Abstract
Introduction
Kras mutations have been thought to play an important role in pancreatic cancer progression. In this study, we evaluated how serially passaging primary pancreatic tumors with and without Kras mutations, in nude mice, can generate more aggressive variants of human pancreatic cancer.
Materials & Methods
Orthotopic mouse models of human pancreatic cancer were established by injecting 1×106 cells of the Kras wildtype BxPC-3 cell line, expressing red fluorescent protein (RFP) or the Kras mutant Panc-1 cell line expressing green fluorescent protein (GFP), into the pancreas. Pancreatic tumors were harvested from premorbid mice to establish cell lines. One million passaged cells were then orthotopically injected into another set of mice. Serial passaging continued until decreasing lifespan of the implanted mice stabilized, which occurred by 6 passages. Mice harboring serially-passaged cell lines were followed with weekly imaging.
Results
Serially passaging generated more aggressive variants of both human pancreatic cancer cell lines, one which was Kras wild-type (BXPC-3) and the other Kras mutant, Panc-1, which displayed faster tumor growth and shortened survival time. Overall survival decreased from 18 weeks in mice with the parental cell line (P0) tumor to ~6 weeks in mice by the sixth passage (P6). Average time to metastasis was shortened from 14 weeks to ~3 weeks or less. At termination, mice with the passaged tumor demonstrated a greater extent of distant metastasis.
Conclusions
Serial passaging of tumor creates more aggressive variants of human pancreatic cancer cell lines regardless of Kras mutation. The aggressive variants can be used to study the molecular basis of highly malignant pancreatic cancer and to screen for effective agents against this disease.
Keywords: pancreatic cancer, orthotopic mouse models, in vivo selection, Kras, metastasis, variants, survival
INTRODUCTION
Our laboratory developed the first patient-like orthotopic mouse models of pancreatic cancer (1, 2). In an effort to help develop this patient-like model, orthotopic transplantation of histologically intact pancreatic-cancer specimens, including patient specimens, in nude mice was used. The model resembles the clinical picture including (i) extensive local tumor growth, (ii) extension of the locally growing tumor to the nude mouse stomach and duodenum, (iii) metastases to liver and regional lymph nodes, and (iv) distant metastases to the adrenal gland, diaphragm, and mediastinal lymph nodes. Immunohistochemical analysis of the antigenic phenotype of the transplanted human pancreatic tumors showed a similar pattern of expression to the original patients of two different human tumor-associated antigens (1). We have developed another orthotopic mouse model using a passaged human pancreatic cancer line, PANC-4. The tumor grew with subsequent invasive local tumor growth and liver and peritoneal metastases (2).
Subsequently, the genetic reporters green fluorescent protein (GFP) and red fluorescent protein (RFP) were used to produce imageable models of pancreatic cancer. Human pancreatic tumor cell lines, BxPC-3 and MiaPaCa-2, were engineered to stably express high-levels of GFP. The GFP-expressing pancreatic tumor cell lines were surgically orthotopically implanted as tissue fragments in the body of the pancreas of nude mice. Whole-body optical images visualized real-time primary tumor growth and formation of metastatic lesions that developed in the spleen, bowel, portal lymph nodes, omentum, and liver. Intravital imaging was used for quantification of growth of micrometastasis on the liver and stomach (3).
A highly-fluorescent RFP-expressing pancreatic cancer model was orthotopically established in nude mice with the MIA-PaCa-2 human pancreatic cancer cell line. Fluorescent tumor fragments were surgically transplanted onto the nude mouse pancreas. Groups treated with intraperitoneal gemcitabine or intravenous irinotecan were sequentially and non-invasively imaged to compare, in real time, the antimetastatic and antitumor effects of these agents compared with untreated controls. Gemcitabine highly improved survival by inducing transient tumor regression over the first 3 weeks. However, at this time, growth and dissemination occurred despite continued treatment, suggesting the development of tumor resistance (4). In this model we showed that metronomic gemcitabine was more effective (5).
