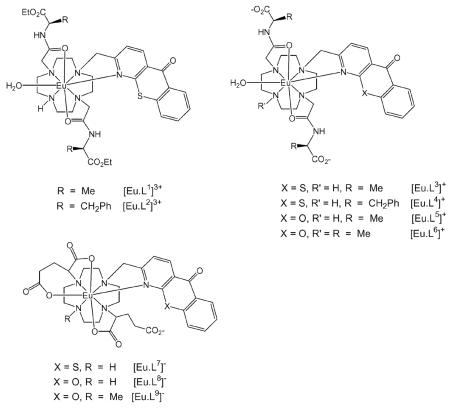
Abstract
Ratiometric methods of analysis have been developed for the selective determination of lactate or citrate in microlitre samples of human serum, urine or prostate fluids following comparison of anion binding affinities for a family of nine luminescent europium(III) complexes.
Lactate and citrate are essential oxy-anions in nature. Their determination is typically based on multi-component enzymatic methods of analysis, using lactic acid dehydrogenase (LDH) or citrate lyase,1 most commonly linked to absorption spectrophotometric analysis of NAD+ at 340 nm. The limit of sensitivity of the spectrophotometric assay is about 0.1 mM; and therefore requires a relatively high sample volume. The assay sensitivity can be improved to ~1 μM by employing fluorimetric detection of NADH. However, in each case, sample pre-treatment and deproteination are required to eliminate interfering sample components.
Lactate and citrate are key metabolites in the intermediary metabolism of the cell; lactate is the important end product of anaerobic and hypoxic glucose metabolism via glycolysis. It is also the major product of aerobic glycolysis that characterizes the metabolism of tumour cells. Lactate analysis of serum is important in sports medicine and in clinical and experimental medicine; for example, lactate is elevated in liver damage and liver disease. Citrate is the key metabolite in the Krebs cycle (citric acid cycle) of virtually every aerobic cell. The synthesis and oxidation of citrate provides the major energy supply (~70%) of cells. Citrate is also the source of acetyl CoA that is required for fatty acid and cholesterol synthesis which is essential for proliferating cells, tissue regeneration, embryogenesis and steroid hormone synthesis. Determination of citrate levels is important in clinical conditions and in normal cell metabolism. Diminished citrate levels in urine have been linked to various aspects of kidney dysfunction, for example in the pathogenesis of nephrolithiasis and nephrocalcinosis.2
Citrate levels are markedly reduced in malignant prostate cancer tissue and provide the most consistent characteristic change in the onset and progression of prostate cancer.3a,3b Citrate represents a significant and much needed biomarker that may provide a reliable method for the screening, detection and monitoring of subjects with prostate cancer. The analysis of citrate concentration of expressed prostatic fluid can provide a simplified, accurate and relatively non-invasive screening/diagnostic procedure for prostate cancer.3b Citrate concentrations in malignant prostate tissue decrease from the normal tissue value of ~10 000–12 000 nmols/gram to ~1000–3000 nmols/gram. Concentrations in prostatic fluid samples thus decrease from the normal range of ~50–200 mM in healthy males to cancer levels of ~2–20 mM. These normal and clinical relationships illustrate the need to develop simple, fast and chemoselective methods to determine the concentration of these oxy-anions in biological fluids, such as cell extracts, serum, urine and particularly in prostate or seminal fluid samples (seminal fluid is normally composed of 50% prostate fluid thereby defining the citrate level).
To address this issue, we report direct, non-enzymatic methods for measurement of lactate and citrate in microlitre samples of biological fluids, using single component emissive europium(III) complexes. We have been developing sensitive luminescence methods of analysis based on the reversible binding of anions to an emissive metal centre. Examples of reversible anion binding in water to single-component, well-defined metal complexes are of considerable current interest.4–8 Of particular note is the chelation of lactate and citrate to lanthanide(III) centres, involving cooperative ligation of the hydroxyl and α-carboxylate groups—verified by luminescence, X-ray and NMR studies.4–7 This process involves reversible displacement of lanthanide bound water molecules. The associated change in lanthanide coordination environment is signalled by modulation of the lifetime and spectral form of the europium luminescence. The sensitivity of the electric dipole allowed ΔJ = 2 (around 616 nm) and ΔJ 4 (700 nm) europium emission bands to changes in the Eu coordination environment is well known.
