Abstract
Purpose
Hepatocellular cancer (HCC) is a deadly and most rapidly increasing cancer in the USA and worldwide. The etiology and factors involved in development of HCC remain largely unknown. A marked decrease in zinc occurs in HCC. Its role and involvement in HCC has not been identified. We investigated the relationship of cellular zinc changes to the development of malignancy, and the identification of potential zinc transporters associated with the inability of hepatoma cells to accumulate zinc.
Methods
The detection of relative zinc levels in situ in normal hepatic cells vs. hepatoma was performed on normal and HCC tissue sections. ZIP1, 2, 3, and 14 transporters were identified by immunohistochemistry.
Results
Intracellular zinc levels are markedly decreased in HCC hepatoma cells vs. normal hepatic cells in early stage and advanced stage malignancy. ZIP14 transporter is localized at the plasma membrane in normal hepatocytes, demonstrating its functioning for uptake and accumulation of zinc. The transporter is absent in the hepatoma cells and its gene expression is downregulated. The change in ZIP14 is concurrent with the decrease in zinc. ZIP1, 2, 3 are not associated with normal hepatocyte uptake of zinc, and HCC zinc depletion. HepG2 cells exhibit ZIP14 transporter. Zinc treatment inhibits their growth.
Conclusions
ZIP14 downregulation is likely involved in the depletion of zinc in the hepatoma cells in HCC. These events occur early in the development of malignancy possibly to protect the malignant cells from tumor suppressor effects of zinc. This provides new insight into important factors associated with HCC carcinogenesis.
Keywords: Hepatocellular cancer, Zinc, ZIP transporters, ZIP14, Zinc transporters
Introduction
Liver cancer (hepatocellular carcinoma/HCC) is the most rapidly increasing cancer in the USA and worldwide [1]. It is projected that 24,120 new HCC cases and 18,910 deaths will occur in the USA in 2010. HCC is typically diagnosed in advanced stages with a median survival of 6–20 months following diagnosis. The lack of information and understanding of the early events in hepatocarcinogenesis impedes progress in the identification of effective early biomarkers and in the development of more effective therapeutic options for HCC.
Danielsen and Steinnes [2] 40 years ago reported a 62% decrease in the concentration of zinc in hepatoma vs. adjacent normal tissue in liver biopsies, which has been confirmed in several reports [3–8]. A cellular decrease in zinc of this magnitude will result in significant changes in cell growth, cell function, and cell metabolism, which are important for the development of malignancy. Consequently, it is surprising that reported studies are lacking regarding the role and implication of zinc in HCC. This present report shows for the first time (1) that the in situ decrease in zinc in the hepatoma cells occurs early in the development of malignancy and persists in advancing stages of malignancy; (2) identifies ZIP14 as a likely zinc uptake transporter involved in the lost ability of the malignant cells to accumulate zinc; and (3) treatment with physiologic concentration of zinc results in cellular accumulation and inhibition of growth of HepG2 cells. The concept is proposed that zinc is a tumor suppressor agent in HCC, and the silencing of ZIP14 expression in the malignant cells could be involved in preventing zinc accumulation in order to avoid its adverse effects.
Material and Methods
Zinc and Zip transporter levels were determined and compared in tissue micro-array (TMA) slides containing normal liver and HCC cores (obtained from US Biomax, Inc) and in archived liver tissue samples provided by Dr. Desouki (Medical University of South Carolina). For in situ staining of zinc levels, the slides were exposed to dithizone (DTZ) or to Zinquin ester. DTZ staining followed the method described by Sternberg et al. [9] with modification. The reagent consists of 100 ug DTZ dissolved in acetone, to which an equal volume of Zn-free water is added. The slides were exposed to DTZ reagent for up to 15 min, followed by a Zn-free water rinse, covered with a cover slip, and examined by light microscopy. We employed a seven-headed Reichert Omega Model #4000 microscopy system equipped with a high-definition, two-megapixel cameras and a desktop computer for capturing and storing digital images. After obtaining required pictures, the slide was prepared for H&E staining for identification and confirmation of the histopathology of the TMA cores and tissue sections. Zinquin ester was kindly provided by Dr. Peter Zalewski (University of Adelaide). Zinquin ester was prepared as a 5-mM stock in DMSO and diluted to a final concentration of 25 µM working solution in PBS immediately before applying to the slides at room temperature. The slides were washed twice with PBS to remove excess Zinquin, dried, and mounted with anti-fade mounting medium. Zinquin fluorescence was visualized by confocal microscopy at excitation 351–358 nm and emission at 460 nm.
The relative abundance of ZIP1, ZIP2, and ZIP3 transporters was determined by immunohistochemistry (IHC), which employed corresponding antibodies that we developed and described in previous studies [10–12]. ZIP14 IHC was performed with ZIP14 antibody kindly provided by Dr. Robert Cousins (University of Florida). ZIP14 in situ RT-PCR was conducted by Dr. Bagasra (Claflin University) as previously described [10, 11] with the following primers: forward 5′-GTCTGGCCTTTGGCATCCT-3′; reverse 5′-AGGGAACATATCAGCCAGAGAAAT-3′; probe 5′-{PCBL}-CAGCCACTTCTCTGCCAACT-3′.
HepG2 cells were cultured under standard conditions in DMEM medium supplemented with 2% fetal bovine serum and 1% penicillin–streptomycin mixture. Cells were plated in 12-well plates (3 × 104 cells/well) and cultured overnight. The next day, the medium was removed, the cells were washed, and the medium replaced with DMEM containing various concentrations of ZnCl2 (indicated in results). Cell proliferation was measured using the CyQUANT Cell Proliferation Assay Kit (Invitrogen) according to the manufacturer’s protocol. Cell lysate zinc levels were measured using Zinquin fluorescence read in a Floroskan microplate reader with excitation/emission filter combination of 355/485.
Results
Comparative Cellular Zinc Levels in Normal Liver vs. HCC
In the following description, we employ the “grading system” of malignancy according to the description of USBiomax for their characterization of the TMA cores:
The grade 1–3 (or I–III) in Pathology Diagnosis is equivalent to well-differentiated, moderately differentiated or poorly differentiated, respectively, under microscope.
