gene polymorphism and DMI was found in a recessive genetic model (OR = 1.37, 95% CI 1.04–1.80, P = 0.02). However, no significant association was found either using a dominant model (OR = 1.02, 95% CI 0.76–1.36, P = 0.88) or an additive model (OR = 1.17, 95% CI 0.95–1.43, P = 0.13) in all study populations (Table 2). In the analysis of heterogeneity, all the I2 values of three models for 7 studies were higher than 50% (recessive model: 61.5%, dominant model: 56.1%, and additive model: 72.5%, respectively). Therefore, the random-effects model was used for synthesis of the data.
Figure 2. Forest plot of the overall risk of DMI associated with the ICAM-1 gene K469E polymorphism.
Table 2. Pooled measures on the relation of ICAM-1 gene K469E polymorphism with DMI, DR and DN.
| Group | Data | Inherited model | Number of cases/controls | Pooled OR (95% CI) | I 2 (%) |
| All | All relevant articles | Dominant | 1770/1601 | 1.02 (0.77–1.36) | 56.1 |
| Recessive | 1770/1601 | 1.37 (1.04–1.80)* | 61.5 | ||
| Additive | 1770/1601 | 1.17 (0.95–1.43) | 72.5 | ||
| Excluded for DHWE | Dominant | 1574/1367 | 1.05 (0.75–1.46) | 63.4 | |
| Recessive | 1574/1367 | 1.38 (1.00–1.91)* | 67.5 | ||
| Additive | 1574/1367 | 1.19 (0.93–1.52) | 77.0 | ||
| Asian | All relevant articles | Dominant | 757/606 | 1.22 (0.94–1.58) | 36.6 |
| Recessive | 757/606 | 1.79 (1.13–2.81)* | 62.5 | ||
| Additive | 757/606 | 1.45 (1.06–1.99)* | 68.3 | ||
| Caucasian | All relevant articles | Dominant | 1013/995 | 0.82 (0.65–1.02) | 45.8 |
| Recessive | 1013/995 | 1.10 (0.79–1.54) | 57.2 | ||
| Additive | 1013/995 | 0.95 (0.75–1.22) | 68.2 | ||
| Excluded for DHWE | Dominant | 817/761 | 0.71 (0.40–1.25) | 71.3 | |
| Recessive | 817/761 | 0.97 (0.58–1.62) | 73.0 | ||
| Additive | 817/761 | 0.87 (0.59–1.30) | 81.5 | ||
| DR | All relevant articles | Dominant | 952/749 | 1.13 (0.71–1.82) | 69.2 |
| Recessive | 952/749 | 1.49 (0.94–2.37) | 73.8 | ||
| Additive | 952/749 | 1.26 (0.89–1.79) | 81.2 | ||
| DN | All relevant articles | Dominant | 818/852 | 0.91 (0.71–1.16) | 0.0 |
| Recessive | 818/852 | 1.25 (1.02–1.55)* | 0.0 | ||
| Additive | 818/852 | 1.07 (0.93–1.22) | 0.0 |
Abbreviations and definitions: DHWE: deviated from HWE in cases and/or in controls; DR, diabetic retinopathy; DN, diabetic nephropathy.
P<0.05.
After exclusion of the article deviating from HWE in cases and in controls, the A allele was still found to be significant associated with an increase risk of DMI in a recessive model (OR 1.38, 95% CI 1.00–1.91, P = 0.05). The dominant model and the additive model were remains non-significant (P values>0.05).
Subgroup analyses
In the subgroup analyses by ethnicity, significant associations between the A allele and increased risk of DMI were found among Asians both in a recessive (OR = 1.79, 95% CI 1.13–2.81, P = 0.01) and an additive (OR = 1.45, 95% CI 1.06–1.99, P = 0.02) model, but not in a dominant model (OR = 1.32, 95% CI 0.91–1.91, P = 0.14). However, the significant association between the ICAM-1 K469E gene polymorphism and DMI was not found among Caucasians (Table 2).