The affinity of the anion for the Eu(III) centre is determined by Coulombic attraction (lactate: pKa 3.86; citrate: 3.13, 4.76 and 6.40, 298 K, I = 0) and by the steric demand at the metal created by the other coordinated ligands. With this background in mind, and based on our appreciation of selective anion binding at Ln(III) centres in complexes of macrocyclic heptadentate ligands,6,9 we have examined the anion binding behaviour of a series of Eu(III) complexes, [EuLn], (n = 1–9), varying complex charge and ligand steric demand in order to modulate affinity for lactate and citrate. The desired affinity constant and working range of the measurement is determined by the nature and ionic composition of the background medium, typically made up of potentially interfering anions, protein (e.g. albumin 0.7 mM) and certain cations, (e.g. Ca2+, 1.2 mM; Zn2+, 10 μM, Mg2+, 0.9 mM) that can perturb the equilibrium speciation.
Each of the complexes examined† is based on a common macrocyclic core and includes a heterocyclic azaxanthone (λexc 337 nm) or azathiaxanthone (λexc 380 nm) moiety in which the pyridyl nitrogen is bound to Eu, facilitating the sensitisation of lanthanide emission.10 In addition, in the cationic complexes [Eu.L1]3+–[Eu.L6]+, two trans-related amide carbonyl donors are incorporated, derived from Ala or Phe (either as an ethyl-ester or carboxylate), to make up the heptadentate array. For the series of mono-anionic complexes, [Eu.L7]−–[Eu.L9]−, each glutarate substituent gives rise to coordination of the α-carboxylate and one of the δ-carboxylate groups binds to Eu(III), making the ligand an 8-coordinate donor. This intramolecular coordination has previously been established to occur in related complexes (Eu, Gd, Tb) that possess this glutarate side arm.11,12 Competitive displacement of the intramolecularly bound carboxylate by added oxy-anions can occur and changes the coordination environment at the Eu(III) centre, leading to modulation of the Eu emission spectral form. In two cases, [Eu.L6]+ and [Eu.L8]− the ring secondary amine group is methylated (via an Eschweiler–Clarke reaction of the precursor secondary amine†). Such N-methylated complexes are less hydrated in solution (absence of a H-bond NH donor) and the change from H to Me also increases the steric demand at the metal centre. The lower solvation of the N-methylated complexes enhances the apparent affinity of the complex for a given anion (typically by 5–8 kJ mol−1 at Eu/Tb6b). An increase in steric demand about the metal lowers the binding constant, especially for bulkier species (e.g. citrate3−) compared to lactate. Overall, in the series [Eu.L1]3+–[Eu.L9]− a gradation of binding affinities for lactate and citrate is expected, due to the interplay of the effects of charge, steric demand and competitive ligation.
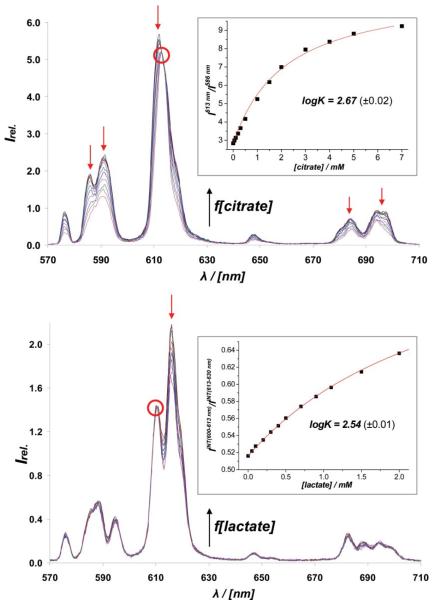
Affinity constants for lactate, citrate and bicarbonate with [Eu.L1]3+–[Eu.L9]− were determined in saline solution (298 K, 0.1 M NaCl, 4 mM KCl, 0.9 mM Na2HPO4, pH 6.55), observing the change in the intensity ratio of the Eu3+ emission bands at 616/686 nm as a function of added anion, Table 1.† With lactate and citrate, measurements were also made in a `simulated prostate fluid' background, prior to analyses in prostate fluid clinical samples. This medium contained various MCl2 salts (M = Mg, Ca, Zn, CtotM2+ = 11 mM), human serum albumin (0.3 mM) and 3 mM NaHCO3, Table 1.13 The added M2+ salts not only compete for the oxy-anion but also appear to stabilise the ternary [Eu.L(citrate)] adducts. For example, with [Eu.L2]3+, titrations of citrate (pH 7.4, 0.1 HEPES, 0.1 M NaCl, 0.9 mM NaH2PO4, 30 mM NaHCO3 and 2.3 mM sodium lactate) in the absence and presence of 2 mM MCl2 (M = Ca, Zn and Mg) revealed an apparent affinity constant that increased by about two log units.