Grade 1 or well-differentiated: Cells appear normal and are not growing rapidly.
Grade 2 or moderately differentiated: Cells appear slightly different than normal.
Grade 3 or poorly differentiated: Cells appear abnormal and tend to grow and spread more aggressively.
In addition, this is appropriate since the characterization of the progression of malignancy from early stage to advanced stage is important and relevant to this presentation.
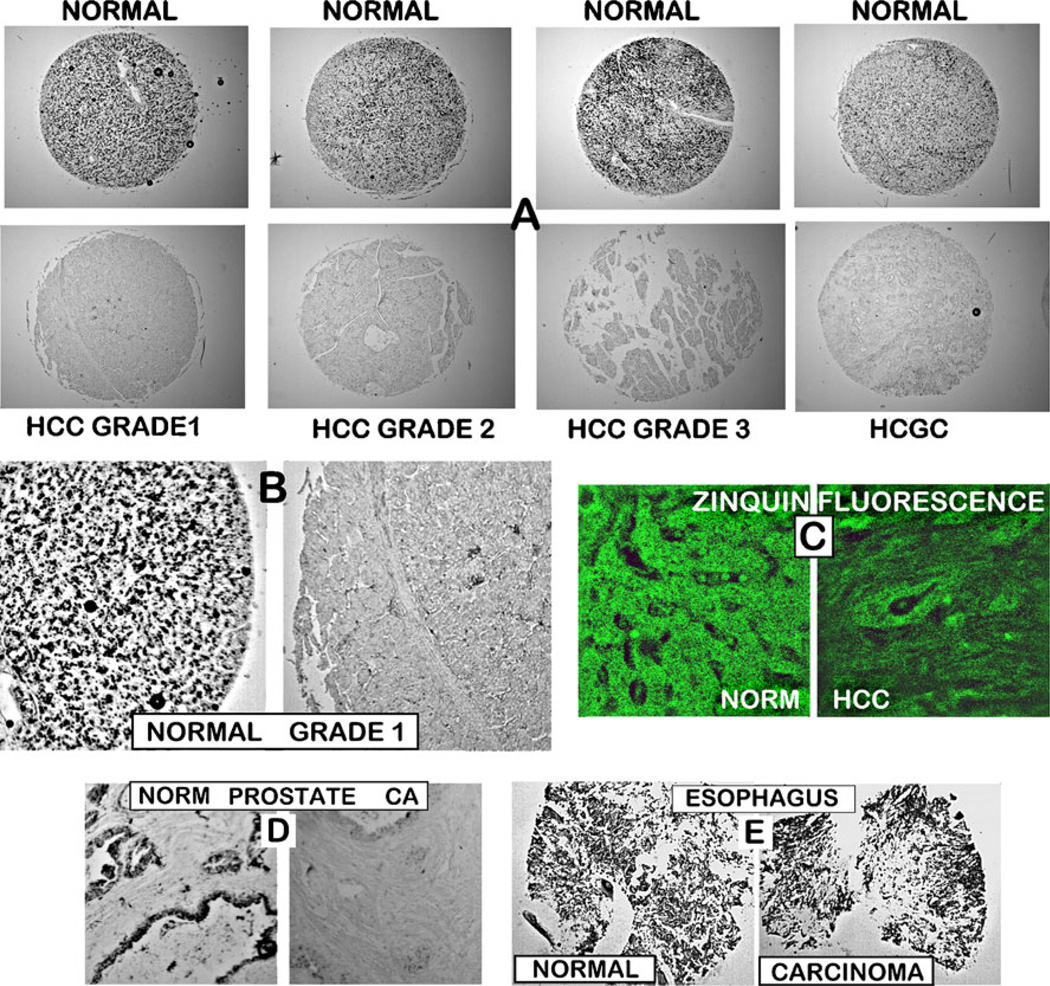
Figure 1 shows the representative results of the zinc staining of TMA slides that contained normal liver and HCC cores. Figure 1a and b reveals that the HCC cores exhibit a marked depletion of ZnDTZ staining as compared to the high ZnDTZ staining of the normal cores; and the association of ZnDTZ in normal hepatic cells and its absence in the hepatoma cells. The depletion of zinc is evident in well-differentiated, moderately differentiated, and poorly differentiated malignancy. These results demonstrate that the depletion of zinc occurs early in the development of malignancy and persists in the progression of malignancy. To corroborate the accuracy of the zinc changes detected with DTZ, staining was performed with prostate tissue sections and with esophageal TMA cores. Normal prostate acini contain high levels of zinc, and prostate adenocarcinoma involves a marked depletion of zinc. This difference is well-identified by DTZ staining shown in Fig. 1d. In contrast, esophageal squamous cell carcinoma exhibits little or no change in zinc compared to normal esophagus [13]. This is also revealed by the absence of any detectable difference in ZnDTZ staining in carcinoma vs. normal cores (Fig. 1e). Consequently, the zinc differences observed with DTZ staining of the liver normal vs. HCC cores reflect the major changes in their respective zinc levels.
Fig. 1.
In situ staining of zinc levels in normal versus HCC cores on a tissue micro-array slide. a Representative examples of DTZ staining of normal and malignant liver cores. Black pigments in normal liver cores are due to ZnDTZ, which is absent in malignant cores. b Enlargement to show that ZnDTZ staining is dominant in the normal liver parenchyma (hepatocytes) and is absent in HCC. c Confocal microscopy of Zinquin fluorescence of zinc levels, which confirms the loss of zinc in HCC compared to normal liver. d DTZ staining of normal and malignant prostate tissue section, which shows the loss of ZnDTZ staining that characterizes prostate cancer depletion of zinc. e DTZ staining of normal esophageal tissue cores and esophageal squamous carcinoma cores, which shows no difference in ZnDTZ staining consistent with the absence of changes in zinc levels in esophageal cancer
Additional corroboration of the decrease in cellular zinc in the hepatoma cells was obtained with Zinquin staining (Fig. 1c). We employed the Zinquin stain to determine which of the cellular pools of zinc is involved in its depletion. Zinquin binds zinc with a formation constant of log Kf∼10, so that it detects relatively loosely bound forms of zinc. This is the cellular pool of zinc that constitutes exchangeable reactive zinc that is responsible for the cellular effects of zinc. In contrast, ZnDTZ with a log Kf∼15 detects tightly bound immobile zinc as well as mobile reactive zinc; thus, it is a relative estimate of total cellular zinc. Consequently, the Zinquin-detected loss of zinc reveals that the decrease in total cellular zinc includes a major decrease in the mobile reactive pool of zinc in the hepatoma cells. The significant decrease in this pool of zinc will result in altered cellular activities that are due to zinc effects.