With regard to the specific type of DMI, we also conducted the subgroup analyses stratified by DN or DR. The results showed that the A allele was found to be significantly associated with an increase risk of DN in a recessive model (OR = 1.25, 95% CI 1.02–1.55, P = 0.04). Moreover, the heterogeneity of the subgroup disappeared (I 2 = 0.0%). However, in the subgroup of DR, no significant association with the A allele was found in all genetic models (all P values>0.05).
Publication bias
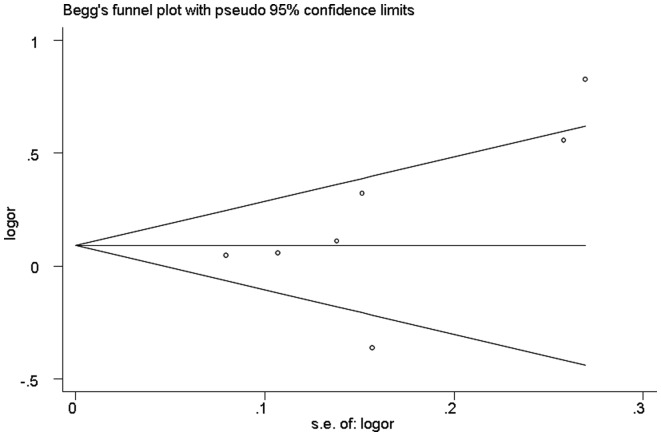
The publication bias of the included studies was assessed by the Begg’s funnel plot and Egger’s test. The funnel plot for additive model in all studies showed no evidence for asymmetry (Figure 3). And Egger’s linear regression test revealed no significant publication bias was observed (P = 0.22).
Figure 3. Funnel plot of publication bias for the ICAM-1 gene K469E polymorphism.
Discussion
The incidence of microvascular complications is increasing among the growing number of patients suffering from diabetes mellitus [32], [33]. The prevalence of overall microvascular complication is 30% to 40% at the time of diagnosis [34]. Although microvascular complications are the results of multiple causative factors, the genetic contribution of the individual plays a significant role in determining susceptibility to DMI [35]. The ICAM-1 gene, encoding intercellular adhesion molecule 1, might play a crucial role in the pathogenesis of DMI including DN and DR [13], [36], [37]. The association between ICAM-1 gene K469E (rs5498) polymorphism and DMI risk was first reported in an East Asian population [23]. However, as discussed above, conflicting data regarding the role of ICAM-1 gene K469E polymorphism in DMI susceptibility have been reported [24], [25], [26], [27], [28], [29]. Against this backdrop, we performed a meta-analysis to clarify the relationship between ICAM-1 gene K469E polymorphism and DMI risk.
This current meta-analysis of 7 studies including 1810 DMI cases and 1601 diabetic controls systematically evaluated the association between ICAM-1 gene K469E polymorphism and DMI risk. With the use of results provided in previous studies, we compared the minor allele to major allele in additive model, recessive model and dominant model. A significant relationship was found between ICAM-1 gene K469E polymorphism and increased DMI risk under a recessive model (OR = 1.37, 95% CI 1.04–1.80; P = 0.02). However, no significant association between ICAM-1 gene K469E polymorphism and DMI risk was found in dominant and additive models (OR = 1.02, 95% CI 0.77–1.36; P = 0.88; OR = 1.17, 95% CI 0.95–1.43; P = 0.13, respectively). Furthermore, ethnic-specific meta-analysis showed that no significant association existed between ICAM-1 gene K469E polymorphism and DMI in Europeans (all P values>0.05). In contrast, DMI risk was increased in Asians in recessive and additive models (OR = 1.78, 95% CI 1.13–2.81, P = 0.01; OR = 1.45, 95% CI 1.06–1.99, P = 0.03, respectively), suggesting a possible influence among environmental exposures and different genetic backgrounds. In the stratified analysis by the specific type of microvascular complication, the significant association was observed in DN subgroup in recessive model (OR = 1.25, 95% CI 1.02–1.55; P = 0.04). Nevertheless, no significant association of ICAM-1 gene K469E polymorphism with DR subgroup was found (all P values>0.05). Since this meta-analysis included only two studies using DN population, the false positive association between ICAM-1 gene K469E polymorphism and DN could not be ruled out because studies with small sample size may have random error to detect a slight effect. Additional future studies should be performed to focus on DN population. More studies are warranted to further validate microvascular complication type difference in the effect of this polymorphism on DMI susceptibility.
