Abstract
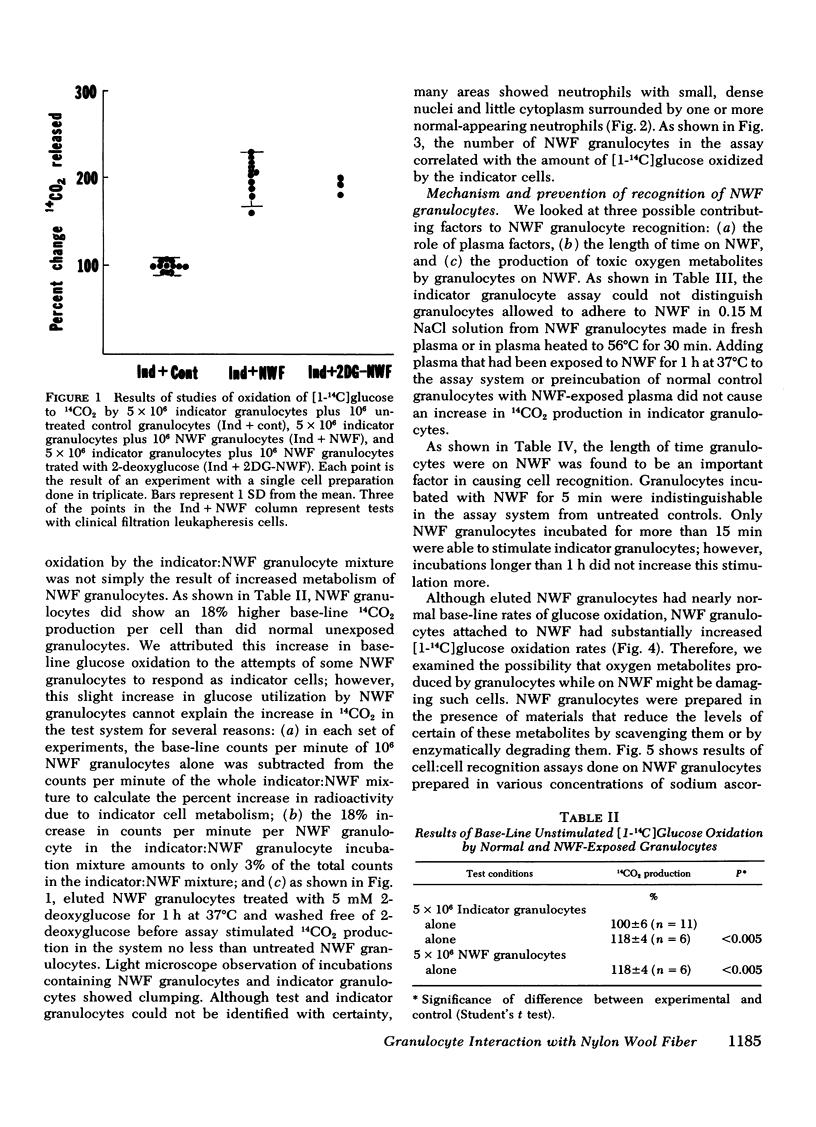
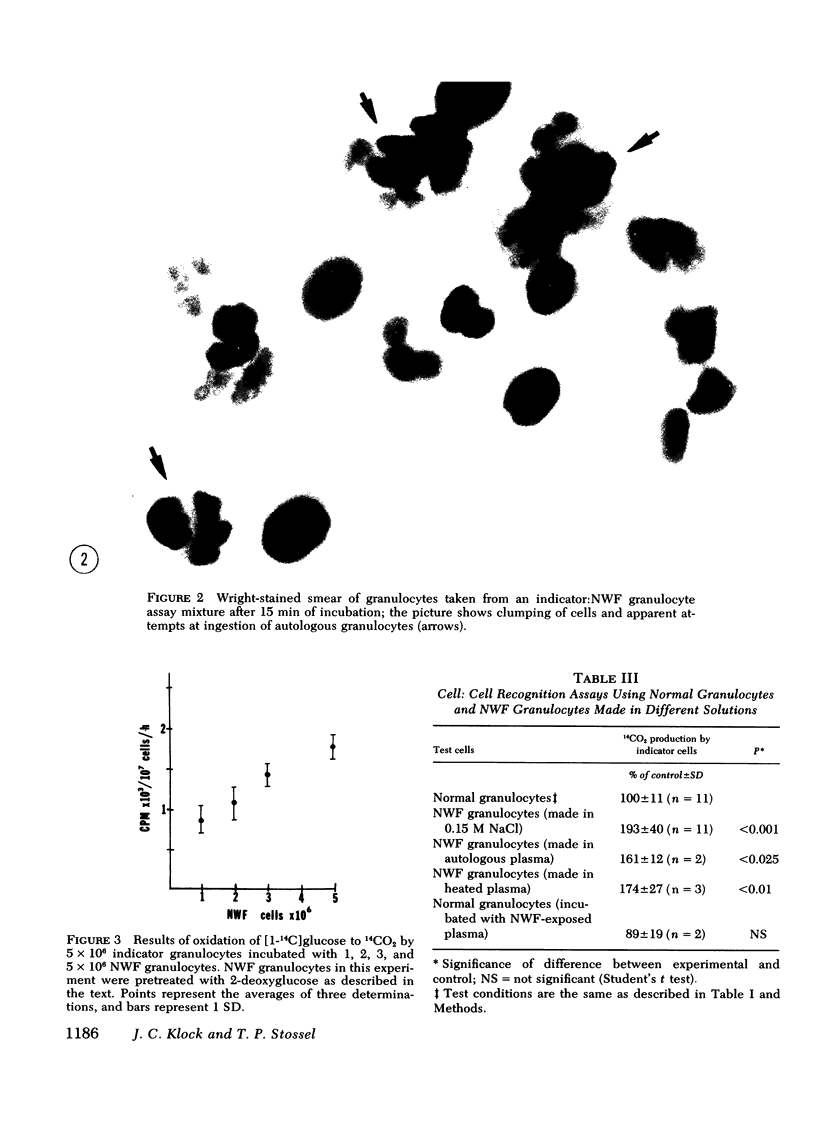
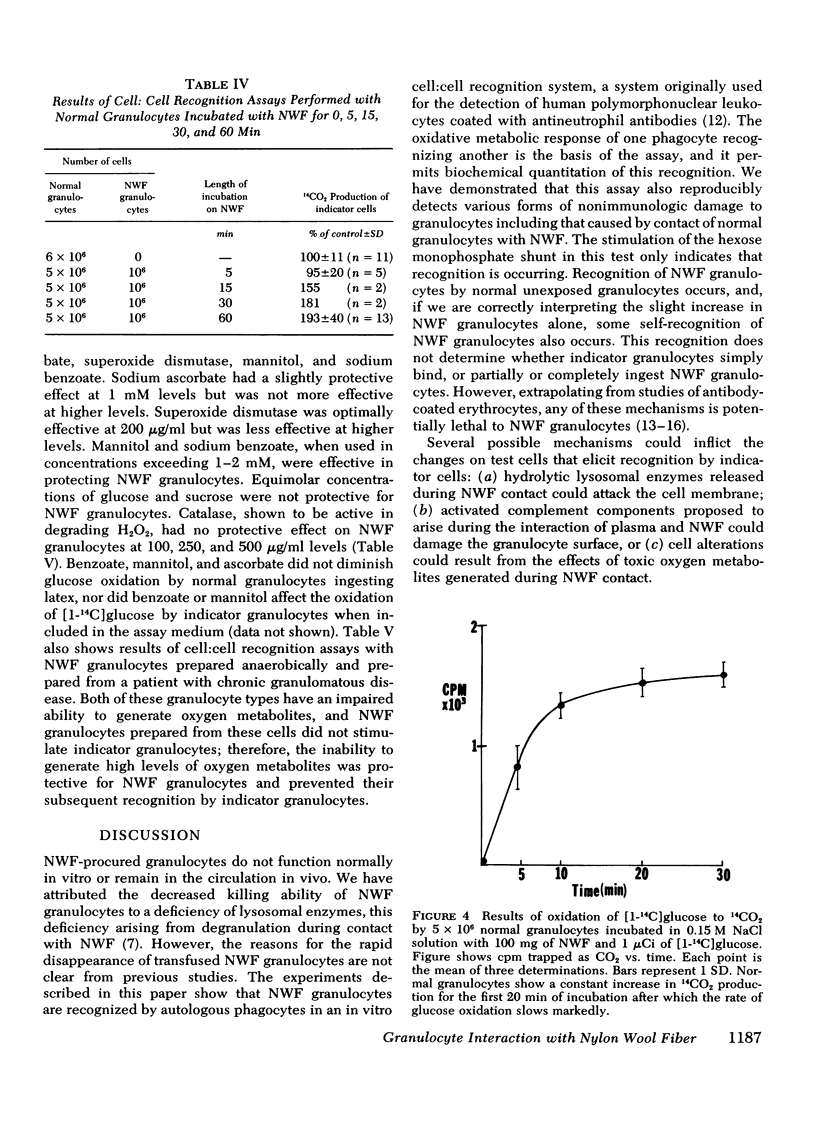
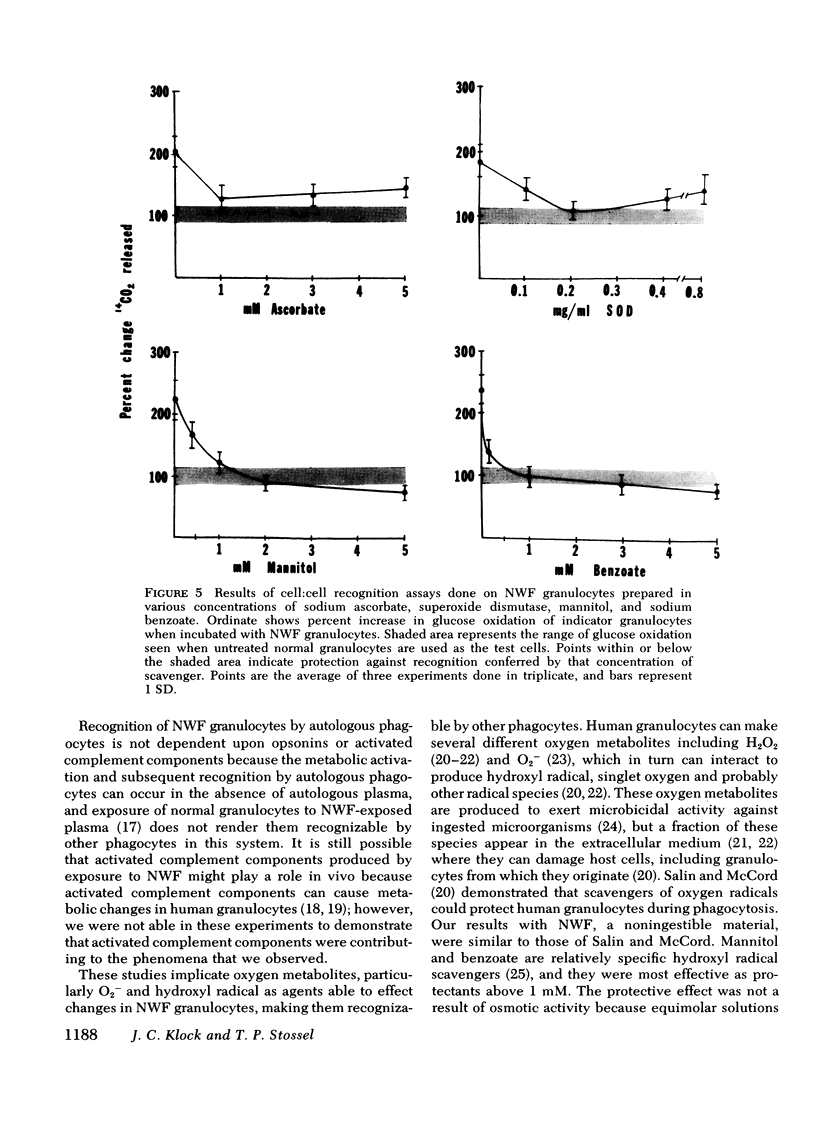
Granulocytes collected by reversible adhesion to nylon wool fiber (NWF) function relatively well in standard in vitro tests; however, they have an abnormally shortened survival time in the circulation. Assuming that this rapid disappearance represents clearance and that recognition by phagocytes is important for such clearance, we used an autologous in vitro cell:cell recognition assay to determine whether phagocytes can detect cellular changes induced by exposure of normal granulocytes to NWF. Human granulocytes incubated with NWF 1 h at 37°C, eluted with 20% acid citrate dextrose plasma, and washed stimulated the hexose monophosphate shunt activity of normal granulocytes an average of twofold (193±40% of controls), indicating a recognition response. NWF-induced granulocyte recognition was not dependent on plasma factors or activated complement components but was dependent on the time that the granulocyte was on the NWF and was maximal by 60 min of exposure. After elution from NWF, granulocytes demonstrated resting glucose oxidation rates only slightly higher than normal; however, during the first 20 min of exposure to NWF, granulocytes increased their rate of 14CO2 production from [1-14C]glucose three- to five-fold. Therefore, experiments were performed to determine whether toxic oxygen metabolites produced by NWF-adherent cells might contribute to recognition. The results