Tumors from pancreatic cancer patients were established in NOD/SCID mice immediately after surgery and subsequently passaged orthotopically in transgenic nude mice ubiquitously expressing GFP. The primary patient tumors acquired GFP-expressing stroma. Subsequent liver metastases, and disseminated peritoneal metastases maintained the stroma from the primary tumor, and possibly recruited additional GFP-expressing stroma, resulting in their very bright fluorescence of the metastases. The GFP-expressing stroma included cancer-associated fibroblasts and tumor-associated macrophages in both the primary and metastatic tumors (6). The tumors were then passaged orthotopically to non-transgenic nude mice. It was possible to longitudinally image the brightly fluorescent tumors noninvasively as they progressed in the nontransgenic nude mice (7).
Fidler’s group selected metastatic variants from the human Colo-357 cell line by serial orthotopic passage. Cells were injected into the spleen or pancreas of nude mice. Hepatic metastases were harvested, and tumor cells were reinjected into the spleen or pancreas. This cycle was repeated several times to yield cell lines L3.6sl (spleen to liver) and L3.6pl (pancreas to liver). The variant cells produced significantly higher incidence and number of lymph node and liver metastases than the parental cells (8).
Since the pancreatic cancer cell lines used by Fidler were already aggressive, it was thought by using less aggressive human pancreatic cancer cell lines that a wider range of metastatic variants could be isolated and that these would be useful to study metastasis of this disease. In addition, the cell lines used in this report express RFP or GFP, enabling facile real-time imaging of tumor progression and metastasis in order to rapidly characterize variants.
In this study, serial passage of pancreatic tumor, in nude mice selected for more aggressive variants of two human pancreatic cancer epithelial-like cell lines, BxPC-3 and Panc-1. Panc-1 contains the Kras mutation while BxPC-3 does not (9). However, both cell lines demonstrate, with in vivo selection, increasingly more aggressive tumor biology as evident by faster tumor growth and earlier metastasis with each in vivo passage. The results suggest that highly aggressive pancreatic cancer variant can be selected from either Kras wild-type or mutant cell line.
MATERIALS AND METHODS
Cell Culture
The human pancreatic cancer cell lines BxPC-3 and Panc-1 were obtained from the ATCC and stably transfected with either RFP or GFP using protocols previously described by our laboratories (10–12). BxPC-3-DsRed, with a normal Kras gene, and Panc-1-GFP, with a mutant Kras gene, were maintained in RPMI 1640 media or in DMEM (Gibco-BRL, Grand Island, NY), respectively, supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). The cell culture medium was supplemented with penicillin or streptomycin (Gibco-BRL), sodium pyruvate (Gibco-BRL), sodium bicarbonate (Cellgro, Manassas, VA), L-glutamine (Gibco-BRL), and minimal essential medium nonessential amino acids (Gibco-BRL). Cells were incubated at 37°C with 5% carbon dioxide. The cell lines were not previously passaged more than 4 times in vitro our laboratory.
Animal Care
Female athymic nu/nu nude mice (AntiCancer, Inc., San Diego, CA) were maintained in a barrier facility on high-efficiency particulate air filtered racks. The animals were fed with autoclaved laboratory rodent diet (Tecklad LM-485; Western Research Products, Orange, CA). All surgical procedures were performed under anesthesia with an intramuscular injection of 100 μl of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine. For each procedure, 20 μl of 1 mg/kg buprenorphine was administered for pain control. Euthanasia was achieved by 100% carbon dioxide inhalation, followed by cervical dislocation. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Animals under assurance number A3873–01.
Orthotopic Implantation of Pancreatic Cancer Cells
Human BxPC-3-RFP and Panc-1-GFP cancer cells were harvested by trypsinization and washed twice with serum-free medium. Viability was verified to be greater than 95% using the Vi-Cell XR automated cell viability analyzer (Beckman Coulter, Brea, CA). The cells were resuspended at 106 cells per 10 μl of serum-free medium. Orthotopic human pancreatic cancer xenografts were established in nude mice by direct injection of fluorescent BxPC-3-RFP or Panc-1-GFP tumor cells into the pancreas. A small 6 to 10-mm transverse incision was made on the left flank of the mouse through the skin and peritoneum. The tail of the pancreas was exposed through this incision and 1×106 cells, mixed 1:1 with matrigel (BD Biosciences, Bradford MA) in a 10-μL final volume, were injected into the pancreatic tail using a Hamilton syringe (Hamilton Co, Reno NV). Upon completion, the pancreas was returned to the abdomen and the incision was closed in two layers using 6.0 Ethibond non-absorbable sutures (Ethicon Inc., Somerville, NJ).