The polymorphism K469E (rs5498) is a non-synonymous single nucleotide polymorphism and resides in the fifth immunoglobulin-like domain of ICAM-1 that is essential for dimerization, surface presentation and solubilisation of the protein [38]. It results in a change in the amino acid sequence of the Ig-like domain 5 (from glutamic acid to lysine). This domain is of crucial importance for the activity of ICAM-1 protein in its interactions with LFA-1 and for the adhesion of B cells [39]. Previously study has demonstrated that this polymorphism affects ICAM-1 mRNA splicing pattern and TPA-induced apoptosis [40]. A recent genome wide association study has demonstrated a strong correlation between K469E polymorphism and sICAM-1 levels [20]. Thus, the K469E polymorphism of ICAM-1 gene may play an important role in inflammation and atherosclerosis.
As the publication of findings often depends on the expectation of researchers, false-negative results may be suppressed or false positive results magnified [41]. Although the results of this study did not show significant publication bias, the number of studies included in this meta-analysis was small and large inter-study heterogeneity was observed. Significant heterogeneity existed in overall comparisons in each genetic model. The observed heterogeneity could be attributed to differences in several factors such as microvascular complication type, diabetes duration, ethnic variations, environmental factors and methodological factors in design and conduct of the studies. In addition, DMI is a complex etiology generated by combined effect of genetic and environmental risk factors. Due to different racial or ethnic populations with different frequencies of alleles, different genetic backgrounds may affect DMI susceptibilities. It has been reported that the K allele was risk factor for DR in Asian, whereas the E allele was risk factor for DR in Caucasian. This may reflect the genetic heterogeneity between different ethnicities.
The present meta-analysis had several limitations that must be taken into account. Firstly, only published data which were included by the published studies and unpublished studies which had null results were missed, which might bias the results, while our statistical tests may not have totally shown it. Secondly, the overall outcomes were based on individual unadjusted genotype data, while a more precise evaluation should be made by adjusting other potentially suspected factors including age, sex, and environmental factors. Thirdly, data were not stratified by other factors such as gender status and the clear diagnosis methods of DMI, because sufficient information could not be extracted from the original studies. Fourthly, because of the complex nature of DMI, it is unlikely that a SNP in one single gene would be obviously associated with an increase in DMI risk, without consideration of any other polymorphic susceptible genes. Fifthly, although there are many similarities in the coexistence of DR and DN being both as microvascular disease and microscopically both have capillary basement membrane thickening, they still have somewhat different pathogenesis. DR is characterized by a spectrum of lesions within the retina. These include changes in vascular permeability, capillary microaneurysms, capillary degeneration, and excessive formation of new blood vessels. However, the development and progression of DN is highly complex given the diversity of cell populations present within the kidney and the various physiological roles of this organ. High glucose concentrations induce specific cellular effects, which affect various resident kidney cells including endothelial cells, smooth muscle cells, mesangial cells, podocytes, cells of the tubular and collecting duct system, and inflammatory cells and myofibroblasts. Sixthly, both types of diabetes were enrolled in our study, and differences in pathogenesis between type 1 and type 2 diabetes are existed. Finally, since the number of studies included in the subgroup analyses was small, the results lacked sufficient reliability to confirm or refute an association in a definitive manner.
