Abstract
Emmetropization is a vision dependent mechanism that attempts to minimize refractive error through coordinated growth of the cornea, lens and sclera such that the axial length matches the focal length of the eye. It is generally accepted that this visually guided eye growth is controlled via a cascade of locally generated chemical events that are initiated in the retina and ultimately cause changes in scleral extracellular matrix (ECM) remodeling which lead to changes in eye size and refraction. Of much interest, therefore, are the molecular mechanisms that underpin emmetropization and visually guided ocular growth. The choroid, a highly vascularized layer located between the retina and the sclera is uniquely situated to relay retina-derived signals to the sclera to effect changes in ECM synthesis and ocular size. Studies initiated by Josh Wallman, Ph.D., clearly demonstrate that the choroid plays an active role in emmetropization, both by modulation of its thickness to adjust the retina to the focal plane of the eye (choroidal accommodation), and well as through the release of growth factors that have the potential to regulate scleral extracellular matrix remodeling. His discoveries prompted numerous investigations on the molecular composition of the choroid and changes in gene expression associated with visually guided ocular growth. This article will review molecular and functional studies of the choroid to provide support for the hypothesis that the choroid is a source of sclera growth regulators that effect changes in ocular growth in response to visual stimuli.
Keywords: choroid, emmetropization, sclera, myopia
1. Introduction
It is generally accepted that visually guided eye growth is controlled via a cascade of locally generated chemical events that are initiated in the retina and ultimately cause changes in scleral extracellular matrix (ECM) remodeling. In both chicks and mammals, scleral remodeling in response to visual stimuli is characterized by changes in proteoglycan synthesis, collagen synthesis, and matrix metalloproteinase activity (Rada et al., 1992, 1999, 2000; Norton and Rada, 1995; Nickla et al., 1997). In chicks, increases in proteoglycan synthesis and accumulation within the cartilaginous layer of the sclera at the posterior pole result in increased scleral growth, ocular elongation and myopia development (Rada et al., 1992; 2002). In contrast, myopia development in tree shrews and primates is associated with decreases in scleral proteoglycan synthesis and accumulation (Norton and Rada, 1995; Rada et al., 2000; Moring et al., 2007), decreased collagen accumulation (Norton and Rada, 1995), and increased matrix metalloproteinase activity (Guggenheim and McBrien 1996). These extracellular matrix changes in mammals result in a thinner, more extensible sclera that elongates excessively in response to uniaxial tension as compared with sclera of emmetropic eyes (Siegwart and Norton, 1999). In both chicks and mammals the visually regulated scleral response is rapid (within hours) in chicks (Summers Rada and Hollaway, 2011), days in tree shrews (Siegwart and Norton, 1999) and weeks in marmosets (Troilo et al., 2006), and, in the case of form deprivation is restricted to the posterior pole (Rada et al., 1994; Norton and Rada, 1995; Rada et al., 2000).
Because of its proximity to the sclera, the choroid, a highly vascularized layer located immediately adjacent to the sclera, has been implicated in the regulation of scleral metabolism during visually guided ocular growth (Wallman et al., 1995; Marzani and Wallman, 1997; Rada and Palmer, 2007). This article will review functional aspects of the choroid to provide support for the hypothesis that the choroid is a source of scleral-growth regulators that affect changes in ocular growth in response to visual stimuli.
2. Choroid development, structure and general functions
The choroid is the vascular layer of the eye, located between the retinal pigment epithelium (RPE) and the sclera and extends from the ora serrata anteriorly to the optic nerve posteriorly. The primary function ascribed the choroid is to supply oxygen and nourishment to the outer retina. Additionally the choroid has been shown to play a significant role in the regulation of intraocular pressure (IOP) by providing an additional pathway for drainage of aqueous fluid from the anterior chamber (the uveoscleral pathway) (Alm and Nilsson, 2009) (Alm and Nilsson 2009) as well as through the regulation of choroidal blood flow (Polska et al., 2007). To meet the nutritional demands of the retina, the choroid has the highest rate of blood flow of any structure in the human body, including the brain and kidneys (Alm and Bill, 1973). The choroid consists of a layer of choriocapillaries that are adjacent to Bruch’s membrane and larger blood vessels nearer the sclera. Additionally, the chick choroid contains numerous thin-walled, endothelial-lined vessels that exhibit structural features of lymphatic vessels (lymphatic lacunae) (Junghans et al., 1996; De Stefano and Mugnaini, 1997). The lacunae are largest and most prominent toward the sclera in a region termed the suprachoroidea, and they contain a clear liquid, suprachoroidal fluid (Pendrak et al., 2000). Similar sparse lymphatic-like structures have occasionally been reported in the primate choroid eye (Krebs and Krebs, 1988; Sugita and Inokuchi, 1992). Located between the lymphatic and blood vessels is the extravascular choroidal stroma, consisting of loose connective tissue containing fibroblasts, non-vascular smooth muscle cells, melanocytes and various leukocytes. Additionally, the choroidal stroma contains a population of disperse neurons, intrinsic choroidal neurons (ICNs) (Schrodl et al., 2003: Stubinger et al., 2010), that receive input from the sympathetic and parasympathetic nervous system and may also function autonomously, similar to the ganglion cells of the enteric nervous system (Hansen, 2003). The choroid is adherent to the sclera by connective tissue strands and by numerous blood vessels and nerves that enter the choroid from the sclera. Additionally small amounts of choroidal tissue may extend into the scleral canals through which ciliary vessels and nerves enter the eye (Green, 1986).
In humans, formation of the choroidal vasculature is an early event that commences at the time of optic vesicle invagination, within the first trimester. The choroid differentiates from the mesenchymal capsule that surrounds the optic cup and is homologous in origin with the pia mater and arachnoid membranes investing the brain (the leptomeninges). The principal cells of the choroid; choroidal stromal fibroblasts, melanocytes, ICNs, pericytes, and extravascular smooth muscle cells differentiate from neural crest cell–derived mesenchyme while vascular endothelial cells are derived from mesoderm (Noden 1982; Torczynski 1982; Etchevers et al., 2001). Although the molecular mechanisms governing the formation and survival of the choroid are unclear, the development of the choroidal vasculature appears to depend on the presence of differentiated RPE and its production of inductive signals including VEGF (Zhao and Overbeek, 2001; Saint-Geniez et al., 2009).
3. Role of the choroid in emmetropization
In addition to its roles in nourishing the retina and regulating IOP, studies initiated by Wallman et al. (1995; Marzani and Wallman, 1997) clearly demonstrated that the choroid also plays an active role in emmetropization, both by modulation of its thickness to adjust the retina to the focal plane of the eye (choroidal accomomodation), and well as through the release of growth factors that have the potential to regulate scleral proteoglycan synthesis.