The combination of normal and cancer cores contained in the TMA slides that we employed and represented in the results described above (Fig. 1) included a total of 26 cancer cases (52 cores) and eight normal cases (16 cores). The cancer cases included five cases (10 cores) of Grade 1: two cases (four cores) of Grades 1 and 2; 13 cases (26 cores) of Grade 2; four cases (10 cores) of Grade 3; two cases (four cores) of hepatocholangioadenocarcinoma (HCGC). No quantitative differences in the relative zinc levels associated with the grade of cancer were apparent. The normal cores are described by USBiomax as from subjects that had no history of HCC. A major advantage of the employment of the TMA slides in this study is that multiple cancer and normal specimens are treated simultaneously and identically for comparative zinc levels.
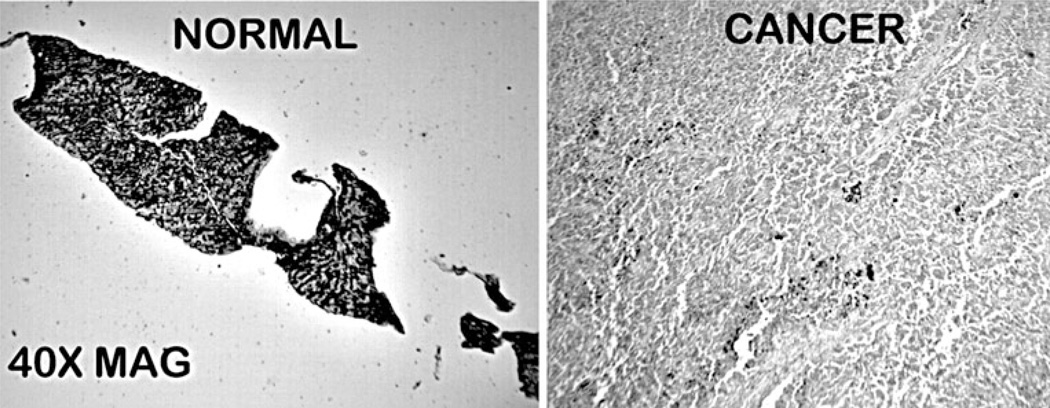
Figure 2 shows the DTZ staining of archived tissue sections that contained normal liver and cancer. The results clearly show that the normal liver contained high ZnDTZ staining, whereas HCC exhibited a major depletion of zinc. Thus, the results confirm the changes observed with the TMA cores.
Fig. 2.
DTZ staining of tissue sections of normal liver and HCC. Black ZnDTZ reveals high zinc in normal liver hepatocyte parenchyma which is absent in HCC
The Relative Levels of ZIP14 Transporter in Normal Liver and HCC
We proceeded to investigate the possible association of zinc uptake transporters with the depletion of zinc in HCC. The concentration of zinc in mammalian cells is dependent largely (with exception) on the presence and activity of plasma membrane zinc uptake transporters, especially ZIP family (Slc39a) transporters. We elected to employ immunohistochemical analysis of selected ZIP transporters in normal and HCC liver tissue sections. Transporter abundance and plasma membrane localization are necessary to identify both expression and functionality; which cannot be determined by gene expression.
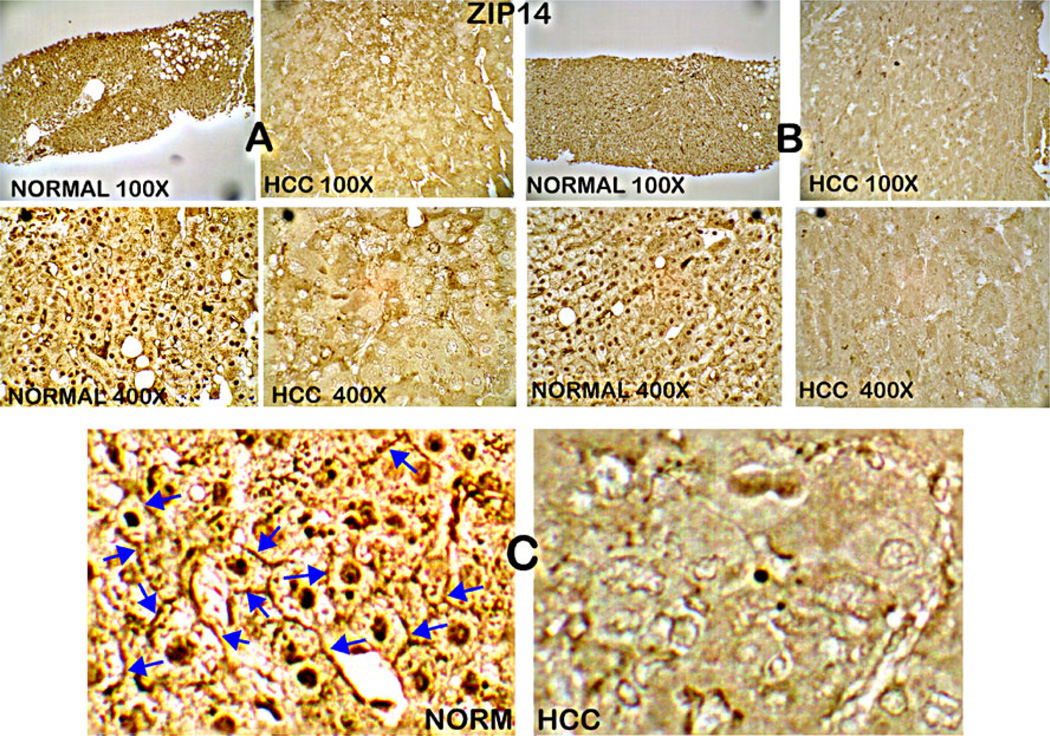
ZIP14 has been identified as a functional plasma membrane zinc uptake transporter in hepatic cells [14–16], which prompted us to determine if it might be associated with HCC. ZIP14 IHC was performed on serial sections from the normal and HCC liver tissues that were employed for the zinc study shown in Fig. 2. The results shown in Fig. 3 reveal that ZIP14 transporter abundance is high in normal hepatic cells, and is essentially absent in the hepatoma cells. It is important to note that Fig. 3b shows that the transporter is localized at the cell membrane in the normal cells, whereas no demonstrable membrane ZIP14 transporter exists in the malignant cells. Localization at the plasma membrane is a requirement for a transporter to function as a zinc uptake transporter, and this demonstrates that ZIP14 is involved in the zinc uptake process of human normal hepatocytes. Thus, the results shown in Figs. 2 and 3 demonstrate that the loss of ZIP14 transporter accompanies the loss of zinc.