Table 1.
Comparison of apparent binding constants (log K) for europium(III) complexes with the stated anion (298 K, 20 μM complex, λexc 380 (azathioxanthone) or 337 (azaxanthone) nm, pH 6.55, standard deviations in parenthesis) in saline solutiona and in a `simulated prostate fluid' backgroundb [values in italics] that is used to establish a calibration curve for the clinical samples
| Complex | citrate | lactate | bicarbonate |
|---|---|---|---|
| [Eu.L1]3+ | 5.26(03) [5.20](02) |
3.31(02) [3.41](03) |
2.81(02) |
| [Eu.L2]3+ | 5.22(02) [4.88](04) |
3.27(01) [2.97](03) |
3.07(01) |
| [Eu.L3]+ | 4.58(03) [4.51](01) |
2.94(03) [3.20](02) |
2.55(03) |
| [Eu.L4]+ | 4.01(02) [4.20](03) |
2.79(04) [2.82](03) |
2.23(02) |
| [Eu.L5]+ | 4.19(02) [3.89](03) |
3.08(02) [3.43](05) |
2.14(02) |
| [Eu.L6]+ | 4.36(01) [3.97](02) |
3.49(01) [3.82](01) |
2.80(01) |
| [Eu.L7]− | 2.52(02) [3.12](02) |
2.46(02) [2.90](01) |
2.11(05) |
| [Eu.L8]− | 2.54(03) [1.54](03) |
3.03(01) [2.65](02) |
1.27(02) |
| [Eu.L9]− | 1.69(01) [2.02](02) |
3.18(02) [3.33](01) |
1.23(03) |
contains: 0.1 M NaCl, 4 mM KC1,0.1 M HEPES and 0.9 mM NaH2PO4.
contains: 0.1 M NaCl, 4 mM KC1, 4 mM CaCl2, 2 mM ZnCl2, 5 mM MgCl2, 0.3 mM HSA, 3 mM NaHCO3, and 0.1 M HEPES.
Pronounced citrate/lactate selectivity was observed with the positively charged complexes, e.g. for [Eu.L3]+, the ratio of affinity constants is 42:1. Lactate binding is preferred with the more sterically demanding mono-anionic complexes. For [Eu.L9]−, the lactate/citrate ratio was 30/1. These complexes were selected for further study, analysing for citrate or lactate respectively in human serum, urine, saliva, seminal and prostate fluid samples using the luminescence method, and comparing the data obtained to measurements made using enzyme kits.14 For lactate and citrate analyses using the Eu emission method, a calibration curve must be determined in an appropriate background medium, allowing the working range of the measurement to be defined. Thus, in a `simulated prostate fluid' background containing 0.3 mM HSA, 0.1 M NaCl, 4 mM KCl, 3 mM NaHCO3, 4 mM CaCl2, 2 mM ZnCl2, 5 mM MgCl2 (pH 6.5, 0.1 M HEPES), Fig. 1, modulation of Eu emission (10 μM concentration) is evident over the lactate concentration range zero to 1 mM. Typically, a 5 μL sample of fluid, (seminal or prostate) was diluted into pH 6.5 buffer by a factor of 10, and the solution analysed in a 50 μL optical cuvette (λexc 336 nm). The intensity ratio of the 692/619 or 613/622 or 613/619 nm emission bands in [Eu.L9]− was measured and the lactate concentration deduced by reference to the calibration curve. Samples of lactate in urine (3.5 mM), seminal fluid (3.8 mM) prostate fluid (4 mM) and reconstituted human serum (1.9 mM) were found to be within 10% of the concentration deduced enzymatically.† In saliva, which contains high levels of lactic acid dehydrogenase, each method gave a zero reading for lactate (± 0.2 mM).
Fig. 1.