showed that (a) normal granulocytes exposed to NWF in the presence of scavengers of superoxide anion (superoxide dismutase) or free radicals (ascorbate, mannitol, or benzoate) and washed before assay did not stimulate glucose oxidation of indicator granulocytes; and (b) NWF granulocytes prepared from cells unable to generate high levels of toxic oxygen metabolites, i.e. cells prepared anaerobically or from a patient with chronic granulomatous disease, also failed to stimulate indicator granulocytes. Human granulocytes placed in contact with NWF show an oxidative burst and become recognizable to other phagocytes. Free radical scavengers are effective in minimizing this recognition conferred on NWF-procured granulocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum F. R., Norton L., Graw R. G., Jr Migration of transfused granulocytes in leukopenic dogs. Blood. 1977 Mar;49(3):483–488. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Stossel T. P. Effects of anti-human neutrophil antibodies in vitro. Quantitative studies. J Clin Invest. 1974 Jun;53(6):1534–1545. doi: 10.1172/JCI107704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. L. The behaviour of phagocytic cell receptors in relation to allergic red cell destruction. Ser Haematol. 1974;7(3):348–357. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Djerassi I., Kim J. S., Suvansri U., Mitrakul C., Ciesielka W. Continuous flow filtration--leukopheresis. Transfusion. 