To our knowledge, this study was the first comprehensive meta-analysis to assess the relationship between ICAM-1 gene K469E polymorphism and DMI susceptibility. It provided evidences of the association between ICAM-1 gene K469E polymorphism and DMI risk, and supported the hypothesis that the ICAM-1 gene K469E polymorphism might be a susceptibility marker for DMI. Since potential biases and confounders could not be ruled out completely in this meta-analysis, additional large case-control studies are required to validate our findings. Meanwhile, further studies regarding other SNPs (or haplotypes) in the ICAM-1 gene and DMI susceptibility are also encouraged to help better understand the role of ICAM-1 gene in DMI. Moreover, gene-gene and gene-environment interactions should also be considered in future studies.
Acknowledgments
We thank all our colleagues working in the Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Funding Statement
The authors have no support or funding to report.
References
- 1. Nathan DM (2010) Navigating the choices for diabetes prevention. N Engl J Med 362: 1533–1535. [DOI] [PubMed] [Google Scholar]
- 2. Reusch JE (2003) Diabetes, microvascular complications, and cardiovascular complications: what is it about glucose? J Clin Invest 112: 986–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenson RS, Fioretto P, Dodson PM (2011) Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis 218: 13–18. [DOI] [PubMed] [Google Scholar]
- 4. Estacio RO, Dale RA, Schrier R, Krantz MJ (2012) Relation of reduction in urinary albumin excretion to ten-year cardiovascular mortality in patients with type 2 diabetes and systemic hypertension. Am J Cardiol 109: 1743–1748. [DOI] [PubMed] [Google Scholar]
- 5. Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, et al. (2000) Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raman R, Gupta A, Krishna S, Kulothungan V, Sharma T (2012) Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 27). J Diabetes Complications 26: 123–128. [DOI] [PubMed] [Google Scholar]
- 7. Tian C, Fang S, Du X, Jia C (2011) Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis. Diabetologia 54: 803–811. [DOI] [PubMed] [Google Scholar]
- 8. McDonough CW, Bostrom MA, Lu L, Hicks PJ, Langefeld CD, et al. (2009) Genetic analysis of diabetic nephropathy on chromosome 18 in African Americans: linkage analysis and dense SNP mapping. Hum Genet 126: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abhary S, Hewitt AW, Burdon KP, Craig JE (2009) A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes 58: 2137–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lebedeva T, Dustin ML, Sykulev Y (2005) ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol 17: 251–258. [DOI] [PubMed] [Google Scholar]
- 11. Hopkins AM, Baird AW, Nusrat A (2004) ICAM-1: targeted docking for exogenous as well as endogenous ligands. Adv Drug Deliv Rev 56: 763–778. [DOI] [PubMed] [Google Scholar]
- 12. Blankenberg S, Barbaux S, Tiret L (2003) Adhesion molecules and atherosclerosis. Atherosclerosis 170: 191–203. [DOI] [PubMed] [Google Scholar]
- 14. King GL (2008) The role of inflammatory cytokines in diabetes and its complications. J Periodontol 79: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 15. Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, et al. (2003) Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 16. Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH (2005) Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol 16: 1711–1722. [DOI] [PubMed] [Google Scholar]
- 17. Zhu Y, Zhang XL, Zhu BF, Ding YN (2012) Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol Biol Rep 39: 3727–3735. [DOI] [PubMed] [Google Scholar]
- 18. Mao D, Lu S, Li N, Zhang Y, Long M (2011) Conformational stability analyses of alpha subunit I domain of LFA-1 and Mac-1. PLoS One 6: e24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gadek TR, Burdick DJ, McDowell RS, Stanley MS, Marsters JC Jr, et al. (2002) Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 295: 1086–1089. [DOI] [PubMed] [Google Scholar]