3.1 Choroidal accommodation
Wallman et al. (1995) was the first to demonstrate that the eye is able to change its refractive state by as much as 7 D by pushing the retina forward or pulling it back in response to imposed myopic or hyperopic defocus, respectively. These changes in the position of the retina are effected through modulation of the thickness of the choroid. Similar changes in choroid thickness have been observed in guinea pigs (Howlett and McFadden, 2009), tree shrews (Siegwart and Norton, 1998), and primates (Hung et al., 2000; Troilo et al., 2000) but to a lesser degree. Wallman’s group also demonstrated that choroid thickness could change following as little as 10 minutes of imposed defocus (Zhu et al., 2005), suggesting that the eye can directly determine the sign of defocus as opposed to a trial-and-error method in order to correct for refractive error. Moreover the choroid response to imposed defocus occurs more rapidly and more accurately than changes in scleral ECM synthesis and ocular elongation. Based on these observations, Wallman suggested that choroid thickness changes are in place to rapidly bring the eye close to emmetropia in order to prevent overshooting of the correct focal length when the eye is attempting to compensate for either myopic or hyperopic defocus (Wallman and Winawer, 2004) (Fig. 1).
Figure 1.
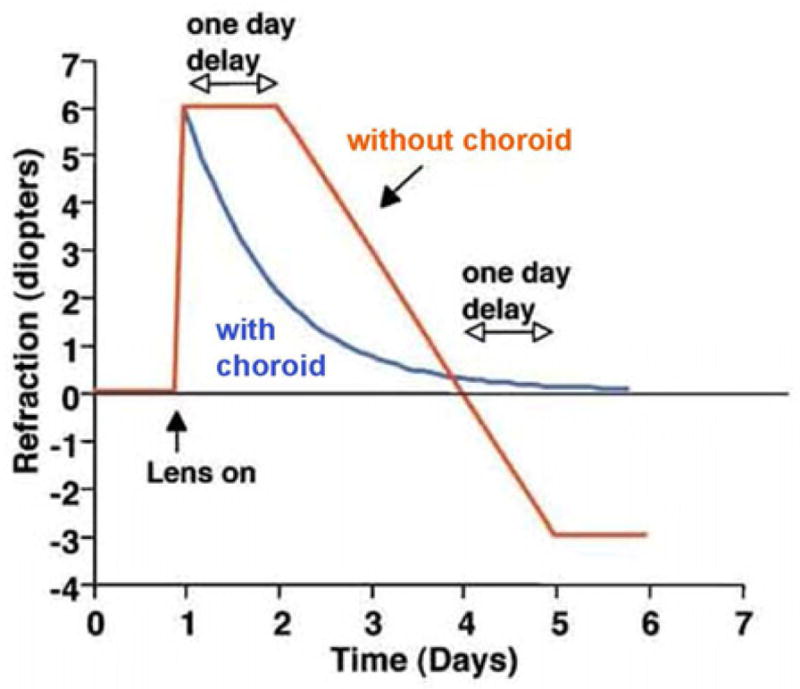
Role of the choroid in compensation for lens induced defocus. In a real eye (blue line), changes in choroid thickness allow rapid and accurate compensation for induced refractive error. In the absence of a choroidal response (red line), ocular compensation for defocus would be delayed by at least 1 day and would also overshoot the refractive target. From: Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron 43;447–486, 2004. Reproduced with permission © Cell Press.
Wallman speculated that changes in choroid thickness may be the result of changes in choroidal blood flow, vascular permeability, the contraction and relaxation of nonvascular smooth muscle cells in the choroidal stroma, and/or in the production of osmotically active molecules, such as glycosaminoglycans, that draw water into lymphatic lacunae present in the choroidal stroma (Wallman et al., 1995). This latter theory has been supported by studies in our lab (Rada et al., 2010) demonstrating dramatic increases in hyaluronic acid synthesis and accumulation in the choroids of eyes undergoing myopic defocus as a result of prior form deprivation induced myopia (recovery from myopia) (Fig. 2). Moreover, increased hyaluronic acid synthesis appears to be mediated by increased gene and protein expression of the enzyme, hyaluronic acid synthase 2 (HAS2) by an unidentified cell type within the choroid following as little as 6 hrs of recovery (Rada et al., 2010). Unknown are the cellular and molecular events that initiate the changes in choroidal thickness in response to visual stimuli, and whether these thickness changes have additional roles, aside from modulation of the focal plane, in the emmetropization process. For example, in addition to modulating the position of the retina, choroid thickness may provide a mechanism for regulating the delivery of bioactive molecules released from the retina, RPE or choroid to the sclera. In this capacity, a thicker choroid would be predicted impede the transfer of anabolic growth factors to the sclera, slow scleral matrix synthesis, and thereby slow the rate of ocular elongation.
Figure 2.
Hyaluronic acid in control (upper) and recovering (lower) chick choroids. Hyaluronic acid is visualized using biotinylated-HABP (HA-binding region of aggrecan protein) and fluorescein-streptavidin. Significant HA accumulation (indicated by green labeling) is detected in the choroidal stroma of treated eyes between blood vessels (BV) and lymphatic channels (asterisks) following four days of recovery and throughout the treatment period. A, D, F, H) contralateral control choroids. B, E, G, I) recovering choroids. C; Negative control in which the biotinylated-HABP was omitted Blue staining represents the 4,6-diamidino-2-phenylindole (DAPI) staining of the nuclei. Scale bar= 50 μm. From: Summers Rada, et al., Increased hyaluronan synthase-2 mRNA transcripts and hyaluronan accumulation accompany choroidal thickening during the recovery from induced myopia. Invest. Ophthalmol. Vis. Sci. 51;6172–6179. 2010. Reproduced with permission © Association for Research in Vision and Ophthalmology.