1972 Mar-Apr;12(2):75–83. doi: 10.1111/j.1537-2995.1972.tb05889.x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw R. G., Jr, Herzig G., Perry S., Henderson E. S. Normal granulocyte transfusion therapy: treatment of septicemia due to gram-negative bacteria. N Engl J Med. 1972 Aug 24;287(8):367–371. doi: 10.1056/NEJM197208242870801. [DOI] [PubMed] [Google Scholar]
- Guerry D., Kenna M. A., Schrieber A. D., Cooper R. A. Concanavalin A-mediated binding and sphering of human red blood cells by homologous monocytes. J Exp Med. 1976 Dec 1;144(6):1695–1700. doi: 10.1084/jem.144.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig G. P., Root R. K., Graw R. G., Jr Granulocyte collection by continuous-flow filtration leukapheresis. Blood. 1972 Apr;39(4):554–567. [PubMed] [Google Scholar]
- Higby D. J., Yates J. W., Henderson E. S., Holland J. F. Filtration leukapheresis for granulocyte transfusion therapy. Clinical and laboratory studies. N Engl J Med. 1975 Apr 10;292(15):761–766. doi: 10.1056/NEJM197504102921501. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klock J. C., Bainton D. F. Degranulation and abnormal bactericidal function of granulocytes procured by reversible adhesion to nylon wool. Blood. 1976 Jul;48(1):149–161. [PubMed] [Google Scholar]
- Koj A., Chudzik J., Pajdak W., Dubin A. The occurrence of common inhibitors of trypsin and of leucocyte neutral proteinase in human serum. Biochim Biophys Acta. 1972 Apr 7;268(1):199–206. doi: 10.1016/0005-2744(72)90215-x. [DOI] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Production of O2- in photolyzed water demonstrated through the use of superoxide dismutase. Photochem Photobiol. 1973 Feb;17(2):115–121. doi: 10.1111/j.1751-1097.1973.tb06340.x. [DOI] [PubMed] [Google Scholar]
