Abstract
Objective
The aim of this study was to examine the accuracy of a new sternal skin conductance (SSC) device for the measurement of hot flashes, and secondly, to assess the acceptability of the device by women.
Methods
Three small descriptive pilot studies were performed utilizing two sequential prototypes of the SSC device developed by an engineering device company in the Midwest. The devices were worn either in a monitored setting for 24 hours or in an ambulatory setting for 5 weeks. During the study period, women recorded hot flashes in a prospective hot flash diary and also answered questions about the acceptability of wearing the SSC device.
Results
The first prototype was not able to collect any analyzable skin conductance data due to various malfunction issues; including poor conductance and battery failure. However, 16 patients did wear the device for 5 weeks and reported that wearing the device was acceptable, although 31% stated that it did interfere with daily activities. Hot flash data from the second prototype revealed a concordance rate between patient reported and device recorded hot flashes of 24%.
Conclusions
Findings from these studies support the discordance between SSC recorded and patient reported hot flashes. In addition, the studies reveal further limitations of SSC monitoring, including difficulties with data collection and lack of consistency in interpretation. Based on these results and other recent trials identifying issues with SSC methodology, it is time to find a better physiologic surrogate measure for hot flashes.
Keywords: sternal skin conductance, hot flash measurement
Introduction
Hot flashes are one of the most common and distressing symptoms of menopause, occurring in over 75% of menopausal women.1 Although they are not life threatening, hot flashes can have a significant negative impact on functional ability and quality of life. Hot flashes are especially problematic in breast cancer survivors, as they can be precipitated by premature menopause due to chemotherapy and anti-estrogenic medications.
There have been several effective treatments identified for hot flashes using retrospective and prospective self report measures. Hormonal therapy is the most effective treatment for hot flashes and treatment with estrogen compounds can decrease hot flashes by 80% to 90%.2 However, due to its possible risks, it is recommended that estrogen is used at the smallest possible dose, for the shortest time and, in breast cancer survivors, it is recommended that estrogen should be avoided. Other therapies, including serotonergic antidepressants and gabapentin, have shown benefit in the treatment of hot flashes with decreases around 50–60%,3 yet the search for a more effective treatment for hot flashes continues, with the goal of higher efficacy and better side effect profiles. There is some thought that hot flash measurement could be improved to facilitate the discovery of better treatments.
Currently, there are multiple tools used for the assessment of hot flashes in clinical research. The most common method of measurement is subjective patient-reported data in the form of questionnaires or diaries. Early studies used a recollection type of questionnaire in which women would retrospectively report hot flashes over the past several days. This method was fraught with inaccuracy and bias. A more recent hot flash diary was developed as a prospective, real time measurement of hot flashes. This diary is a well-accepted method of measuring hot flashes having been successfully used in multiple trials evaluating pharmacologic treatments.4 Event monitors are another method of subjective patient reporting, where patients record a hot flash when it occurs by pushing a button on a device. Recently, there has been concern that subjective patient reporting of hot flashes may be subject to bias and under and/or over-reporting. As a result there has been an interest in finding an appropriate objective measure of hot flashes.5
The development of a valid physiologic surrogate measure for hot flashes is challenging, due largely to the fact that the physiology of hot flashes is not definitively known. Currently, the most researched method of objectively measuring hot flashes is sternal skin conductance (SSC). Previous studies have shown that hot flashes are accompanied by large changes in skin conductance and increased sternal skin conductance was noted to precede changes in peripheral or core temperature.6,7 Early studies in laboratory settings reported a high correlation between SSC and self-reported hot flashes.7,8 Based on these studies, an increase in SSC of 2 μmho in a 30 second period was determined to be associated with a self-reported hot flash.7,9 Although early studies supported the correlation between subjective hot flashes and SSC recorded hot flashes, later studies, particularly in ambulatory settings, have failed to demonstrate such high levels of concordance.10,11 Consequently, the use of SSC in hot flash clinical trials remains controversial.5,11
The most commonly used SSC monitor is the Biolog 3991, which is comprised of electrodes attached to the sternum and transmitted though lead wires to a Biolog monitor, which is worn over clothing, such as on a belt. The monitor cannot be used while bathing; it is large, and therefore, can interfere with daily activities. In addition, the recording capacity is limited to 24 hours and the device costs around $2,300 each.12
In 2004 a request for application (RFA) was released, entitled Improving Measurement Tools for Sternal Skin Conductance and Hot Flashes. This RFA offered small business innovation research grants to conduct research to improve measurement tools or devices for sternal skin conductance. An engineering device company in the Midwest received one of these grants and developed a new SSC device. The device was smaller and lighter than the Biolog 3991. The new device attaches directly to the subject using a standard electrode adhesive pad and has short leads to two electrodes. In addition, the device was developed to record data for minimum of 4 weeks between battery charges. However, it still could not be worn while showering or bathing. The engineering device company was awarded two grants initially which resulted in two prototypes, the second building on knowledge from the first.