3.2 Choroid-mediated tissue interactions: The choroid as a pluricellular paracrine organ
As a highly vascular tissue, the choroid is responsible for the synthesis of a number of growth factors that are necessary for the development, growth and maintenance of its elaborate vasculature. For example, choroidal endothelial and stromal cells have been shown to synthesize vascular endothelial growth factor (VEGF) (Saint-Geniez et al., 2006), basic fibroblast growth factor (bFGF or FGF-2) (Frank et al., 1996; Ogata et al., 1996) and hepatocyte growth factor (HGF) (Grierson et al., 2000). These growth factors are necessary to promote and/or inhibit endothelial cell differentiation, proliferation, and migration, as well as vascular maturation, stabilization, maintenance and permeability. While these factors are necessary for choroid growth and development under normal conditions, they can also be involved in the pathogenesis of choroidal neovascularization associated with age-related macular degeneration (AMD) (Kvanta et al., 1996; Spilsbury et al., 2000). Moreover, the choroid has also been shown to synthesize the matrix metalloproteinases MMP1, MMP2 and MMP3, in association with choroidal capillaries and in the choroidal stroma (Steen et al., 1998). Changes in MMP activity within the choroid stroma, regulated in part by choroidal expression of TIMP3, occur in association with neovascularization and may play a role in formation of drusen deposits associated with AMD (Leu et al., 2002). The aforementioned growth factors, MMPs and TIMPs are secreted proteins and exert their effects through receptor-mediated interactions with neighboring cells. Therefore, in addition to their role in maintaining the choroidal vasculature, it is conceivable that these proteins could have effects on cells and tissues outside of the choroid.
In several developing systems, the choroid has been demonstrated to be the source of growth factors that can act not only within the choroid, but also on neighboring tissues. In 1991, Coulumbe and Nishi demonstrated that cells isolated from the chick choroid produced a secreted, soluble macromolecule of >10 kDa that stimulated somatostatin expression in choroidal neurons of the ciliary ganglion. They speculated that the choroid, the target tissue of the choroidal ciliary neurons in vivo, supported the development and differentiation of a subpopulation of ciliary neurons via the secretion of a diffusible factor that stimulated somatostatin expression by choroidal ciliary neurons. Moreover, because vascular smooth muscle in the choroid layer is the normal target in vivo for somatostatin-containing ciliary ganglion neurons, Coulumbe speculated that choroidal smooth muscle cells were the source of the somatostatin-stimulating factor (Coulombe and Nishi, 1991). Coulumbe subsequently identified the choroid cell-derived, somatostatin-stimulating activity as activin a, protein member of the TGF-B superfamily (Coulombe and Kos, 1997).
More recently, the choroid was demonstrated to be essential for the process of retinal regeneration in the newt (Cnops pyrrhogaster) and in the African clawed frog, (Xenopus laevis). In the newt, a complete neural retina can be regenerated from the RPE after surgical removal in adult animals. Using an in vitro culture model of newt retinal regeneration, Mitsuda et al. (2005) demonstrated that the choroid plays an essential role in newt RPE transdifferentiation to neural retina, and that in the absence of the choroid this transdifferentiation does not occur. Results of in vitro studies, demonstrated that the RPE-to-retina transdifferentiation was mediated by diffusible factors from the choroid and did not require direct contact of the two tissues. Although the diffusible factor(s) have not been definitively identified, FGF2 was considered a likely mediator, possibly acting synergistically with IGF-1 (Yoshii et al., 2007). Conversely, in the African clawed frog, transdifferentiation of RPE to retina only occurred when the choroid was removed from the RPE (Kuriyama et al., 2009). Moreover, disruption of the RPE/choroid tissue interaction by separation of the two layers in vitro induced expression of Pax6 by RPE and subsequent transdifferentiation of the RPE into a retinal laminar structure. In these studies, the molecular basis for the choroid/RPE tissue interaction in this model was not determined, however it was speculated that under normal conditions, the presence of the choroid suppressed Pax6 expression by RPE cells and disruption of the RPE/choroid results in Pax 6 expression and activation of RPE cells that subsequently can undergo FGF2-mediated transdifferentiation into cells of the neural retina.
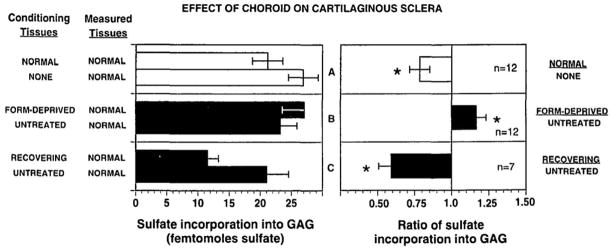
Marzani and Wallman (1997) were first to demonstrate that the secreted molecules from from the choroid can inhibit scleral proteoglycan synthesis and thereby have the potential to regulate the rate of ocular elongation (Fig. 3). Chick sclera, which consist of an outer fibrous layer and inner cartilaginous layer, were isolated, their two layers separated and placed in co-cultures with choroids isolated from normal chick eyes, eyes of chicks during the development of form deprivation myopia, as well as from eyes recovering from a previous period of form deprivation. Following co-culture, proteoglycan synthesis was measured in the scleral layers. This study demonstrated that co-culture of sclera with choroids from untreated eyes inhibited proteoglycan synthesis in the cartilaginous layer of the chick sclera. Moreover, scleral proteoglycan synthesis was more greatly inhibited by choroids isolated from recovering eyes. Conversely, sclera co-cultured with choroids isolated from myopic (form deprived) eyes demonstrated an increased rate of proteoglycan synthesis relative to that of sclera co-cultured with untreated choroids. Since the changes in scleral proteoglycan synthesis induced by co-culture with choroids under different growth conditions mimicked those changes observed in sclera under the same visual conditions in vivo (Rada et al., 1992), these studies provided the first evidence that the choroid could be the source of scleral growth regulators involved in visually guided ocular elongation.
Figure 3.
Co-culture of chick choroid and cartilaginous sclera. A) Co-culture of cartilaginous sclera (measured tissue) with normal chick choroids (conditioning tissue) resulted in an inhibition of scleral proteoglycan synthesis as compared with that of sclera not cultured with choroids. B) Co-culture of cartilaginous sclera with choroids from form deprived eyes resulted in less inhibition of scleral proteoglycan synthesis as compared with that of scleral co-cultured with untreated choroids. C) Co-culture of cartilaginous sclera with choroids from recovering eyes resulted in greater inhibition of scleral proteoglycan synthesis as compared with that of scleral co-cultured with untreated choroids. From: Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest. Ophthalmol. Vis. Sci. 38:1726–1739. 1997. Reproduced with permission © Association for Research in Vision and Ophthalmology.