Fig. 3.
ZIP14 immunohistochemistry of normal liver and HCC tissue sections. a, b High abundance of ZIP14 in normal hepatocytes, which is absent in hepatoma cells. c Enlargement shows the abundant localization of ZIP14 at the plasma membrane, which is absent in hepatoma cells
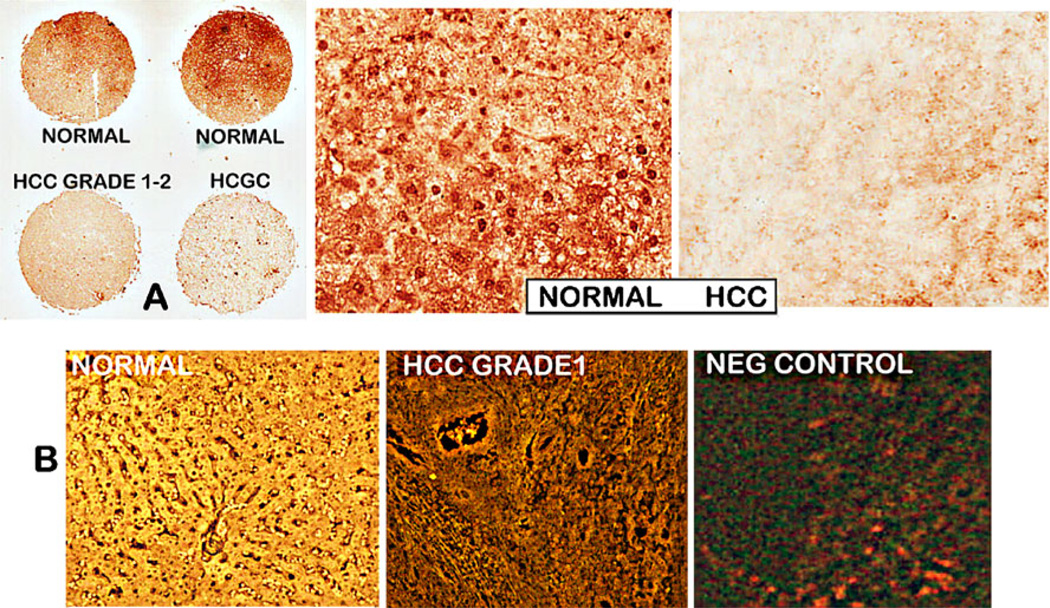
We also performed ZIP14 IHC on a serial TMA slide that was employed for the zinc staining shown in Fig. 1. Figure 4a also shows the major decrease in ZIP14 in HCC and HCGC as compared to normal liver cores. To determine if the loss of ZIP14 transporter in HCC is due to downregulation of ZIP14 gene expression, in situ RT-PCR was performed. This was conducted independently by Dr. Bagasra at Claflin University. Figure 4b shows that ZIP14 gene expression is virtually absent in the malignant core compared to high expression in the normal hepatocytes.
Fig. 4.
ZIP14 immunohistochemistry and in situ RT-PCR of a tissue micro-array slide containing normal liver and HCC cores. a The normal cores exhibit high abundance of ZIP14 transporter; the HCC and HCGC cores exhibit loss of ZIP14 transporter. b In situ RT-PCR that shows the high ZIP14 expression in normal hepatic cells and downregulation of expression in HCC hepatoma cells
ZIP14 in HepG2 Cells
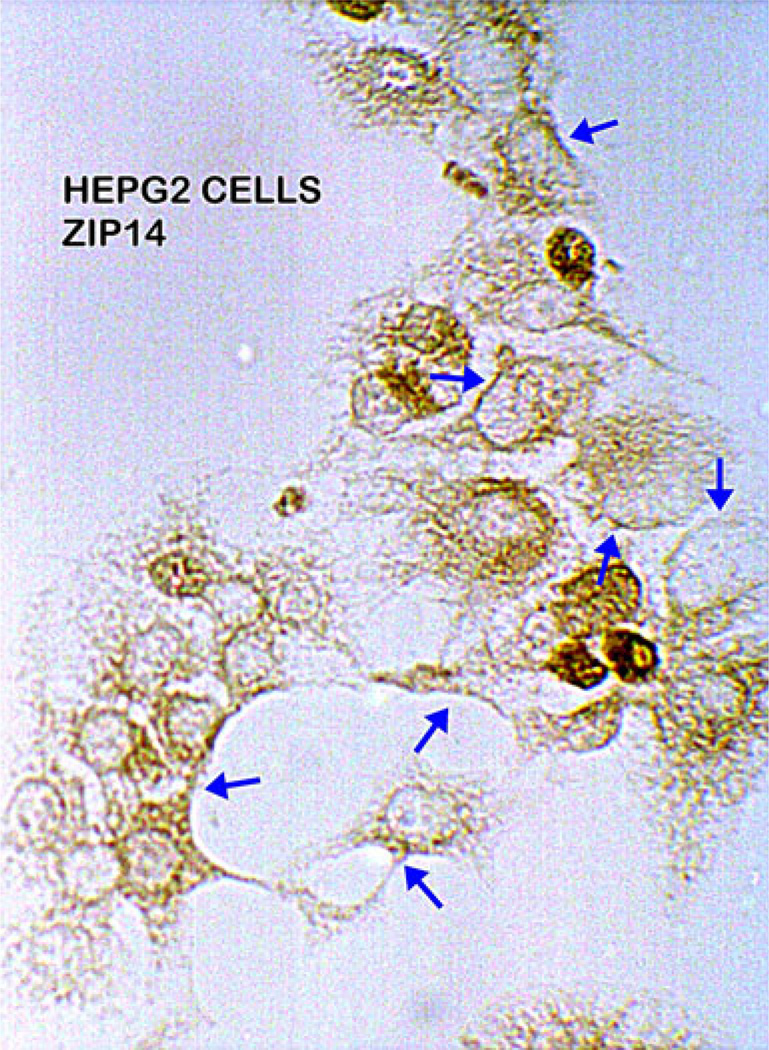