Lower: calibration curve for lactate analysis of prostate or seminal fluids using [Eu.L9]− showing the variation of Eu3+ emission bands as a function of added lactate (pH 6.5, 0.1 M HEPES, λexc 365 nm, 50 μs time gate, 10 μM complex, 0.1 M NaCl, 0.3 mM NaHCO3, 0.4 mM KCl, 0.03 mM HSA, 0.4 mM CaCl2, 0.2 mM ZnCl2, 0.5 mM MgCl2). The inset shows the ratio of two emission bands vs [lactate], with the fit (line) to the data. Upper: calibration curve and Eu emission spectra for citrate analysis in diluted prostate fluid, using [Eu.L3]+; conditions as above but with 0.1 M Na lactate present.
Similar procedures may be used to analyse the citrate content of clinical biofluids using [Eu.L3]+. In this case, typically a 1 μL sample of prostatic fluid is diluted ×200. To obviate any interference due to variations in lactate concentration in male prostate fluid samples, calibration measurements were made (λexc 365 nm, 10 μs gate time, 50 μL optical cell) in `simulated prostate fluid' solution, that contained a large excess of lactate (0.1 M) Fig. 1.
Citrate was determined in seventeen samples of prostate fluid from healthy male human volunteers, and values ranging from 12 mM to 160 mM were measured. These were confirmed (±10%) by independent analysis (Fig. 2) using a commercially available citrate lyase enzyme kit, for which the amount of bio-fluid required was 25 times greater. Using similar methods, but with a 100-fold dilution of sample, citrate was also determined in the urine of healthy volunteers (range 3.5 to 5.5 mM (±10%)).
Fig. 2.
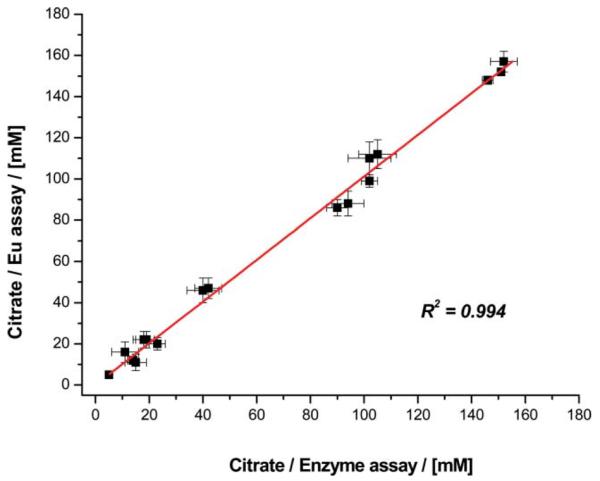
Comparison of citrate concentrations determined in diluted prostate fluid samples from male volunteers, comparing the Eu emission method to results obtained using a citrate lyase kit, (R2 = 0.994).
In summary, rapid luminescence measurements have been developed that allow the determination of citrate or lactate in low volume (<5 μL) samples of various biological fluids. In the citrate analyses using [Eu.L3]+, sample dilution is followed by a time-gated measurement eliminating any interference from concomitant chromophore fluorescence. In particular, this method has been applied to the analysis of citrate in 17 clinical prostate fluid samples, and values varying by an order of magnitude were determined. These studies allow the correlation of measured citrate levels with the health status of the patient. In certain cases, this may be used to confirm or indicate the onset or progression of prostate adenocarcinoma. The optical methods that have been developed are rapid (<5 min) and offer advantages over alternative enzymatic assays that take longer to carry out, require larger sample volumes and need additional sample preparation steps.15
Acknowledgements
We thank EPSRC, NIH (LCC, grants CA79903 and CA71207), the EC network of excellence DiMI and the Royal Society for support, and Dr Andrew Beeby for his assistance with instrumental development.