Three pilot studies were performed with a goal to determine the tolerability and efficacy of these new devices in the measurement of hot flashes. The first study measured the initial prototype in a laboratory type setting for 24 hours and then in the ambulatory setting for 5 weeks. A second prototype was tested over 24 hours in a monitored setting to evaluate the sensitivity and specificity of this improved device. The purpose of this paper is to report the experience of the clinical testing of these two initial prototypes.
Methods
All trials involved postmenopausal women who reported bothersome hot flashes (defined by occurrence ≥4 times per day). Daily hot flashes had to be present for ≥1 month immediately prior to study entry. Participants also had to be ≥18 years of age with excellent performance status. Women were not eligible if they had a history of allergic or adverse reaction to adhesives, current use of implanted pacemakers or metal implants, or current reliance on electronic devices for regular monitoring (i.e. insulin pumps or blood pressure monitors). Volunteers for the study were recruited from advertisements inside Mayo Clinic, Rochester and the surrounding community. Women received payment for their time and effort since this study did not offer an intervention.
The first set of trials utilized the first prototype of the skin conductance monitor. One study was developed with two phases. As this was a feasibility study related to the function of the device and did not involve an intervention, we wanted to keep the sample size as small as possible to meet the objective, hence minimizing burden. Therefore, we estimated a sample size of 20, 3 women for the 24 hour overnight phase, and 17 for the outpatient phase. The three women participating in the overnight phase were admitted to the General Clinical Research Center (GCRC) facility and the hot flash monitor was connected to the participant. The women wore the SSC device for 24 hours, including a 30 minute treadmill exercise test. A hot flash diary was completed continuously for 24 hours and a comfort, bother, and weight questionnaire was completed at the end of the GCRC stay. Another pilot trial of the first prototype investigated the use of the hot flash monitor in the ambulatory setting. Eligible women were provided a skin conductance hot flash device and were instructed to wear it for 5 weeks. They were also given a hot flash diary to fill out daily, in real time, and a comfort, bother, weight questionnaire to complete at the end of week 5. The monitors were attached initially by study personnel. Participants would remove the device once daily for showering. The times the device was unattached and reattached were recorded in the diary. Each patient was contacted by the study nurse or other research personnel by telephone weekly during weeks 1–5 to determine compliance, answer questions, and encourage continued completion of the booklet. The study utilized the Common Terminology Criteria for Adverse Events (CTCAE) for adverse event monitoring and reporting. The goals of these studies were to determine whether hot flash frequency as measured by the skin conductance measure and recording tool, correlates with the patient recorded hot flash frequency per hot flash diary in a controlled and ambulatory setting. Also, it aimed to evaluate comfort and obtrusiveness of using the skin conductance recording tool over 5 weeks. The last trial utilized the same methodology as the first trial, including 24 hours of monitoring in the GCRC, but used the second prototype of the device with upgrades based on what was learned in the first set of trials.
Data recordings from the device were reviewed for each participant and any rapid increase in skin conductance was identified. Based on previous data reported by Freedman et al, a hot flash was defined as an increase in skin conductance level ≥2 μmho within a 30-second period.7 The device-identified hot flash has a quick spike with a slow decent. If the increase in skin conductance appeared to have a quick spike, but was <2 μmho it was identified but not counted as an objective hot flash. Hot flash diaries were then reviewed and if the device-identified hot flash was within 10 minutes of the diary recorded hot flash, they were considered to be concordant. There is no known accepted criteria regarding timing of a diary recorded hot flash and SCC recorded hot flash.