Establishing High Metastatic Variants by Passage
Orthotopic human pancreatic cancer xenografts from pancreatic cancer cell lines BxPC-3-RFP and Panc-1-GFP were initially established in nude mice by direct injection as described above. These tumors were allowed to grow until the animals developed widespread metastases and malignant ascites. Strict criteria were followed to determine premorbidity in which mice were graded on a scale of 0 to 4 for degree of cachexia, ascities and immobility with 4 designated as the highest tumor progression. If a mouse had at least two of the three categories or immobility as a grade 4, the mouse was sacrificed. At the time of sacrifice, the primary tumor was collected and gently dissociated and plated for in vitro culture. After one to two in vitro passages for expansion, the passaged cell line was injected orthotopically into the tail of the pancreas of another set of athymic nu/nu nude mice. Adequate expansion also permitted storage of passaged cells in liquid nitrogen for later analysis. This process of serially passaging tumor in mouse models continued until the decreasing lifespan of tumor-bearing mice plateaued and stabilized.
Animal Imaging
Mice were followed post-implantation to evaluate tumor progression and metastasis. Mice were imaged on a weekly basis using the Olympus OV-100 Small Animal Imaging System (Olympus Corp, Tokyo Japan) (13), containing an MT-20 light source (Olympus Biosystems, Planegg Germany) and DP70 CCD camera (Olympus Corp.). All images were analyzed with Image J v1.440 (National Institutes of Health, Bethesda, MD).
Histology
Primary and metastatic tumor samples were removed en bloc with surrounding tissue at the time of sacrifice, fixed in Bouin’s Solution, and embedded in paraffin prior to sectioning and staining with hematoxylin and eosin (H&E) for standard light microscopy. Frozen samples were also stored in OCT and were later sectioned for fluorescent light microscopy. Lung samples from mice implanted with the parental line as well as the passaged line of BxPC-3-RFP were randomly selected and sectioned. All slides were imaged using the Virtual Microscopy NanoZoomer-XR (Hamamatsu Corporation, Hamamatsu City, Japan) that would capture the entire slide for later analysis of the area of fluorescence using Image J. All images were processed for contrast and brightness with the use of the NanoZoomer viewer and of Photoshop element-4.
Statistical Analysis
PASWStatistics 18.0 (SPSS, Inc.) or R v. 2.11.0 was used for statistical analyses. ANOVA models were used to compare continuous variables between multiple groups. Survival outcomes were compared using log rank tests with confidence intervals computed and Kaplan-Meier curves were generated. A p-value of ≤0.05 was considered statistically significant for all comparisons.
RESULTS
Serial in vivo passaging of primary tumor from human pancreatic cancer-bearing nu/nu mice was used to generate a highly aggressive passage-6 (P6) of BxPC-3-RFP cell line and passage-5 (P5) of Panc-1-GFP cell line. These passaged lines demonstrated more rapid primary tumor growth and faster time to distant metastasis compared to the parental lines.