To our knowledge, no reports exist concerning the expression of ZIP14 in malignant hepatic cell lines. Because future studies regarding the role of zinc and ZIP transporters in HCC will require studies with malignant cell lines, we determined if ZIP14 transporter exists in HepG2 cells. Figure 5 shows that ZIP14 transporter is expressed in the cultured HepG2 cells, and the transporter exhibits localization with the cell membrane. The results mimic the ZIP14 membrane association observed in the in situ normal hepatic cells, rather than exhibiting the loss of ZIP14 observed in the in situ hepatoma cells as shown in Figs. 3 and 4. This observation has relevance to the mechanism involved in the loss of ZIP14 that occurs in the hepatoma cells in situ in HCC, which is discussed below.
Fig. 5.
ZIP14 immunohistochemistry of HepG2 cells. The cells exhibit presence of ZIP14 with localization at the plasma membrane (arrows)
Relative Levels of ZIP1, ZIP2, and ZIP3 in Normal Liver and HCC
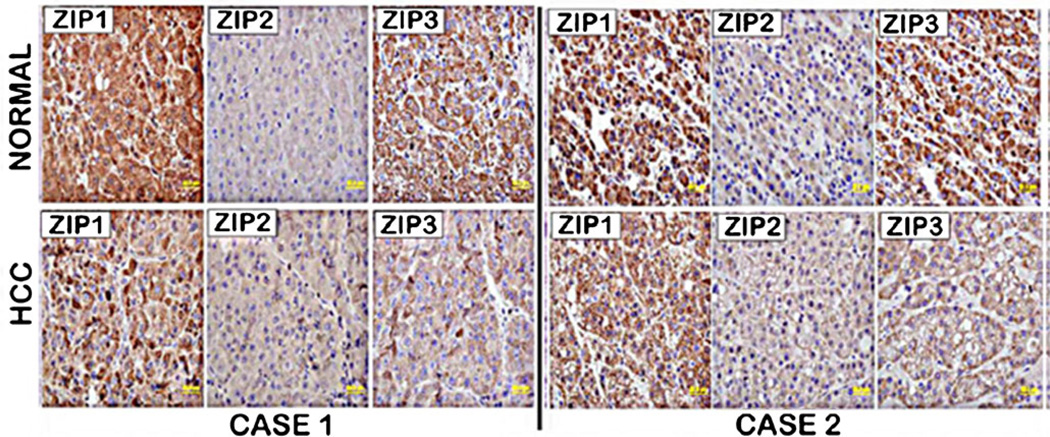
We also determined the relative abundance of ZIP1, ZIP2, and ZIP3 transporters in normal and HCC tissue sections. Figure 6 shows that ZIP1 and ZIP3 transporter abundance is relatively high in normal hepatic cells and is markedly decreased in HCC. In contrast, ZIP2 transporter is low or absent in normal hepatic cells, and there is little, if any, change in HCC. However, the IHC also reveals that in normal hepatic cells, there is no evidence that ZIP1or ZIP3 is localized at the cell membrane. Consequently, it is unlikely that these are functional zinc uptake transporters in the normal hepatoyctes, and their decrease in HCC is not likely to be associated with the decrease in zinc in the hepatoma cells.
Fig. 6.