Descriptive statistics were utilized. Concordance rates were calculated as the percentage of hot flashes identified by both SCC device and patient reported diaries. Under-reported hot flashes were those that were recorded on the SSC but not in patient diary. Over-reported hot flashes were those that were recorded by patients and not identified by the SSC. Sensitivity was calculated as the proportion of SCC defined hot flashes that were also reported by the patient divided by the total number of SCC defined hot flashes. In these calculations SSC measured hot flashes were used as the referent measure.
Results
SSC Prototype 1
All 3 participants enrolled in the first study spent 24 hours in the GCRC and completed the protocol. The median age of the participants was 53. Seventeen women were enrolled in the second study, mean age of 57. They all wore the device for 5 weeks; there were missing data for one patient, which was reportedly lost in the mail. Overall, the device was well tolerated, although, 5/17 (31%) reported that it prevented them from doing activities, specifically showering/bathing, air travel, sex, and water aerobics. Thirteen of 17 (81%) women reported that they would prefer to wear a device rather than fill out a hot flash diary. Unfortunately, hot flash data could not be derived from the first prototype. It was theorized that this was because special conductance gel was not utilized and the electrode patches were not able to appropriately pick up conductance.
SSC Prototype 2
Three patients were enrolled in this trial and all participants spent 24 hours in the GCRC. Despite the use of conductance gel, only one device recorded an entire 24 hours of data. The other two participants have device data for 5 and 6 hours, respectively. The device recorded during exercise for all patients. Table 1 reviews the concordance rate and sensitivity of the device. A total of 21 hot flashes were recorded in the daily diaries over 24 hours. Five diary recorded hot flashes were detected by the device, resulting in a concordance rate of 24%. Six diary recorded hot flashes corresponded with a rapid rise in skin conductance on the device that was < 2 μmho. Therefore, if the recordings were judged utilizing less strict spike criteria, that would be < 2 μmho in magnitude, then 11 diary recorded hot flashes were identified by the device. Samples of data from the skin conductance device are shown in Figure 1.
Table 1.
Concordance between a sternal skin conductance device and diary-recorded hot flashes
| Detected by Device | Indicated in Diary | |
|---|---|---|
| Yes | No | |
| Yes | 5 (6)a | 6 (5)a |
| No | 16 | |
| Study Characteristics | ||
| % Concordance | 5/21 (24%) | |
| % False-Positives (under-reporting) | 6/21 (29%) | |
| % False-Negatives (over-reporting) | 16/21 (76%) | |
| Sensitivity | 0.45 | |
Rise in skin conductance level lower than 2 μmho.
Figure 1.
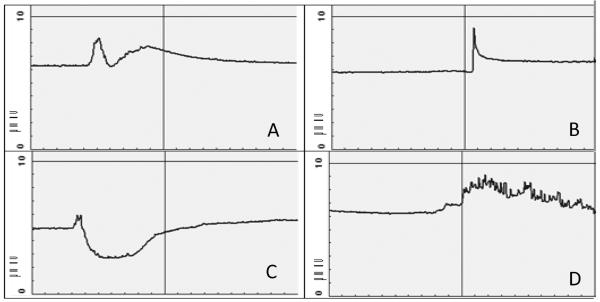
Sample Skin Conductance Data; Each image displays 10 minutes.
A. Skin conductance data for hot flash, recorded using diary
B. Skin conductance data for hot flash, not recorded using diary
C. Skin conductance data (<2 μmho) for hot flash, recorded using diary
D. Skin conductance data during exercise
Overall, wearing the device was acceptable and 2/3 patients would prefer to wear the device rather than keep a hot flash diary. However, one patient reported that the device affected her ability to shower and the electrodes made her skin itch.
Discussion
Despite improvements from lessons learned in the first prototype, only one device in one patient functioned as expected over the study period. However, we were able to collect data on the acceptability of wearing this device for 5 weeks in the outpatient setting. This is the longest period of time that a SSC device has been wore in an ambulatory setting, as previous studies of SSC devices only have patients wear it for 24–36 hour periods. This study shows that it is feasible and acceptable for women to wear a small device over a 5 week period in the outpatient setting without significant impairment in their quality of life. However, patients still did report some difficulties with wearing the monitor including interference with bathing, traveling, sex, and limitation of clothing selection. In addition, some developed skin irritation from the adhesives and many patients still found the device to be bulky and requested a smaller more discrete monitor. Interestingly, women preferred the device over having to keep a paper diary in real time to capture their hot flashes.