3.3 Changes in choroid protein synthesis and vascular permeability during recovery
In an attempt to identify choroid-derived factor(s) that could modulate scleral proteoglycan synthesis, several studies were carried out comparing the profiles of newly synthesized and secreted proteins from choroids of chick eyes recovering from induced myopia with that of control eyes. These studies identified ovotransferrin (conalbumin), as a protein synthesized and secreted by choroids in relatively large amounts following 7 – 21 days of recovery (Rada, et al. 2001). Moreover, incubation of chick sclera with ovotransferrin (6 uM) resulted in a significant inhibition of scleral proteoglycan synthesis in vitro (↓62%). These results were later corroborated by a microarray analysis (discussed below) that identified overtransferrin as one of a few genes significantly upregulated in chick choroids following four days of recovery (Rada and Delrow, 2004). The increase in ovotransferrin accumulation and release was not simply a reflection of choroidal thickening because ovotransferrin levels in culture medium returned to control levels by 28 days of recovery, whereas choroidal thickness remained significantly elevated throughout the 50 day recovery period.
Based on a previous report that demonstrated marked increases in choroidal vascular permeability during recovery from myopia (Pendrak et al., 2000), we speculated that increased protein secreted into the culture medium from choroids of recovering eyes was a reflection of a temporary increase in choroidal permeability to serum proteins that occurs following 4 to 7 days of recovery (Rada and Palmer, 2007). Moreover, we speculated that changes in choroidal vascular permeability could potentially regulate the delivery of scleral growth regulators. Subsequent studies comparing scleral proteoglycan synthesis following brief periods of recovery suggested that increased choroidal permeability and release of serum proteins was not directly responsible for initial changes in scleral proteoglycan synthesis, since significant changes in proteoglycan synthesis were detected within 12 hrs of recovery, whereas significant changes in choroidal permeability were observed only with longer recovery periods (Summers Rada and Hollaway, 2011).
3.4 Microarray analyses of choroid gene expression
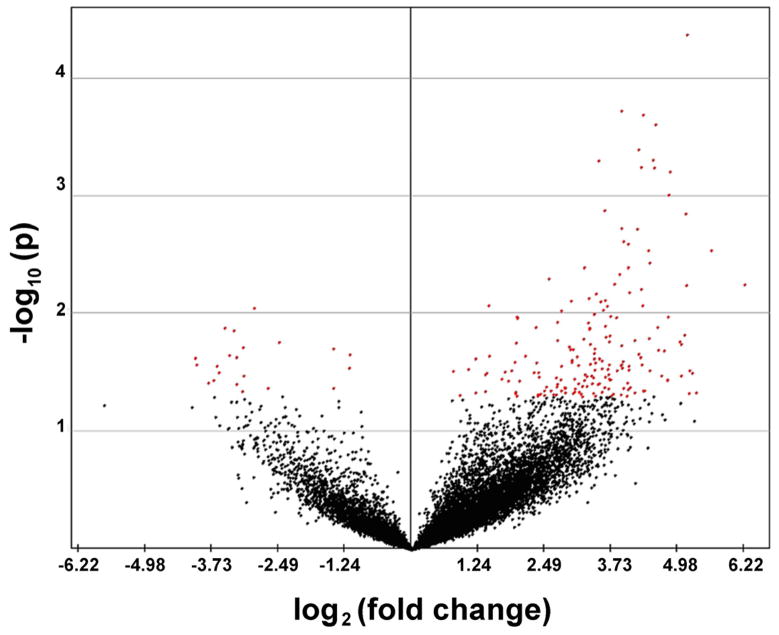
Analyses of gene expression changes associated with myopia and emmetropization have provided clues as to the nature of the chemical cascade that translates image quality to eye length (recently reviewed by Stone and Khurana, 2010). The emphasis of most gene expression profiling studies over the last decade has been on the retina. Only one full length publication reported on changes in choroid gene expression in response to imposed defocus (Shelton et al., 2008). In this study, contact lenses of equal power but opposite sign (+5D and −5D) were applied to both eyes of the common marmoset (Callithrix jacchus) for 62 – 92 days to induce opposite growth states in both eyes of the same subject. The choroid/RPEs of each eye were dissected from the sclera and retina, and immediately snap frozen in liquid nitrogen. Total RNA was isolated from the entire marmoset choroid/RPE using TRIZOL Reagent and digested with DNase I. α-33P radiolabeled cDNA probes were generated and used to interrogate a 12,000 human gene array (Atlas™ Plastic Human 12K Array; Clontetech labs, Mountain View, CA). Using these arrays, 204 genes were found to be significantly changed in minus lens-treated as compared with plus lens-treated eyes. Of these 204 genes, 183 were up-regulated in minus lens-treated eyes as compared with plus lens-treated eyes and 21 genes were relatively down-regulated in minus lens-treated eyes as compared with plus lens-treated eyes (Fig. 4). Among the relatively down-regulated genes were cell receptors, including protein tyrosine phosphatase receptor-type B (PTPRB), and relatively up-regulated genes included transcripts for growth factors, including basic fibroblast growth factor 2 (FGF-2) and transforming growth factor beta-induced, 68kD (TGFBI/ßig-h3). These results demonstrated that imposed optical defocus induces significant gene expression changes in the marmoset choroid/RPE. Whether these gene expression changes were occurring in the RPE and/or the choroid and whether these gene expression changes were directly involved in the ocular growth changes resulting from the lens treatment was not determined. In an effort to identify a causal relationship between gene expression and the direction of ocular growth, we recently analyzed RNA isolated from marmoset choroids following a very brief period of lens wear (10 days), prior to detectible changes in eye size and refraction, using a newly developed marmoset-specific DNA microarray (Eumama) (Summers Rada et al., 2011). Following the 10 day period of lens treatment, no significant changes in eye length or refraction were detected between lens treated and control eyes, and relatively small gene expression changes (+/− 1.3 fold) were observed in choroids between lens-treated (+5D or −5D) or control eyes as compared with changes detected following a longer periods of binocular lens induced anisometropia. Taken together, these results suggest that if changes choroid gene expression directly modulate ocular elongation in marmosets, these genes are differentiatlly expressed at relatively low fold-change levels. We also suspect, therefore, that the larger fold changes in choroidal/RPE gene expression previously identified may be a reflection of the choroidal response to changes in eye size and refraction, rather than the cause of the induced ocular changes.
Figure 4.