ZIP1, ZIP2, and ZIP3 immunohistochemistry of normal liver and HCC tissue sections. Normal liver hepatocytes exhibit ZIP1 and ZIP3 transporter, which is markedly decreased in HCC. However, neither ZIP1 nor ZIP3 transporter is localized to the plasma membrane. ZIP2 transporter is not detectable in normal liver hepatocytes with little, if any, difference in HCC
Effect of Zinc Treatment on Proliferation of HepG2 Cells
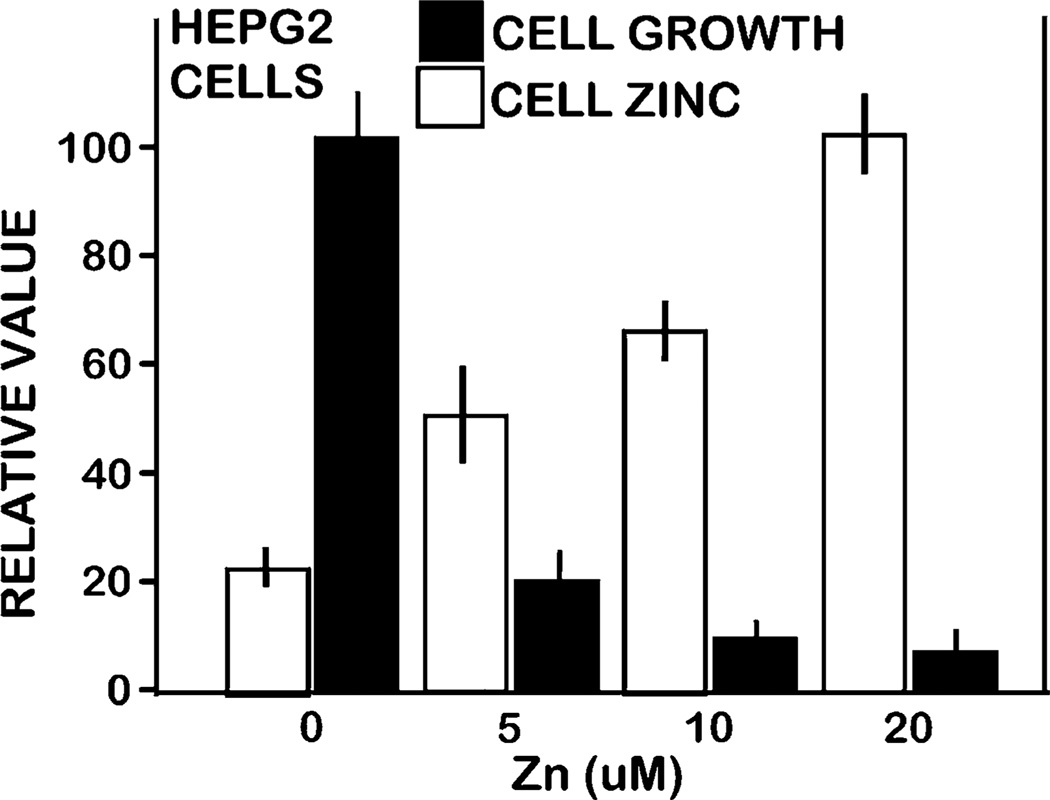
The results presented above suggest the implication of zinc as a tumor-suppressor agent in the HCC malignant cells, as has been identified in some other cancers (discussed below). To obtain initial information, we determined the effects of zinc treatment on the growth of HepG2 cells. The results (Fig. 7) show that treatment with the physiological concentration of 5 µM zinc increased cellular zinc ∼110%, which resulted in ∼80% inhibition of cell growth. Therefore, the HepG2 cells are highly sensitive to the growth-inhibitory effects of increased cellular zinc. Since we employed a Zinquin assay, the observed effect is due to an increase in the mobile reactive form of zinc, and this is the pool of zinc that is decreased in HCC hepatoma cells (Fig. 1).
Fig. 7.
Effect of zinc treatment on growth and cellular zinc level of HepG2 cells. Pyrithione (1 µM) and zinc were added to medium, and the cells were incubated for 24 h. After treatment, cell number and zinc level were determined using the CyQUANT Cell Proliferation Assay kit and by Zinquin fluorescent indicator
Discussion
In situ staining for zinc levels in tissue sections has the advantage of providing a visualization of zinc changes in the cellular components of the tissue. One can identify with certainty that the zinc changes are associated specifically with the normal hepatocytes vs. the hepatoma cells. Zinc measurements with tissue extracts or X-ray fluorescence of tissues, as were conducted in the earlier reported studies do not provide such information. In addition, the in situ identification of relative zinc levels and changes in hepatocytes and hepatoma cells allows for identification of comparative and concurrent changes in other parameters such as ZIP transporters, and also the association of changes in the hepatoma cells with the progression of malignancy.
In this study, we now confirm with visualized in situ zinc staining of normal and HCC tissue sections that cellular zinc is markedly decreased in the hepatoma cells as compared to the normal hepatic cells. In addition, we show for the first time that the decrease in zinc exists in early stage malignancy and persists in the advancing stages. When coupled with the earlier reports of decreased zinc in HCC [2–8], compelling evidence now establishes that zinc depletion is involved in the development and progression of HCC. It is also notable that the composite of all of the earlier studies and this study must include a spectrum of HCC cases that involve various liver pathologies such as cirrhosis, hepatitis, and other conditions and with none of these conditions. Yet, the decrease in zinc in HCC is common to essentially all cases, which strongly indicates that the zinc relationship exists exclusive of other accompanying pathology. This would suggest that the loss of zinc is a consistent event in the process of HCC carcinogenesis.
The reported ∼55–65% zinc decrease in HCC [2–8] is confirmed by the dramatic loss of zinc identified by in situ staining of the hepatoma cells in HCC (Figs. 1 and 2). The earlier reports involved methods that determine the change in the total zinc concentration in the tissues. However, the effects of cellular zinc on cellular activities results from the exchangeable reactive pool of zinc, which constitutes ∼5% of the cell’s total zinc. In addition to corroborating the large decrease in total zinc, we now establish that a major decrease occurs in the important reactive pool of zinc, and this will have significant implications on cellular activities of the normal hepatocytes vs. the hepatoma cells in HCC. The likely consequence of this decrease in zinc is the removal of tumor suppressor effects of zinc on the hepatoma cells as has been identified in other malignant cells [17, 18]. This possibility is corroborated by our observation that the cellular accumulation of mobile reactive zinc inhibited the cell growth of HepG2 cells. Lemire et al. [19] also reported that zinc exhibited metabolic cytotoxic effects on HepG2 cells.
The concentration of zinc in mammalian cells is generally predominantly dependent upon the cellular uptake of zinc from ISF (derived from blood plasma); which is achieved manly by the presence of ZIP-family plasma membrane zinc uptake transporters. We identified ZIP14 as a plasma membrane transporter in normal human liver hepatic cells in situ, which indicates that it is a likely functional zinc uptake transporter in the normal human hepatocytes in situ. ZIP14 has been shown and suggested [14–16] to be a functional zinc uptake transporter in hepatic cells in animals. Also, our identification of the loss of ZIP14 transporter and its gene expression in HCC is consistent with microarray data deposited in the oncomine database (https://www.oncomine.org/resource/login.html), which suggest that ZIP14 is among the top 1% of genes under-expressed in HCC compared to normal liver. The concurrent loss of ZIP14 transporter with the decrease in zinc in the hepatoma cells in early stage and progressing malignancy would suggest that ZIP14 downregulation is a possible causative factor for the loss of zinc in the transformation of normal hepatocytes to HCC hepatoma cells. Further studies are necessary to confirm such a relationship. While we showed that ZIP1, ZIP2, and ZIP3 transporters are not likely candidates involved with the loss of zinc in HCC, we do not dismiss the possibility of the involvement of other zinc transporters.