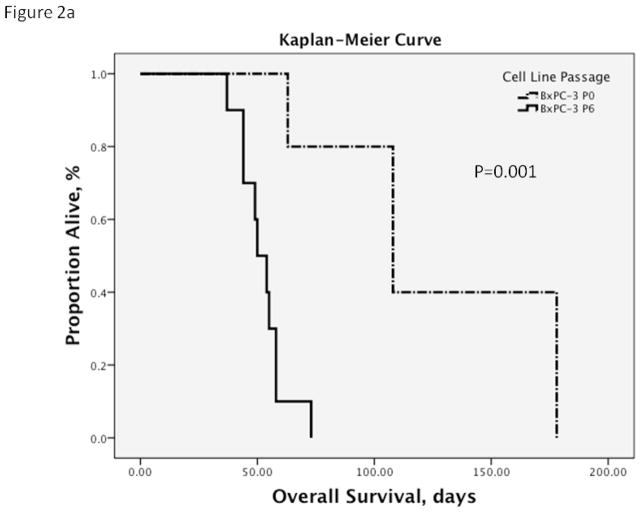
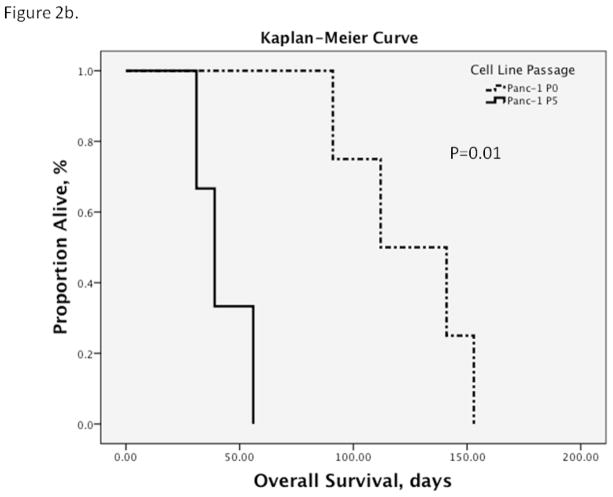
With passage, the overall survival of mice harboring tumors shortened significantly (p=0.002). The decrease in overall survival of tumor-bearing mice continued until the 5th passage in which both pancreatic cancer cell lines resulted in a plateau in the lifespan of the mouse. Additional passages after this point failed to decrease the overall survival, indicating stabilization of overall survival in tumor-bearing mice (Figure 1). By the 6th in vivo passage of BxPC-3-RFP and the 5th in vivo passage of Panc-1-GFP, the overall survival in tumor-bearing mice was significantly decreased as described above (Figure 2a&b). Mice implanted with BxPC-3-RFP (P6) experienced a significantly shorter overall survival of 52 days compared to 127 days of mice implanted with the parental line (p=0.001). A similar impressive difference was witnessed in mice implanted with Panc-1-GFP (P5) in which overall survival was decreased from 124 days in the parental cell line groups to 42 days (p=0.01) in the (P5) cell line group.
Figure 1. Passing pancreatic cancer cell lines decreases survival time of tumor-bearing animals.
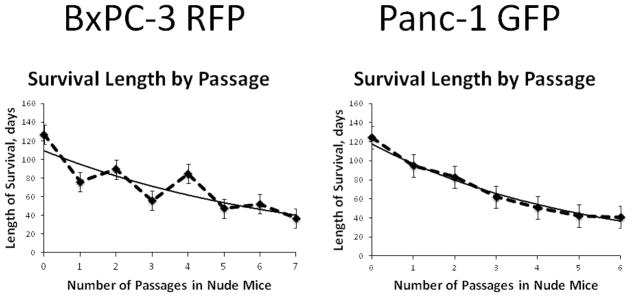
The lifespan of tumor-bearing mice shortened with each passage (p=0.002) but started to plateau by the 5th passage. Passaging beyond this point did not decrease the lifespan of tumor-bearing mice and overall survival stabilized in both pancreatic cancer cell lines.
Figure 2. Kaplan-Meier Survival Curves.
a. By the 6th in vivo passage of BxPC-3, the overall survival in P6 tumor-bearing mice had decreased from an average of 127 days (95% CI 83.1, 170.9) to 52.2 days (95% CI 46.0, 58.4) compared to the parental cell line (P0). The median survival decreased from 108 days of the parental line (95% CI 59.7, 156.3) to 50 days (95% CI 42.3, 57.7; p=0.001).
b. There was a similar effect with serial in vivo passaging of Panc-1. Overall survival in P5 tumor-bearing mice decreased from an average of 124.3 days of the parental line (95% CI 96.7, 151.8) to 42 days (95% CI 27.6, 56.4) compared to the parental cell line (P0). Median overall survival shortened from 112 days (95% CI 63, 161) to 39 days (95% CI 26.2, 51.8; p=0.01).