Volcano plot of marmoset choroid/RPE microarray data. Scatter plot represents a summary of the t-tests for the individual genes. The fold change (log2) in gene expression between the minus lens-treated group and plus lens-treated group (x axis) as plotted against the p value (−log10) for a given gene associated with the Student t-test comparison of the two groups of samples (n = 4). Using a p value of 0.05 as the cutoff threshold, 21 genes were significantly down-regulated in minus lens-treated eyes as compared with plus lens-treated eyes (negative value, red dots to left of center line) and 183 genes were significantly up-pregulated in minus lens-treated eyes as compared with plus lens-treated eyes (positive value, red dots to right of center line). Each point represents an individual gene in the array; genes not significantly altered are depicted in black. Those genes appearing on the upper left or right region have a large fold-change and a smaller p value. From: Shelton L., et al., Microarray analysis of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular growth and refraction Mol. Vis. 14:1465–1479. 2008. Reproduced with permission © Molecular Vision
Recently, choroidal gene expression changes were analyzed in tree shrews undergoing treatment with minus lenses, recovery, and plus lenses (He et al., 2012). Of 55 genes assessed, 4 ECM-related genes were significantly up-regulated during lens-induced myopia, including insulin- like growth factor 2 (Igf2), retinaldehyde-binding protein 1 (Rlbp1), transforming growth factor beta-induced, 68kD (Tgfb1), and hypoxia inducible factor 1, alpha subunit (Hif1A). During recovery and plus lens wear, different populations of genes, primarily signalling and transcriptional regulatory genes were shown to be significantly up- or down-regulated in treated choroids as compared with untreated choroids. The identification of Tgfb1 as a gene up-regulated in choroids during the development of myopia by two independent studies and in two different animal models (marmosets and tree shrews) (Shelton et al., 2008; He et al., 2012) suggests Tgfb1 may play a fundamental role in the choroidal response to imposed defocus.
Two microarray studies have been reported in abstract form on changes in the retina/rpe/choroid of chicks during the recovery from induced myopia. Using a 4000 immune system gene array, Rada and Delrow (2004) identified avian thymic hormone (Pvalb) and ovotransferrin (Ltf) as two genes significantly up-regulated in avian choroid/RPE/retinas following 1 and 4 days, respectively. Among the 37 down-regulated genes were several ribosomal proteins (acidic ribosomal phosphoprotein (Rplp0), ribosomal protein L10 (Rpl10) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (13 replicates on the array), suggesting a general down-regulation of gene expression in the choroid/rpe/retina during recovery from induced myopia. Rada and Wiechmann (2009) subsequently confirmed avian thymic hormone as largely expressed in the choroid as well as in a subpopulation of cell bodies located in the inner nuclear layer (INL) of the retina. The function of ATH in the retina and choroid is unknown. Recently, Giummarra et al., (2010) assessed gene expression changes in retina/RPE/choroid associated with recovery using the chicken Affymetrix™ gene-chip array. Following 24 hr of recovery, significant up-regulation of endothelin (End1), serum/glucocorticoid kinase (Sgk1) and myeloid/lympohoid leukaemia 3 (MII3) mRNA was detected, potentially mediating changes in retina/choroid/RPE osmoregulatory processes and changes in choroidal permeability during the recovery from induced myopia.
Based on differences in experimental paradigms employed in the aforementioned microarray studies (e.g. animals models, tissue sources, microarray platforms) it is difficult to integrate these results into a comprehensive understanding of the role of the choroid in emmetropization. However, these studies clearly demonstrate that 1) choroid gene expression can be modulated by changes in the visual environment (i.e. the refractive state of the eye), and 2) some choroidal gene expression changes identified in chicks, such as avian thymic hormone, occur within one day of visual manipulation, suggesting a direct role for the choroid in the retina- sclera signaling cascade.
3.5 Choroidal retinoic acid synthesis
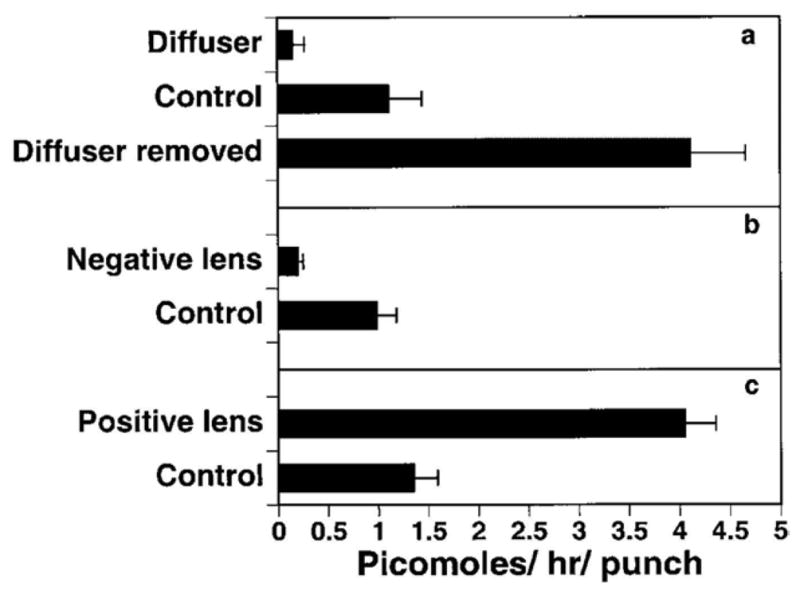
The most significant advance toward the identification of a choroid derived scleral growth regulator was made by Mertz and Wallman (2000) when they published that the chick choroid synthesizes relatively high levels of all-trans-retinoic (atRA) acid as compared with the retina or liver, and the rate of atRA synthesis is dramatically affected by the refractive state of the eye (Fig. 5). Choroidal synthesis of atRA was shown to be increased in chick eyes during recovery from induced myopia and during compensation for imposed myopic defocus (using plus lenses), and atRA was shown to be decreased in eyes undergoing form deprivation myopia and compensation for hyperopic defocus (using minus lenses). Interestingly, the time course of the increase in choroidal atRA synthesis was remarkably similar to that of the decrease in rate of sclera proteoglycan synthesis observed in the early phase of recovery from induced myopia (Summers Rada and Hollaway, 2011) (Fig. 6) suggesting a causal relationship between choroidal atRA synthesis and scleral proteoglycan synthesis. Recently, using an ultrasensitive method of quantification [LC(liquid chromatography)/MS/MS], endogenous and newly synthesized atRA were measured in choroids in organ culture (Rada et al., 2012). In agreement with Mertz and Wallman (2000), atRA concentration was significantly higher in cultures of choroids from eyes recovering for 24 hrs – 15 days than in cultures of paired controls. Moreover, the concentrations of atRA generated by choroids in vitro were within the range to produce significant inhibition of scleral proteoglycan synthesis (Fig. 7) (Rada et al., 2012). Taken together, these studies suggested that choroidal synthesis of atRA in response to visual stimuli may modulate scleral proteoglycan synthesis. In guinea pigs and primates, atRA synthesis is increased in the choroid/sclera (McFadden et al., 2004) and RPE/choroid (Troilo et al., 2006), respectively, during the development of myopia, a condition that is also associated with decreased scleral proteoglycan synthesis. However, in contrast to chicks, decreased proteoglycan synthesis in the mammalian sclera is associated with increased axial elongation (Norton and Rada, 1995; Rada et al., 2000). Similar to chicks, atRA has been demonstrated to inhibit proteoglycan synthesis in the primate sclera (Troilo et al., 2006). Therefore, in both chicks and primates, the visually induced changes in choroidal atRA synthesis and concentration are consistent with the known changes in scleral proteoglycan synthesis that occur during visually guided ocular growth and may represent an evolutionarily conserved mechanism for visually guided ocular growth regulation. How the same visual stimuli (such as hyperopic defocus) can cause opposite changes in choroidal atRA synthesis in chicks and primates is unknown, but suggests the presence of additional regulatory proteins in the cascade between the retina and choroid that differ between primates and chicks.