Although the decrease in zinc in HCC has been reported and confirmed over the past 40 years, the potential importance of this relationship has been largely ignored. Consequently, information regarding the implication of zinc and its role and mechanisms in HCC is seriously lacking. Weaver et al. [21] reported that ZIP4 is upregulated in HCC and, under specific experimental conditions, can promote malignant cell growth and invasiveness. The authors did recognize that zinc has been reported to be decreased in HCC, which is inconsistent with a role of ZIP4 in increasing zinc in HCC hepatoma cells. Weaver et al. suggest that the upregulation of ZIP4 is in response to the depletion of zinc in the hepatoma cells. However, our studies now show that the decrease in zinc is sustained in the advancing stages of malignancy so that a recovery of zinc levels is not evident. Their HCC IHC figure does not show any evidence of membrane association of ZIP4 transporter that would be required for functional cellular uptake of zinc. Consequently, the up regulation of ZIP4 expression in HCC does not appear to be involved in the loss of zinc in hepatoma cells that occurs in the development of HCC and is sustained in advancing malignancy. Their conclusion, based on experimental studies, that increased zinc protects against HCC malignancy is not supported by clinical evidence or by our study and is highly questionable.
It is relevant that we also showed that HepG2 cells exhibit the expression of ZIP14 and the localization of the transporter at the plasma membrane, which is identical to the in vivo relationship in normal hepatocytes and opposite to the in situ relationship in the malignant cells in HCC. This reveals that the loss of ZIP14 transporter in HCC does not result from deletion or permanent mutation of the ZIP14 gene. Therefore, the reappearance of ZIP14 in HepG2 cells under standard in vitro culture conditions is likely due to the absence of the in situ factors/conditions required for induction of ZIP14 gene silencing. Thus, the mechanism of regulation of ZIP14 gene expression in relation to HCC now needs to be established.
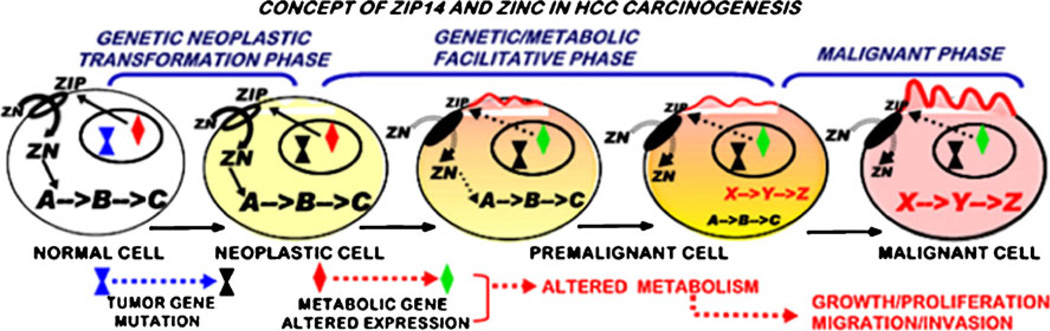
The consistent concurrent downregulation of ZIP14 and depletion of zinc in hepatoma cells in early stage and advancing malignancy strongly suggests a coupling of these events in HCC. The fact that loss of ZIP14 transporter and depletion of zinc are fully apparent in well-differentiated malignancy establishes that these events are initiated early in the transformational stages (i.e. premalignant lesions) leading to malignant cells and malignancy. Added to these relationships is the possibility that the depletion of zinc is necessary to eliminate the tumor suppressor effects of zinc. These observations support the proposal that ZIP14 down-regulation and zinc depletion constitute an important coupled event in HCC carcinogenesis. Carcinogenesis is a multi-step progressive process (Fig. 8) [18, 22, 23]. It requires the initiating tumor promoting oncogene transformation of the normal cell to a neoplastic malignant cell type. This neoplastic cell is not malignant and must undergo additional down-stream genetic/metabolic transformations that lead to premalignant lesions and ultimately to malignant cells. These genetic/metabolic transformations are essential to provide the requirements in support of the malignant process. This relates to the activity differences of normal cells versus malignant cells. The metabolic state of a cell is dependent upon and determined by the existing activity of the cell, such as its functional activity, growth/ proliferation, and differentiation. The genetic/metabolic state of a normal cell is generally unsuitable and even adverse to support the requirements for the malignant process of malignant cells (e.g., bioenergetic/synthetic metabolism to support growth/proliferation and migration/invasion). In the absence of the genetic/metabolic transformations, the neoplastic cell cannot progress to fulfill its malignant potential. We propose that ZIP14 silencing with resulting decrease in zinc is one of the essential genetic/metabolic transformations required for the manifestation of the development of malignancy in HCC (Fig. 8). A consequence of the transformation of the normal cell to the neoplastic cell is that the evolutionary conditions (still unknown) that protect normal cells in situ from the potential adverse effects of zinc do not exist in the malignant cells.
Fig. 8.
The concept of carcinogenesis and the role of zinc and ZIP14 gene expression in hepatocarcinogenesis
In HCC, the premalignant cells/lesions that give rise to the malignant cells are unknown. However, small cell and large cell dysplastic foci/nodules are thought to be likely lesions that give rise to malignancy [24–27]. The expression of ZIP14 along with zinc levels in normal hepatocytes and their changes in malignant cells would be an important link to the identification of premalignant lesions and for early biomarker identification of development of HCC. Also, we show that treatment of HepG2 cells with physiological levels of zinc results in zinc accumulation and inhibition of cell growth. However HepG2 cells exhibit plasma membrane ZIP14 transporter so that zinc uptake from medium can occur. This is not the condition in the hepatoma cells in situ in HCC. As a potential therapeutic approach, the delivery of zinc must be accompanied by a process that will permit the entry of zinc into the malignant cells, which do not express the functional zinc uptake transporter (i.e. ZIP14). If further studies demonstrate that zinc exhibits tumor suppressor effects against malignant hepatic cells, this will be a viable therapeutic approach to pursue.