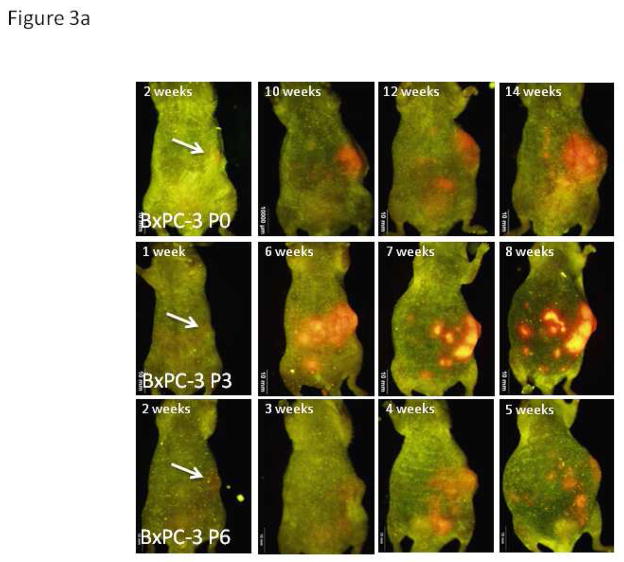
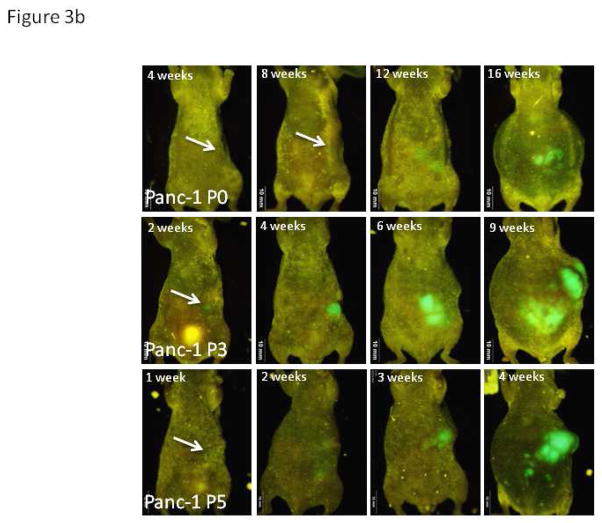
The significantly shorter survival of mice harboring the passaged pancreatic cancer cell lines was not only a result of the faster primary tumor growth but more importantly from more widespread and early distant metastasis (Figure 3). The tumors generated from the parental cell lines metastasized slowly with eventual metastasis, on average, by 14.5 weeks for BxPC-3-RFP and 13.3 weeks for Panc-1-GFP (range 10 to 18 weeks). This widespread metastasis that would eventually develop in the parental cell line groups resulted in premorbid mice that were very cachectic with large ascites. Repeated in vivo passaging of primary tumor expedited this process in which mice would experience widespread, distant metastatic disease much sooner. As early as two weeks after implantation, mice harboring these more aggressive variants of human pancreatic cancer had evidence of widespread metastatic disease. By four weeks, all mice with BxPC-3-RFP (P6) or Panc-1-GFP (P5) tumors had distant and severe metastatic disease that resulted in criteria for premorbidity to be met in all mice by 8 weeks.
Figure 3. Weekly Whole-body Images.
a. Representative images of mice implanted with differently passaged cells of BxPC-3-RFP demonstrate the more aggressive tumor biology of cancer cells generated with in vivo passaging. Serial in vivo passaging of BxPC-3 in mice decreased the average time until metastasis was detected by whole body imaging from 14.5 weeks in the parental line (95% CI 10.5, 18.5) to 3.3 weeks in passage 6 (95% CI 2.7, 3.9; p=0.001).
b. Representative images of mice implanted with differently-passaged cells of Panc-1-GFP demonstrate the more aggressive tumor biology of cancer cells generated with in vivo passaging. Serial in vivo passaging of Panc-1 in mice decreased the average time until metastasis was detected on whole body imaging from 13.3 weeks in the parental line (95% CI 11.1, 15.4) to 3.7 weeks in passage 5 (95% CI 0.9, 1.9; p=0.01). As evident in Figure 3a and 3b, images after one week demonstrate a single area of fluorescence in the left upper quadrant of tumor-bearing mice indicating the presence of a primary tumor in the orthotopic model. The lack of additional fluorescence throughout the abdomen indicates absence of spread due to poor techniques or ruptured tumor. All mice were imaged weekly starting week 1 after implantation of cells and all images strongly suggest the above. Non-invasive imaging was carried out with the OV100 Small Animal Imaging System (Olympus).