Overall the SCC device proved to be unreliable and only limited data were available to determine concordance rate, which demonstrated that only 24% of hot flashes recorded by women were identified by the device as a rise in conductance > 2 μmho. Although early studies reported a high concordance between SSC and patient reported hot flashes,7,9 more recent studies report significantly lower concordance rates.10,13,14 A recent meta-analysis included 24 studies which utilized SSC monitors to measure hot flashes and found that the overall concordance rates between objective and subjective measurements of hot flashes were low, around 29%, although there was significant heterogeneity between studies.10 This is similar to the rate in this study experience.
Under-reporting (hot flashes recorded by SSC monitor and not recorded by patients) is a common cause of discordance between objective and subjective hot flash measurements, with rates between 17%– 42%, depending on setting and time of day.10 However, there are multiple possible explanations for the occurrence of under-reporting. One possibility is that patients do not document all of their experienced hot flashes, whether this is from forgetting, noncompliance, or inability to document due to circumstance (driving, sleeping etc.). This theory can be supported by evidence that concordance rates improve in a nonambulatory setting when it is proposed that patients may have less distracting factors.10 In addition, concordance rates tend to decrease during sleep, a time when patients may not wake up to record their hot flashes.15,16 However, another possibility is that patients do not perceive these episodes as hot flashes and therefore are not recording them. In addition, other traits, such as coping style or optimism may affect reporting.17
Moreover, as the SSC measures skin conductance induced by sweating and/or sympathetic activation, it is possible that the SSC documented hot flashes which are not recorded by patients are not truly hot flashes, but conductance changes related to other causes of sympathetic activation. In our study, 3 patients wore the SSC monitor during physical exercise and had recorded activity on the monitor that had similarities to that recorded during a hot flash (Figure 1). This is the only study, to our knowledge, that recorded SSC data during exercise. However, an older study did report that premenopausal women had hot flashes recorded on the SSC when they did not subjectively report the hot flash and these occurred with exertion and stress. These recorded hot flashes were similar to that of the hot flashes seen in postmenopausal women.18 It has been proposed that spikes recorded by the SSC must be true hot flashes since they met the 2 μmho change and are similar in appearance to previous hot flashes that were concordant with hot flashes recorded by patients.18 Yet, there remains a lack of evidence that the recorded SSC spikes are not a result of other causes of sympathetic activation.
The other cause of decreased concordance rates between SSC and subjective reporting are hot flashes recorded by the patient, but not recorded by the device, this has been referred to as over-reporting or false-positives, and this is the most significant contributing factor to the low concordance in our experience. However, this must be interpreted carefully. One possible explanation for this occurrence is that patients are truly over-reporting hot flashes. This is supported by data that false-positive hot flash reports are more likely after elevated frustration and decreased feelings of control.19 However, it may also be that the SSC is not sensitive enough to record mild hot flashes. One study showed that nearly 50% of subjective distress from hot flashes occurred when the sternal skin conductance magnitude was less that the established cutoff point of 2 μmho within 30 seconds.6 In our study, 6 of the subjectively recorded hot flashes occurred with changes in SSC magnitude less than 2 μmho (Figure 1) and the majority of hot flashes recorded by patients and not by the SSC monitor were rated as mild. These findings are consistent with other studies which show that subjective reported hot flashes not documented on the SSC monitor, tend to be mild or moderate, not severe.13 In addition, differences in sensitivity of SSC monitoring can occur based on the electrolyte media used for the measurements.20 Moreover, rises in skin conductance have varying appearances as shown in Figure 1. Although there have been proposed recommendations for interpretation of SSC monitoring, there appears to be a lack of any definitive algorithm to define a hot flash, making interpretation of the data difficult.
Another important limitation of SSC monitors are that they do not provide any data on severity. Severity is important in the measurement of hot flashes, as severity ratings take into account different aspect of the hot flash experience, including duration, physical symptoms, emotional symptoms, and interference with functioning. It has been shown that hot flash severity is directly related to greater perceived hot flash interference, measured via the Hot Flash Related Daily Interference Scale.21 Currently, SSC monitors have no ability to measure hot flash severity or distress. Carpenter et al. investigated this relationship and reported that the amount of change in SSC during the 30 second rise associated with each hot flash did not correspond to subjective hot flash intensity.6
Conclusions
Overall, the accuracy and utility of SSC in the measurement of hot flashes is controversial and recent data are showing that in addition to technical issues, the use of skin conductance as a physical surrogate for hot flashes may not indeed be as specific a measure as required for rigorous studies. Recently, a large trial involving 395 breast cancer survivors collected hot flash data for 24–36 hours using SSC, event monitors, and patient reported dairies. Researchers, including leaders in the field of hot flash measurement, reported that the objective measurement with SSC had considerable measurement error and may be a less reliable indicator of hot flash symptoms than self-reports.11 Meanwhile, studies continue to demonstrate effective interventions for hot flashes22–24 and women continue to be able to articulate their need for treatment of this bothersome symptom. Therefore, if the need for a valid physiologic measure is still deemed important for hot flash research to continue, it is time to identify a more reliable and specific surrogate.