Acknowledgements
We are grateful to Dr. Robert Cousins (University of Florida) for kindly providing us with the ZIP14 antibody employed in this study. We are also grateful to Dr. Peter Zalewski (University of Adelaide, Australia) for kindly providing us with the Zinquin ester employed in this study. These studies were supported by NIH grants DK076783 and CA79903.
Contributor Information
Renty B. Franklin, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, 650 West Baltimore Street, Baltimore, MD 21201, USA rfranklin@umaryland.edu The University of Maryland Greenebaum Cancer Center,Baltimore, MD 21201, USA.
Bernard A. Levy, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, 650 West Baltimore Street, Baltimore, MD 21201, USA
Jing Zou, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, 650 West Baltimore Street, Baltimore, MD 21201, USA.
Nader Hanna, The University of Maryland Greenebaum Cancer Center,Baltimore, MD 21201, USA; Division of Surgical Oncology, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Mohamed Mokhtar Desouki, Department of Pathology and Laboratory Medicine, Medical University of South Carolina, Charleston, SC 29425, USA.
Omar Bagasra, South Carolina Center for Biotechnology,Orangeburg, SC 29115, USA; Biology Department, Claflin University, Orangeburg, SC 29115, USA.
Leslie A. Johnson, South Carolina Center for Biotechnology,Orangeburg, SC 29115, USA Biology Department, Claflin University, Orangeburg, SC 29115, USA.
Leslie C. Costello, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, 650 West Baltimore Street, Baltimore, MD 21201, USA Email: lcostello@umaryland.edu; The University of Maryland Greenebaum Cancer Center,Baltimore, MD 21201, USA.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med. 1970;11:260–264. [PubMed] [Google Scholar]
- 3.Ebara M, Fukuda H, Hatano R, Saisho H, Nagato Y, et al. Relationship between copper, zinc and metallothionein in hepato-cellular carcinoma and its surrounding liver parenchyma. J Hepatol. 2000;33:415–422. doi: 10.1016/s0168-8278(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 4.Liaw KY, Lee PH, Wu FC, Tsai JS, Lin-Shiau SY. Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am J Gastroenterol. 1997;92:2260–2263. [PubMed] [Google Scholar]
- 5.Tashiro H, Kawamoto T, Okubo T, Koide O. Variation in the distribution of trace elements in hepatoma. Biol Trace Elem Res. 2003;95:49–63. doi: 10.1385/BTER:95:1:49. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro-Itoh T, Ichida T, Matsuda Y, Satoh T, Sugiyama M, et al. Metallothionein expression and concentrations of copper and zinc are associated with tumor differentiation in hepatocellular carcinoma. Liver. 1997;17:300–306. doi: 10.1111/j.1600-0676.1997.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurusamy K, Davidson BR. Trace element concentration in metastatic liver disease: a systematic review. J Trace Elem Med Biol. 2007;21:169–177. doi: 10.1016/j.jtemb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Al-Ebraheem A, Farquharson MJ, Ryan E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. Appl Radiat Isot. 2009;67:470–474. doi: 10.1016/j.apradiso.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg CA, Philips FS. Histochemical demonstration of zinc in the dorsolaterl prostate of the rat:studies with oxine and dithizone. Am J Pathol. 1965;47:325–337. [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin RB, Feng P, Milon BC, Desouki MM, Singh KK, et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005 doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC, et al. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007 doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipman TO, Diamond A, Mellow MH, Patterson KY. Esophageal zinc content in human squamous esophageal cancer. J Am Coll Nutr. 1987;6:41–46. doi: 10.1080/07315724.1987.10720164. [DOI] [PubMed] [Google Scholar]
- 14.Taylor KM, Morgan HE, Johnson A, et al. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Lichten LA, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Ann Rev Nutr. 2009;29:153–176. doi: 10.1152/ajpgi.90676.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liuzzi JP, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Ann Rev Nutr. 2004;24:151–172. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006 doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemire J, Mailloux R, Appanna VD. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J Appl Toxicol. 2008;28:175–182. doi: 10.1002/jat.1263. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi T, Matsui T, Chujo M, Nagao M. Restraint stress upregulates expression of zinc transporter Zip14 mRNA in mouse liver. Cytotech. 2008;57:181–185. doi: 10.1007/s10616-008-9148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, et al. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS One. 2010 doi: 10.1371/journal.pone.0013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costello LC, Franklin RB. Metabolic transformations of malignant cells: an overview. In: Hyatt MA, editor. Methods of cancer diagnosis, therapy, and prognosis, vol 2 general methods and overviews, lung carcinoma and prostate carcinoma. New York: Springer; 2008. pp. 3–16. [Google Scholar]
- 23.Costello LC, Franklin RB. Integration of genetic, proteomic, and metabolic approaches in tumor cell metabolism. In: Singh KK, Costello LC, editors. Mitochondria and Cancer. New York: Springer; 2009. pp. 79–92. [Google Scholar]
- 24.Anthony PP, Cl V, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973;26:217–223. [PMC free article] [PubMed] [Google Scholar]
- 25.Koo JS, Kim H, Park BK, Ahn SH, Han KH, et al. Predictive value of liver cell dysplasia for development of hepatocellular carcinoma in patients with chronic hepatitis B. J Clin Gastroenterol. 2008;42:738–743. doi: 10.1097/MCG.0b013e318038159d. [DOI] [PubMed] [Google Scholar]
- 26.Le Bail B, Bernard PH, Carles J, Ahn SH, Han KH, et al. Prevalence of liver cell dysplasia and association with HCC in a series of 100 cirrhotic liver explants. J Hepatol. 1997;27:835–842. doi: 10.1016/s0168-8278(97)80321-2. [DOI] [PubMed] [Google Scholar]
- 27.Podda M, Roncalli M, Battezzati PM, Borzio M, Bruno S, et al. Liver-cell dysplasia and hepatocellular carcinoma. Ital J. 1992;24:39–42. [PubMed] [Google Scholar]