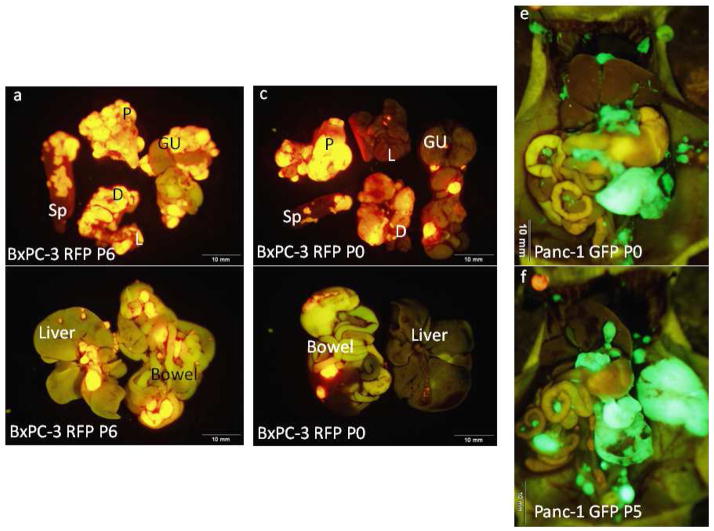
Orthotopic parental and aggressive-variant tumors metastasized to distant sites in a similar distribution and yielded histologically similar lesions (Figure 4). Parental and passaged lines metastasized to the liver, spleen, mesentery, diaphragm, lung and genitourinary system. However, organs of mice implanted with the aggressive-variant tumors had a greater and much earlier burden of metastatic disease at time of termination which at 4 weeks for passage 6 vs 26 weeks for parental line for BxPC-3 (Figure 4a–d) and at 5 weeks for the passage 5 vs 16 weeks for parental Panc-1 (Figure 4e&f). Closer evaluation of lung samples (Figure 5) further illustrated the significant difference in the metastatic potential between the parental line of BxPC-3-RFP and its more aggressive variant (P6). The lung tissue of mice implanted with P6 of BxPC-3-RFP had evidence of more and much earlier metastatic lesions compared to that of mice implanted with the parental line.
Figure 4. Images of Mice at Termination.
Representative mouse implanted with BxPC-3 RFP P6 demonstrates greater metastatic burden at 6 weeks termination (a&b) compared to a representative mouse implanted with the parental line of BxPC-3 RFP at 26 weeks termination (c&d). A similar pattern was seen in the Panc-1 GFP cell line in which the multiple-passaged tumor (f) experienced greater intra-abdominal metastatic burden at 5 weeks termination compared to a mouse implanted with the parental line (e) at 16 weeks termination. Sp, spleen; P, pancreas; GU, genitourinary system; D, diaphragm; L, lung
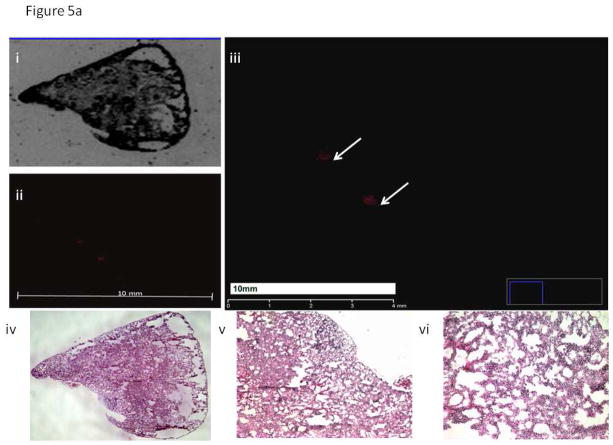
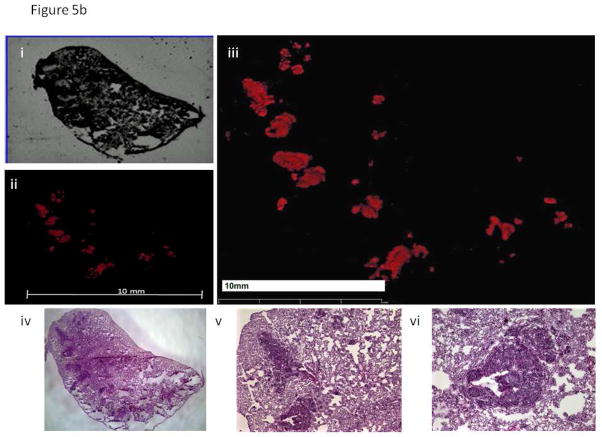
Figure 5. Histology Images of Lung Samples.