- 20. Pare G, Ridker PM, Rose L, Barbalic M, Dupuis J, et al. (2011) Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet 7: e1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim K, Brown EE, Choi CB, Alarcon-Riquelme ME, Kelly JA, et al. (2012) Variation in the ICAM1-ICAM4-ICAM5 locus is associated with systemic lupus erythematosus susceptibility in multiple ancestries. Ann Rheum Dis 71: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papa A, Danese S, Urgesi R, Grillo A, Guglielmo S, et al. (2004) Intercellular adhesion molecule 1 gene polymorphisms in inflammatory bowel disease. Eur Rev Med Pharmacol Sci 8: 187–191. [PubMed] [Google Scholar]
- 23. Kamiuchi K, Hasegawa G, Obayashi H, Kitamura A, Ishii M, et al. (2002) Intercellular adhesion molecule-1 (ICAM-1) polymorphism is associated with diabetic retinopathy in Type 2 diabetes mellitus. Diabet Med 19: 371–376. [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Yu Q, Wang H, Zhang SX, Huang C, et al. (2006) Association of intercellular adhesion molecule 1 polymorphisms with retinopathy in Chinese patients with Type 2 diabetes. Diabet Med 23: 643–648. [DOI] [PubMed] [Google Scholar]
- 25. Ma J, Mollsten A, Prazny M, Falhammar H, Brismar K, et al. (2006) Genetic influences of the intercellular adhesion molecule 1 (ICAM-1) gene polymorphisms in development of Type 1 diabetes and diabetic nephropathy. Diabet Med 23: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma J, Zhang D, Brismar K, Efendic S, Gu HF (2008) Evaluation of the association between the common E469K polymorphism in the ICAM-1 gene and diabetic nephropathy among type 1 diabetic patients in GoKinD population. BMC Med Genet 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrovic MG, Osredkar J, Saraga-Babic M, Petrovic D (2008) K469E polymorphism of the intracellular adhesion molecule 1 gene is associated with proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin Experiment Ophthalmol 36: 468–472. [DOI] [PubMed] [Google Scholar]
- 28. Balasubbu S, Sundaresan P, Rajendran A, Ramasamy K, Govindarajan G, et al. (2010) Association analysis of nine candidate gene polymorphisms in Indian patients with type 2 diabetic retinopathy. BMC Med Genet 11: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinita K, Sripriya S, Prathiba K, Vaitheeswaran K, Sathyabaarathi R, et al.. (2012) ICAM-1 K469E polymorphism is a genetic determinant for the clinical risk factors of T2D subjects with retinopathy in Indians: a population-based case-control study. BMJ Open 2. [DOI] [PMC free article] [PubMed]
- 30. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94: 311–321. [DOI] [PubMed] [Google Scholar]
- 33. Tseng LN, Tseng YH, Jiang YD, Chang CH, Chung CH, et al. (2012) Prevalence of hypertension and dyslipidemia and their associations with micro- and macrovascular diseases in patients with diabetes in Taiwan: an analysis of nationwide data for 2000–2009. J Formos Med Assoc 111: 625–636. [DOI] [PubMed] [Google Scholar]
- 34. Kim JH, Kim DJ, Jang HC, Choi SH (2011) Epidemiology of micro- and macrovascular complications of type 2 diabetes in Korea. Diabetes Metab J 35: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murea M, Ma L, Freedman BI (2012) Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev Diabet Stud 9: 6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Long EO (2011) ICAM-1: getting a grip on leukocyte adhesion. J Immunol 186: 5021–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaul K, Hodgkinson A, Tarr JM, Kohner EM, Chibber R (2010) Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Curr Diabetes Rev 6: 294–303. [DOI] [PubMed] [Google Scholar]
- 38. Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, et al. (1995) Intercellular adhesion molecular-1 dimerization and its consequences for adhesion mediated by lymphocyte funtion associated-1. J Exp Med 182: 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown A, Turner L, Christoffersen S, Andrews KA, Szestak T, et al. (2013) Molecular architecture of a complex between an adhesion protein from the malaria parasite and intracelluar adhesion molecular 1. J Biol Chem 288: 5992–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iwao M, Morisaki H, Morisaki T (2004) Single-nucleotide polymorphism g.1548G>A (E469K) in human ICAM-1 gene affects mRNA splicing pattern and TPA-induced apoptosis. Biochem Biophys Res Commun 317: 729–735. [DOI] [PubMed] [Google Scholar]
- 41. Salanti G, Sanderson S, Higgins JP (2005) Obstacles and opportunities in meta-analysis of genetic association studies. Genet Med 7: 13–20. [DOI] [PubMed] [Google Scholar]