- McCullough J., Weiblen B. J., Deinard A. R., Boen J., Fortuny I. E., Quie P. G. In vitro function and post-transfusion survival of granulocytes collected by continuous-flow centrifugation and by filtration leukapheresis. Blood. 1976 Aug;48(2):315–326. [PubMed] [Google Scholar]
- Ohlsson K. Alpha1-antitrypsin and alpha2-macroglobulin. Interactions with human neutrophil collagenase and elastase. Ann N Y Acad Sci. 1975 Jun 13;256:409–419. doi: 10.1111/j.1749-6632.1975.tb36067.x. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer C. A., Buchholz D. H., Aisner J., Betts S. W., Wiernik P. H. Clinical experience with transfusion of granulocytes obtained by continuous flow filtration leukopheresis. Am J Med. 1975 Mar;58(3):373–381. doi: 10.1016/0002-9343(75)90603-8. [DOI] [PubMed] [Google Scholar]
- Seljelid R., Munthe-Kaas A. Cytotoxic effect of human monocytes to mouse red cells. Exp Cell Res. 1973 Aug;80(2):473–476. doi: 10.1016/0014-4827(73)90326-1. [DOI] [PubMed] [Google Scholar]
- Strauss R. G., Mauer A. M., Asbrock T., Spitzer R. E., Stitzel A. E. Stimulation of neutrophil oxidative metabolism by the alternate pathway of complement activation: a mechanism for the spontaneous NBT test. Blood. 1975 Jun;45(6):843–849. [PubMed] [Google Scholar]
- Wright D. G., Kauffmann J. C., Chusid M. J., Herzig, Gallin J. I. Functional abnormalities of human neutrophils collected by continuous flow filtration leukopheresis. Blood. 1975 Dec;46(6):901–911. [PubMed] [Google Scholar]