a. Representative lung sample from a mouse implanted with BxPC-3 RFP P0, small microscopic metastatic lesions are noted.
- Bright-field image of lung section from a mouse implanted with BxPC-3 RFP P0.
- Fluorescence image of lung section from a mouse implanted with BxPC-3 RFP P0.
- Enlarged fluorescence image of lung section from a mouse implanted with BxPC-3 RFP P0. Arrows indicate two small areas of metastatic foci within a section of lung tissue from a mouse harboring the BxPC-3 RFP parental line. Red fluorescence indicates the presence of tumor within the tissue sample.
- H&E image of lung section from a mouse implanted with BxPC-3 RFP P0.
- 4X magnification of H&E image of lung section from a mouse implanted with BxPC-3 RFP P0.
- 10X magnification of H&E image of lung section from a mouse implanted with BxPC-3 RFP P0.
b. Mice implanted with BxPC-3 RFP P6 had significantly more metastatic lesions to the lung than mice implanted with BxPC-3 RFP P0.
- Bright field image of lung section from a mouse implanted with BxPC-3 RFP P6.
- Fluorescence image of lung section from a mouse implanted with BxPC-3 RFP P6.
- Enlarged fluorescence image of lung section from a mouse implanted with BxPC-3 RFP P6. The red fluorescence indicates the presence of metastatic foci within the tissue sample. The area of fluorescence is significantly greater in the section of lung metastasis from a mouse harboring the BxPC-3 RFP P6 line.
- H&E image of lung section from a mouse implanted with BxPC-3 RFP P6.
- 4X magnification of H&E image of lung section from a mouse implanted with BxPC-3 RFP P6.
- 10X magnification of H&E image of lung section from a mouse implanted with BxPC-3 RFP P6.
DISCUSSION
The present study has demonstrated that variants of a human pancreatic cancer cell line can be generated that are much more aggressive than the parental line by simple in vivo passage regardless of initial Kras status. Indeed the BxPC3-RFP passage-6 variants are more tumorigenic than the parent; have a much earlier time to metastasis (3 weeks compared to 12 weeks) and have over a 2-fold decrease in the median survival rate (Table 1). Similar results were seen for the Panc-1-GFP passage-5 variants when compared to the parental cell line (Table 1).
TABLE 1.
| Cell Line Passage | Median time to metastases N, (95% CI) | Median Overall Survival N, (95% CI) | Tumorigenicity |
|---|---|---|---|
|
| |||
| BxPC-3-RFP | |||
| P0 | 12 weeks, (6.8, 17.2) | 108 days, (59.7, 156.3) | 8/13 |
| P6 | 3 weeks, (2.2, 3.8) | 50 days, (42.3, 57.5) | 10/10 |
|
| |||
| Panc-1-GFP | |||
| P0 | 14 weeks, (10.6, 17.4) | 112 days, (63, 161) | 4/8 |
| P5 | 4 weeks, (0.8, 7.2) | 39 days, (26.2, 51.8) | 5/5 |
CI = confidence interval.