Figure 5.
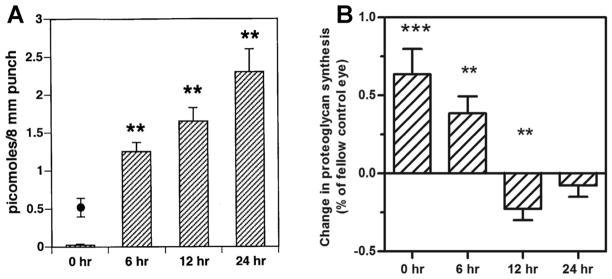
3H all-trans-retinoic acid (atRA) synthesis by isolated choroids cultured in vitro for 2 hrs. A. Choroidal atRA synthesis was reduced in treated eyes of chicks developing form deprivation myopia (diffuser) and increased in treated eyes of chicks recovering from form deprivation myopia (diffuser removed) as compared with contralateral controls. B. Choroidal atRA synthesis was reduced in treated eyes of chicks compensating for negative lens-induced hyperopia(negative lens) as compared with contralateral controls. C. Choroidal atRA synthesis was increased in treated eyes of chicks compensating for positive lens-induced myopia (positive lens) as compared with contralateral controls. From: Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res, 70(4): p. 519–527 2000. Reproduced with permission © Elsevier
Figure 6.
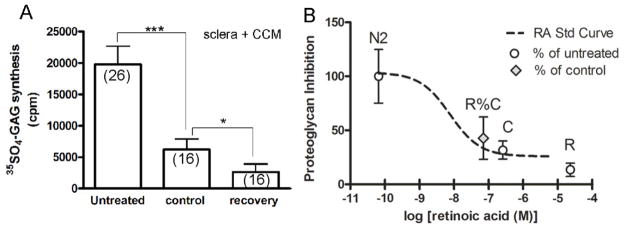
Comparison of changes in choroidal retinoic acid synthesis and scleral proteoglycan synthesis during recovery from induced myopia. A. Time course of increase in choroidal all-trans-retinoic acid (atRA) synthesis in eyes recovering from form deprivation myopia. B. Time course of decrease scleral proteoglycan synthesis in eyes recovering from form deprivation myopia. Fig 6a from: Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res, 70(4): p. 519–527 2000. Reproduced with permission © Elseveir and Fig. 6b adapted from: Summers JA, Hollaway LR. Regulation of the biphasic decline in scleral proteoglycan synthesis during the recovery from induced myopia. Exp. Eye Res. 92(5):394–400 2011. Reproduced with permission © Elsevier.
Figure 7.
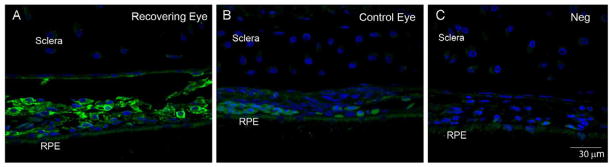
Effect of choroid-conditioned medium (CCM) and retinoic acid (atRA) on scleral proteoglycan synthesis. A. Scleral proteoglycan synthesis was assessed following incubation with untreated medium, medium previously incubated with untreated choroids (controls) and medium incubated with recovering choroids (recovery). B. The relative proteoglycan inhibition by CCM from control and recovering eyes (R%C) was superimposed on a dose-response curve for the effect of atRA on scleral proteoglycan synthesis. The relative inhibition of recovering CCM (43%) corresponded to a concentration of 7.20 × 10−8 M, a value within the range of the measured atRA synthesized by recovering choroids in organ cultures (4 × 10−9 M to 3 × 10−8 M). From:
Summers Rada, J.A., Hollaway, L.Y., Li, N., Napoli, J., Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest. Ophthalmol. Vis. Sci. 53:1649-1662 2012. Reproduced with permission © Association for Research in Vision and Ophthalmology.
Tissue concentrations of atRA are tightly controlled by synthesizing and catabolizing enzymes, binding proteins and nuclear receptors (recently reviewed by Napoli, 2011). Several studies have documented gene and protein expression of retinoic acid binding proteins, retinoic acid receptors (RARs) and retinaldehyde dehydrogenases (RALDHs) in the retinas, choroids and sclera of chick eyes under normal and visually induced ocular growth states (Fischer et al., 1999; Bitzer et al., 2000; Simon et al., 2004; Rada et al., 2012). Two studies identified significant increases in choroidal RALDH2 mRNA expression during recovery from induced myopia and during compensation for lens induced myopia (+7D lens) (Simon et al., 2004; Rada et al., 2012), suggesting that increased synthesis of atRA in choroids of recovering eyes is due to increased RALDH2 enzyme activity. Within the choroid, RALDH2 is present in ovoid and spindle-shaped cells located in the stroma between larger blood and lymphatic vessels as well as in cells immediately adjacent to the sclera (Fischer et al., 1999; Rada et al., 2012) (Fig. 8). In the choroid, some RALDH2 immunopositive cells were observed to co-express alpha-smooth muscle actin, suggesting that myofibroblasts or extravascular smooth muscle cells may be responsible for choroidal synthesis of atRA (Rada et al., 2012).
Figure 8.