Footnotes
Electronic supplementary information (ESI) available: Experimental details of ligand and complex syntheses, characterisation and HPLC analysis; details of the measurement of binding constants and selected Eu emission spectra in various saline solutions and biofluids. See DOI: 10.1039/b901251f
Notes and references
- 1.Li YS, Ju X, Gao XF, Zhao YY, Wu WF. Anal. Chim. Acta. 2008;610:249. doi: 10.1016/j.aca.2008.01.049. [DOI] [PubMed] [Google Scholar]; Duong HD, Rhee JI. Talanta. 2007;72:1275. doi: 10.1016/j.talanta.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Schell-Feith EA, Maerdijk A, van Zweiten PHT, Zanderland HM, Holscher HC, Kist-van Holthe J, von der Heijden BJ. Pediatric Nephrol. 2006;21:1830. doi: 10.1007/s00467-006-0274-4. [DOI] [PubMed] [Google Scholar]; Cebotaru V, Kaul S, Devayst O, Cai H, Racusen L, Guggino WB, Guggino SE. Kidney Int. 2005;68:642.3. doi: 10.1111/j.1523-1755.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Costello LC, Franklin RB. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Costello LC, Franklin RB. Prostate Cancer Prostatic Dis. 2009;12:17. doi: 10.1038/pcan.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard JP, dos Santos CMG, Plush SE, McCabe T, Gunnlaugsson T. Chem. Commun. 2007. p. 129. [DOI] [PubMed] [Google Scholar]; Li C, Wong WT. Tetrahedron Lett. 2004;45:6055. [Google Scholar]
- 5.Terreno E, Botta M, Fedeli F, Mandino B, Milone L, Aime S. Inorg. Chem. 2003;42:4891. doi: 10.1021/ic034321y. [DOI] [PubMed] [Google Scholar]; Botta M, Aime S, Barge A, Bobba G, Dickins RS, Parker D, Terreno E. Chem.-Eur. J. 2003;9:2102. doi: 10.1002/chem.200204475. [DOI] [PubMed] [Google Scholar]; Dickins RS, Badari A. Dalton Trans. 2007. p. 3661. [DOI] [PubMed] [Google Scholar]
- 6.(a) Dickins RS, Aime S, Batsanov AS, Beeby A, Botta M, Bruce JI, Howard JAK, Love CS, Peacock RD, Puschmann H. J. Am. Chem. Soc. 2002;124:12697. doi: 10.1021/ja020836x. [DOI] [PubMed] [Google Scholar]; (b) Bruce JI, Dickins RS, Govenlock LJ, Gunnlaugsson T, Aime S, Botta M. J. Am. Chem. Soc. 2000;122:9674. [Google Scholar]
- 7.(a) Yegorova A, Vityukova E, Beltynkova S, Duerkop A. Microchem. J. 2006;83:1. [Google Scholar]; (b) Parker D, Yu J. Chem. Commun. 2005. p. 3141. [DOI] [PubMed] [Google Scholar]; (c) Lin ZH, Wu M, Schaferling M, Wolfbeis OS. Angew. Chem., Int. Ed. Engl. 2004;43:1735. doi: 10.1002/anie.200353169. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez L, Lima JC, Parola AJ, Pina F, Meitz R, Aucejo R, Garcia-Espana E, Llinares JM, Soriano C, Alarcon J. Inorg. Chem. 2008;47:6173. doi: 10.1021/ic7023956. [DOI] [PubMed] [Google Scholar]; Cabell LA, Best MD, Lavigne JJ, Schneider SE, Perreault DM, Monahan MK, Anslyn EV. J. Chem. Soc., Perkin Trans. 2. 2001:315. [Google Scholar]
- 9.Bretonnierre Y, Cann MJ, Parker D, Slater RJ. Org. Biomol. Chem. 2004;2:1624. doi: 10.1039/b400734b. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson P, Findlay KS, Kielar F, Pal R, Parker D, Poole RA, Puschmann H, Richardson SL, Stenson PA, Thompson AL, Yu J. Org. Biomol. Chem. 2006;4:1707. doi: 10.1039/b601357k. [DOI] [PubMed] [Google Scholar]
- 11.Lowe MP, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E. J. Am. Chem. Soc. 2001;123:7601. doi: 10.1021/ja0103647. [DOI] [PubMed] [Google Scholar]
- 12.Pal R, Parker D. Org. Biomol. Chem. 2008;6:1024. doi: 10.1039/b718993a. [DOI] [PubMed] [Google Scholar]; Pal R, Parker D. Chem. Commun. 2007. p. 474. [DOI] [PubMed] [Google Scholar]
- 13.Citrate forms weak 1:1 complexes with Mg2+, Ca2+ and Zn2+: βMgL, 3.29, βCacit, 4.84, βZucit, 4.85; for lactate: βCalac 1.9; βMglac 0.9; βZnlac 19. NIST Selected Stability Constants of Metal Complexes; v. 8.0. www.nist.gov/srd/nist46.htm.
- 14.Enzyme kits for lactate and citrate were obtained from Megazyme Ltd. (Ireland)
- 15.Poole RA, Kielar F, Parker D, Richardson SL, Stenson PA. Chem. Commun. 2006. p. 4084. A non-enzymatic and ratiometric method based on Eu/Tb emission has also been defined for the analysis of urate in serum and urine samples. [DOI] [PubMed] [Google Scholar]