Bruns et al (8) also isolated high metastatic variants of pancreatic cancer. However, our study demonstrates the isolation of variants with extremely early extensive metastasis observable by as early as 2 weeks, resulting in decreases of survival by approximately 80 days. In addition the number of cells necessary to obtain tumors in all mice from the P6 variant of BxPC-3-RFP is 100 cells or less. The parental BxPC-3 forms no tumors with 100 cells (data not shown). The current study was facilitated by the use of fluorescent protein-based imaging, allowing all metastatic sites to be readily detected.
The importance of the Kras mutation in pancreatic cancer has been suggested (14–16). Pancreatic cancer cell lines were previously subdivided into “Kras mutation” –dependent and “Kras mutation” –independent subsets (17, 18). In the present study, regardless of initial Kras mutation status of the pancreatic cell line, the effects of serial passage were similar on cancer cell biology. While Panc-1 is Kras mutation-dependent, BxPC-3 is not. Yet serial passaging of both cell lines generated in vivo selection of more aggressive variants that demonstrated overall faster tumor growth and earlier metastasis. Furthermore, mice harboring the passaged lines had significantly shorter survival and greater distant metastasis at termination compared to those with the parental tumor. The results of the present study suggest that pancreatic cancer involves much more complex genetic changes than Kras mutations.
Future experiments will determine the Kras status in each isolated variant as well as other genes thought to be possibly involved in panceratic cancer including SMAD4 and p53 and p16. The effects of knocking down Kras with siRNA will also be determined in future experiments. Therapeutics for the high metastatic variants will be investigated in order to identify effective agents for highly aggressive pancreatic cancer in the clinic.
Acknowledgments
Work supported in part by grants from the National Cancer Institute CA142669 and CA132971 (to M.B. and AntiCancer, Inc) and T32 training grant CA121938-5 (to C.A.M.).
Footnotes
Presented at the 2013 Eighth Annual Academic Surgical Congress, February 5-7, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. A novel “patient-like” treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 1993;53:3070–3072. [PubMed] [Google Scholar]
- 3.Bouvet M, Wang J, Nardin SR, Nassirpour R, Yang M, Baranov E, Jiang P, Moossa AR, Hoffman RM. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534–1540. [PubMed] [Google Scholar]
- 4.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–160. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 5.Tran Cao HS, Bouvet M, Kaushal S, Keleman A, Romney E, Kim G, Fruehauf J, Imagawa DK, Hoffman RM, Katz MH. Metronomic gemcitabine in combination with sunitinib inhibits multisite metastasis and increases survival in an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2010;9:2068–2078. doi: 10.1158/1535-7163.MCT-10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, Moriwaki H, Bouvet M, Hoffman RM. Imageable fluorescent metastasis resulting in transgenic GFP mice orthotopically implanted with human-patient primary pancreatic cancer specimens. Anticancer Res. 2012;32:1175–1180. [PubMed] [Google Scholar]
- 7.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, Moriwaki H, Bouvet M, Hoffman RM. Non-invasive fluorescent-protein imaging of orthotopic pancreatic-cancer-patient tumorgraft progression in nude mice. Anticancer Res. 2012;32:3063–3067. [PubMed] [Google Scholar]
- 8.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirivatanauksorn V, Sirivatanauksorn Y, Gorman PA, Davidson JM, Sheer D, Moore PS, Scarpa A, Edwards PA, Lemoine NR. Non-random chromosomal rearrangements in pancreatic cancer cell lines identified by spectral karyotyping. Int J Cancer. 2001;91:350–358. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1049>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman RM, Yang M. Whole-body imaging with fluorescent proteins. Nat Protoc. 2006;1:1429–1438. doi: 10.1038/nprot.2006.223. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman RM, Yang M. Color-coded fluorescence imaging of tumor-host interactions. Nat Protoc. 2006;1:928–935. doi: 10.1038/nprot.2006.119. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nat Protoc. 2006;1:775–782. doi: 10.1038/nprot.2006.109. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]
- 14.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins MA, Brisset JC, Zhang Y, Bednar F, Pierre J, Heist KA, Galban CJ, Galban S, di Magliano MP. Metastatic pancreatic cancer is dependent on oncogenic kras in mice. PLoS One. 2012;7:e49707. doi: 10.1371/journal.pone.0049707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733. e739. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]