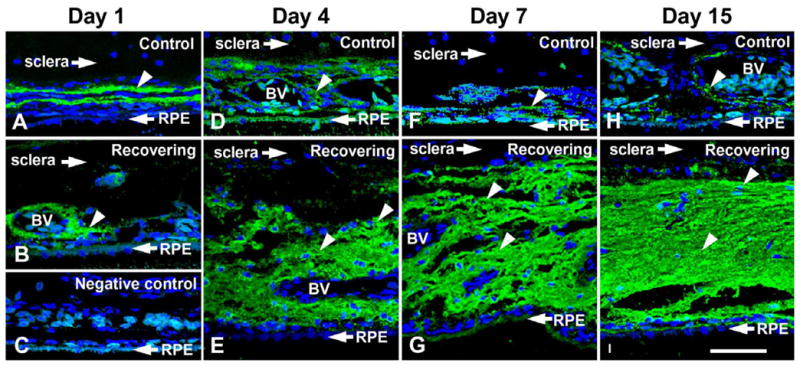
Confocal images of RALDH2 protein (green) in choroids of control and recovering chick eyes following immunolabeling with anti-RALDH2 antibodies. A. robust labeling for RALDH2 is detected in ovoid cells in the stroma of choroids from a recovering eye. B. Minimal RALDH2 protein is detected in choroids of contralateral control eyes. C. Negative control section of a recovering eye (24 hours), where non-immune rabbit IgG was used in the first incubation, followed by incubation in AlexaFluor 488-conjugated goat anti-rabbit immunoglobulin. Nuclei were counterstained with DAPI (blue). From:
Summers Rada, J.A., Hollaway, L.Y., Li, N., Napoli, J., Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest Ophthalmol Vis Sci, 53:1649-1662 2012. Reproduced with permission © Association for Research in Vision and Ophthalmology.
The identification of atRA and its synthesizing enzyme, RALDH2, as possible mediators of visually induced changes in eye size has provided new potential molecular targets for the control of myopia development. However, studies evaluating eye size and refraction following in vivo treatment with atRA agonists and atRA synthesis inhibitors have yielded perplexing results. Ocular elongation is increased following dietary administration of atRA to chicks and decreased following administration of citral, an inhibitor of atRA synthesis (McFadden et al., 2006). Similarly, intraocular administration of the atRA synthesis inhibitor, disulfiram, has been shown to inhibit the development of form deprivation myopia (Bitzer et al., 2000) - results generally opposite of what would be predicated if atRA acts to inhibit ocular elongation in chicks. It is likely that untargeted administration of atRA or atRA synthesis inhibitors leads to multicellular effects that may differ from those mediated by endogenous, choroidally derived atRA. In several developmental systems, atRA concentrations have been shown to be tightly regulated both spatially and temporally by coordinated expression of synthesizing and catabolizing enzymes to generate localized effects (Mey et al., 1997; Hochgreb et al., 2003; Machem 2006; Bok et al., 2011). Similarly, it is likely that atRA concentrations are tightly regulated within specific regions of the choroid to provide local control of scleral proteoglycan synthesis. Further studies are necessary to elucidate the molecular mechanisms involved in the regulation of choroidal atRA synthesis and activity. Results from such studies will likely lead to the development of new therapeutic approaches for the control of myopia through modulation of retinoid signaling in the choroid and/or sclera.
Highlights.
The choroid is a source of growth factors that can act on neighboring tissues.
Visual stimuli can cause changes in choroidal gene expression and choroid thickness.
The choroid secretes scleral growth regulators in response to visual stimuli.
Choroidal RALDH2 activity and retinoic acid synthesis modulate scleral remodeling.
Acknowledgments
Supported by National Eye Institute Grant R01 EY09391 (JAS)
The majority of data presented in this review came from projects funded by the National Eye Institute at the National Institutes of Health. I would like to thank Drs. David Troilo and Terri Young for inviting me to contribute to this special issue of Experimental Eye Research as a tribute to Dr. Josh Wallman. Josh was my mentor, colleague and friend for over 20 years and he will be truly missed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Alm A, Nilsson SF. Uveoscleral outflow--a review. Exp Eye Res. 2009;88:760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res. 2000;70:97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]
- Bok J, Raft S, Kong KA, Koo SK, Drager UC, Wu DK. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci U S A. 2011;108:161–166. doi: 10.1073/pnas.1010547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe JN, Kos K. Target tissue influence on somatostatin expression in the avian ciliary ganglion. Ann N Y Acad Sci. 1997;814:209–225. doi: 10.1111/j.1749-6632.1997.tb46159.x. [DOI] [PubMed] [Google Scholar]
- Coulombe JN, Nishi R. Stimulation of somatostatin expression in developing ciliary ganglion neurons by cells of the choroid layer. J Neurosci. 1991;11:553–562. doi: 10.1523/JNEUROSCI.11-02-00553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Lymphatic vessels. Invest Ophthalmol Vis Sci. 1997;38:1241–1260. [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Wallman J, Mertz JR, Stell WK. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J Neurocytol. 1999;28:597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- Giummarra L, Murphy MJ, Goodyear MJ, Crewther SG. Microarray analysis of chick retina/RPE/choroid during recovery from form deprivation myopia (FDM) Invest Ophthalmol Vis Sci. 2010;51 [ARVO E-abstract #1674] [Google Scholar]
- Green WR. The choroid. In: Spencer WH, editor. Ophthalmic Pathology: An Atlas and Textbook. 3. Philadelphia: W.B. Saunders; 1986. [Google Scholar]
- Grierson I, Heathcote L, Hiscott P, Hogg P, Briggs M, Hagan S. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19:779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1380–1395. [PubMed] [Google Scholar]
- Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1–30. [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myoipa, recovery, and plus-lens wear. Invest Ophthalmol Vis Sci. 2012;53 [ARVO E-abstract #3454] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CY, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hung LF, Wallman J, Smith EL. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41:1259–1269. [PubMed] [Google Scholar]
- Junghans BM, Crewther SG, Liang H, Crewther DP, Wareing L, Pirie B. Lymphatics in the chick choroid? Aust N Z J Ophthalmol. 1996;24:47–49. doi: 10.1111/j.1442-9071.1996.tb00993.x. [DOI] [PubMed] [Google Scholar]
- Krebs W, Krebs IP. Ultrastructural evidence for lymphatic capillaries in the primate choroid. Arch Ophthalmol. 1988;106:1615–1616. doi: 10.1001/archopht.1988.01060140783055. [DOI] [PubMed] [Google Scholar]
- Kuriyama F, Ueda Y, Araki M. Complete reconstruction of the retinal laminar structure from a cultured retinal pigment epithelium is triggered by altered tissue interaction and promoted by overlaid extracellular matrices. Dev Neurobiol. 2009;69:950–958. doi: 10.1002/dneu.20745. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- Leu ST, Batni S, Radeke MJ, Johnson LV, Anderson DH, Clegg DO. Drusen are Cold Spots for Proteolysis: Expression of Matrix Metalloproteinases and Their Tissue Inhibitor Proteins in Age-related Macular Degeneration. Exp Eye Res. 2002;74:141–154. doi: 10.1006/exer.2001.1112. [DOI] [PubMed] [Google Scholar]