Acknowledgments
Supported by Grant Number NIH, R43 EB 06013, “Ambulatory Sternal Skin Conductance Recording Tool; R43 AT004075 NIH “Self—Reported Menopausal Symptomatology and Wireless Skin Conductance Recorder”; UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS) and the United States National Institutes of Health Grant-CA 124477
Footnotes
There are no any conflicts of interest/financial disclosures for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–33. [DOI] [PubMed] [Google Scholar]
- 2.Maclennan AH, Broadbent JL, Lester S, et al. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004:CD002978. doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J Clin Oncol. 2009;27:2831–7. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 5.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JS, Azzouz F, Monahan PO, et al. Is sternal skin conductance monitoring a valid measure of hot flash intensity or distress? Menopause. 2005;12:512–9. doi: 10.1097/01.gme.0000170957.31542.1c. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–9. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 8.Tataryn IV, Lomax P, Meldrum DR, et al. Objective techniques for the assessment of postmenopausal hot flashes. Obstet Gynecol. 1981;57:340–4. [PubMed] [Google Scholar]
- 9.Freedman RR, Norton D, Woodward S, et al. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–8. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 10.Mann E, Hunter MS. Concordance between self-reported and sternal skin conductance measures of hot flushes in symptomatic perimenopausal and postmenopausal women: a systematic review. Menopause. 2011;18:709–22. doi: 10.1097/gme.0b013e318204a1fb. [DOI] [PubMed] [Google Scholar]
- 11.Rand KL, Otte JL, Flockhart D, et al. Modeling hot flushes and quality of life in breast cancer survivors. Climacteric. 2011;14:171–80. doi: 10.3109/13697131003717070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Accessed December 13, 2011]; http://www.ufiservingscience.com/hot_flash.html,
- 13.Hunter MS, Haqqani JR. An investigation of discordance between subjective and physiological measures of vasomotor symptoms. Climacteric. 2011;14:146–51. doi: 10.3109/13697131003735585. [DOI] [PubMed] [Google Scholar]
- 14.Otte JL, Flockhart D, Hayes D, et al. Comparison of subjective and objective hot flash measures over time among breast cancer survivors initiating aromatase inhibitor therapy. Menopause. 2009;16:653–9. doi: 10.1097/gme.0b013e3181a5d0d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–6. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 16.Hanisch LJ, Palmer SC, Marcus SC, et al. Comparison of objective and patient-reported hot flash measures in men with prostate cancer. J Support Oncol. 2009;7:131–5. [PubMed] [Google Scholar]
- 17.Caltabiano ML, Holzheimer M. Dispositional factors, coping and adaptation during menopause. Climacteric. 1999;2:21–8. doi: 10.3109/13697139909025559. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter JS, Andrykowski MA, Freedman RR, et al. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–15. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 19.Thurston RC, Blumenthal JA, Babyak MA, et al. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67:137–46. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 20.Dormire SL, Carpenter JS. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology. 2002;39:423–6. doi: 10.1017.S0048577201393149. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JS, Rand KL. Modeling the hot flash experience in breast cancer survivors. Menopause. 2008;15:469–75. doi: 10.1097/gme.0b013e3181591db7. [DOI] [PubMed] [Google Scholar]
- 22.Barton DL, LaVasseur BI, Sloan JA, et al. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. J Clin Oncol. 2010;28:3278–83. doi: 10.1200/JCO.2009.26.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elkins GR, Fisher WI, Johnson AK, et al. Clinical hypnosis in the treatment of postmenopausal hot flashes: a randomized controlled trial. Menopause. 2012 doi: 10.1097/GME.0b013e31826ce3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267–74. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