- Machem S. Limb development. In: Unsicker K, Kreiglstein K, editors. Cell Signaling and Growth Factors in Development. Wiley-VCH Verlag Gmbh & Co; 2006. pp. 523–618. [Google Scholar]
- Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MH, Mertz JR, Wallman J. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006;83:949–961. doi: 10.1016/j.exer.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P, Drager UC. Retinoic acid synthesis in the developing chick retina. J Neurosci. 1997;17:7441–7449. doi: 10.1523/JNEUROSCI.17-19-07441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda S, Yoshii C, Ikegami Y, Araki M. Tissue interaction between the retinal pigment epithelium and the choroid triggers retinal regeneration of the newt Cynops pyrrhogaster. Dev Biol. 2005;280:122–132. doi: 10.1016/j.ydbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2011;1821:152–167. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16:320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- Noden DM. Periocular mesenchyme: neural crest and mesodermal interactions. In: Jakobiec FA, editor. Ocular anatomy, embryology and teratology. Philadelphia: Harper & Row; 1982. pp. 79–119. [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Ogata NM, Matsushima M, Takada Y, Tobe T, Takahashi K, Yi X, Yamamoto C, Yamada H, Uyama M. Expression of basic fibroblast growth factor mRNA in developing choroidal neovascularization. Curr Eye Res. 1996;15:1008–1018. doi: 10.3109/02713689609017649. [DOI] [PubMed] [Google Scholar]
- Pendrak K, Papastergiou GI, Lin T, Laties AM, Stone RA. Choroidal vascular permeability in visually regulated eye growth. Exp Eye Res. 2000;70:629–637. doi: 10.1006/exer.2000.0825. [DOI] [PubMed] [Google Scholar]
- Polska E, Simader C, Weigert C, Doelemeyer G, Kolodjaschna A, Scharmann J, Schmetterer OL. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest Ophthalmol Vis Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- Rada JA, Delrow J. Changes in gene expression during the recovery from experimental myopia. Invest Ophthalmol Vis Sci. 2004;45 (ARVO E-abstract #1160) [Google Scholar]
- Rada JA, Hollaway LR, Lam W, Li N, Napoli JL. Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest Ophthalmol Vis Sci. 2012;53:1649–1662. doi: 10.1167/iovs.11-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Huang Y, Rada KG. Identification of choroidal ovotransferrin as a potential ocular growth regulator. Curr Eye Res. 2001;22:121–132. doi: 10.1076/ceyr.22.2.121.5525. [DOI] [PubMed] [Google Scholar]
- Rada JA, Johnson JM, Achen VR, Rada KG. Inhibition of scleral proteoglycan synthesis blocks deprivation-induced axial elongation in chicks. Exp Eye Res. 2002;74:205–215. doi: 10.1006/exer.2001.1113. [DOI] [PubMed] [Google Scholar]
- Rada JA, Matthews AL, Brenza H. Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp Eye Res. 1994;59:747–760. doi: 10.1006/exer.1994.1161. [DOI] [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;4:2050–2058. [PubMed] [Google Scholar]
- Rada JA, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest Ophthalmol Vis Sci. 2007;48:2957–2966. doi: 10.1167/iovs.06-1051. [DOI] [PubMed] [Google Scholar]
- Rada JA, Perry CA, Slover ML, Achen VR. Gelatinase A and TIMP-2 expression in the fibrous sclera of myopic and recovering chick eyes. Invest Ophthalmol Vis Sci. 1999;40:3091–3099. [PubMed] [Google Scholar]
- Rada JA, Wiechmann AF. Ocular expression of avian thymic hormone: changes during the recovery from induced myopia. Mol Vis. 2009;15:778–792. [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Wiechmann AF, Hollaway LR, Baggenstoss BA, Weigel PH. Increased hyaluronan synthase-2 mRNA expression and hyaluronan accumulation with choroidal thickening: response during recovery from induced myopia. Invest Ophthalmol Vis Sci. 2010;51:6172–6179. doi: 10.1167/iovs.10-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Maldonado AE, D’Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- Schrodl F, De Laet A, Tassignon MJ, Van Bogaert PP, Brehmer A, Neuhuber WL, Timmermans JP. Intrinsic choroidal neurons in the human eye: projections, targets, and basic electrophysiological data. Invest Ophthalmol Vis Sci. 2003;44:3705–3712. doi: 10.1167/iovs.03-0232. [DOI] [PubMed] [Google Scholar]
- Shelton L, Troilo D, Lerner MR, Gusev Y, Brackett DJ, Rada JS. Microarray analysis of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular growth and refraction. Mol Vis. 2008;14:1465–1479. [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Simon P, Feldkaemper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcriptional changes of retinal and choroidal TGFbeta-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis. 2004;10:588–597. [PubMed] [Google Scholar]
- Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen B, Sejersen S, Berglin L, Seregard S, Kvanta A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–2200. [PubMed] [Google Scholar]
- Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010;50:2322–2333. doi: 10.1016/j.visres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubinger K, Brehmer A, Neuhuber WL, Reitsamer H, Nickla D, Schrodl F. Intrinsic choroidal neurons in the chicken eye: chemical coding and synaptic input. Histochem Cell Biol. 2010;134:145–157. doi: 10.1007/s00418-010-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita A, Inokuchi T. Lymphatic sinus-like structures in choroid. Jpn J Ophthalmol. 1992;36:436–442. [PubMed] [Google Scholar]
- Summers Rada JA, Datson NA, Troilo D. Early changes in gene expression in RPE/Choroid of marmoset eyes in response to image defocus. Invest Ophthalmol Vis Sci. 2011 [ARVO E-abstract #6300] [Google Scholar]
- Summers Rada JA, Hollaway LR. Regulation of the biphasic decline in scleral proteoglycan synthesis during the recovery from induced myopia. Exp Eye Res. 2011;92:394–400. doi: 10.1016/j.exer.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torczynski E. Choroid and suprachoroid. In: Jakobiec FA, editor. Ocular anatomy, embryology, and teratology. Philadelphia: Harper & Row; 1982. pp. 553–585. [Google Scholar]
- Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47:1768–1777. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258. [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–486. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol. 2007;303:45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Zhao S, Overbeek PA. Regulation of choroid development by the retinal pigment epithelium. Mol Vis. 2001;7:277–282. [PubMed] [Google Scholar]
- Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]