Abstract

We have developed an efficient and robust route to synthesize 4,5,7-trisubstituted pyrrolo[3,2-d]pyrimidines as potent kinase inhibitors. This solution-phase synthesis features a SNAr substitution reaction, cross-coupling reaction, one-pot reduction/reductive amination and N-alkylation reaction. These reactions occur rapidly with high yields and have broad substrate scopes. A variety of groups can be selectively introduced into the N5 and C7 positions of 4,5,7-trisubstituted pyrrolopyrimidines at a late stage of the synthesis, thereby providing a highly efficient approach to explore the structure-activity relationships of pyrrolopyrimidine derivatives. Four synthetic analogs have been profiled against a panel of 48 kinases and a new and selective FLT3 inhibitor 9 is identified.
Keywords: Pyrrolopyrimidine, SNAr displacement, Coupling reaction, Reductive amination, N-alkylation
INTRODUCTION
Pyrrolopyrimidines have been broadly used as synthetic pharmacophores in drug discovery due to their structural similarity to purines with numerous associated biological activities. Accordingly, pyrrolopyrimidines have been exploited as inhibitors of kinases (VEGFR,1 FGFR2, HGF,3 Akt,4 MMP5, JAK,6 Lck,7 etc.), methylthioadenosine phosphorylase (MTAP),8 purine nucleoside phosphorylase (PNP),9 and HIV replication.10 In one of our drug discovery programs, we are interested in 4,5,7-trisubstituted pyrrolo[3,2-d]pyrimidines as potential kinase inhibitors. Consequently, it is important to develop facile and efficient methods to synthesize this family of molecules. Retrosynthetic analysis suggests that 4,5,7-trisubstituted pyrrolo[3,2-d]pyrimidines can be synthesized through two general approaches (Scheme 1).
Scheme 1.
Approaches for Synthesis of Pyrrolopyrimidine Derivatives
Approach A is focused on the construction of the pyrrole ring using substituted pyrimidines as the starting materials.11 Although these methods are useful, the yields are poor for some cases.11b In addition, the substituents R1 and R2 on the pyrrolopyrimidine core have to be introduced prior to the ring formation, which renders further modification of these substituents difficult. In contrast, approach B starts with a simple pyrrolopyrimidine core and the substituents are introduced late in the synthesis, enabling exploration of diverse analogues possible at lower cost and in a timely fashion. However, to date there has been no reported method for this approach. In our efforts to employ pyrrolopyrimidines as selective kinase inhibitors, an efficient and robust method has been developed to synthesize 4,5,7-trisubstituted pyrrolo[3,2-d]pyrimidine analogues through approach B.
RESULTS AND DISCUSSION
Compound 1 (Scheme 2) was chosen as the key building block for our synthesis for the following reasons. First, the chloride on the C4 position could be potentially displaced by various nuclophiles to introduce diversified R1 substituents. Second, the NO2/NH2 group at the C7 position could be used as a handle for introduction of N-containing R2 substituents. Third, the R3 group could be attached through N-alkylation at the N5 position.
Scheme 2.

Synthesis of the Key Building block 1 and Its Transformations.
The key building block 1 was synthesized by chlorination of the known compound 7-nitro-3H-pyrrolo[3,2-d]pyrimidin-4(5H)-one with neat phosphorus(V) oxychloride under reflux (Scheme 2). For 2-halopyrimidines, the halogen atom could be replaced smoothly by various nuclophiles.12 However, the additional pyrrole ring in pyrrolopyrimidine makes the replacement reaction more difficult. With 2-(trimethylsilyl)ethoxymethyl (SEM) protecting group at nitrogen of the pyrrole ring, SNAr displacement of chloride at the C4 position of 2 with 4-phenylphenol went smoothly at room temperature in high yield. The direct displacement of chloride in the key building block 1 with phenols, however, was challenging and only occurred after heating at 175 °C for 50 min under microwave irradiation. Because direct displacement of chloride in 1 would circumvent the need for protection and deprotection steps, this reaction was further explored (Table 1).
Table 1.
SNAr Substitution and Coupling Reactions of 1.
 | ||||
|---|---|---|---|---|
| Entry | Reagent | Product | 4 | Yield (%) |
| 1 |  |
4a | 75a | |
| 2 |  |
4b | 76a | |
| 3 |  |
 |
4c | 74a |
| 4 |  |
 |
4d | 32a |
| 5 |  |
4e | 81a | |
| 6 | No desired product | |||
| 7 | n-PrOH |  |
4f | 72b |
| 8 | n-BuNH2 |  |
4g | 92c |
| 9 |  |
4h | 90c | |
| 10 |  |
4i | 85c | |
| 11 |  |
4j | 87d | |
| 12 |  |
 |
4k | 90d |
| 13 |  |
 |
4l | 62d |
| 14 |  |
4m | 90d | |
| 15 |  |
 |
4n | 87d |
| 16 |  |
4o | 64e | |
| 17 |  |
4p | 45e | |
1.5 equiv phenol, 3.0 equiv K2CO3, DMF, µW, 175 °C, 50 min;
1.2 equiv NaH, 2.0 equiv propanol, THF, µW, 120 °C, 20 min;
1.2 equiv aniline/amine, iPrOH, 80 °C, overnight;
1.5 equiv boronic acid, 5% Pd(PPh3)4, DMF/H2O, µW, 150 °C, 15 min;
3 equiv benzylzinc bromide, toluene, 10% Pd(PPh3)4, 100 °C, overnight.
In the presence of potassium carbonate, phenol (entry 1), para-substituted phenols (entries 2 & 5) and meta-substituted phenol (entry 3) underwent the SNAr substitution reactions smoothly with 1 in good yields, while ortho-substituted phenol (entry 4) gave poor yield possibly due to the steric hindrance. The ester functional group didn’t tolerate the reaction conditions (entry 6) and an aliphatic alcohol was not reactive under these conditions. However, conversion to an alkoxide (entry 7) completed the transformation at lower temperature (120 °C vs 175 °C) and in a shorter reaction time (20 min vs 50 min). Amines including aniline (entries 8–10) were better nucleophiles and reacted under much milder reaction conditions (conventional heating at 80 °C for overnight) with higher yields. More importantly, a few cross-coupling reactions also worked using 1 as a reactant which further diversified the available R1 substituents. Accordingly, the R1 substituents of 4 could be introduced either from a boronic acid/ester via Suzuki-Miyaura coupling conditions13 (entries 11–15) or from a zinc reagent via the Negishi coupling reaction14 (entries 16, 17). In general, electron donating groups increase while electron withdrawing groups decrease reactivity of boronic acid in a Suzuki-Miyaura couping reaction. For example, (2-methoxyphenyl)boronic acid (entry 12) provided product 4k with higher yield than (3-fluorophenyl)boronic acid (para-methoxyl) (entry 13). In addition, furan could be introduced to the R1 position (entry 14) and boronic ester (entry 15) also worked under the same reaction conditions. While Suzuki-Miyaura coupling reaction is suitable to introduce aromatic groups at the R1 position, Negishi coupling reaction works better when R1 is an alkyl group. As shown in Table 1, decant yields were obtained when two different zinc reagents were used (entry 16, 17).
Next, we explored the introduction of R2 substituents at the C7 position of pyrrolo[3,2-d]pyrimidines. Since coupling reactions had been successfully applied to introduce the C-substituents, such as aryl, heteroaryl, vinyl, alkynyl and benzyl, at this position in a similar system15, our focus was mainly on the introduction of the diversified N-substituents. The most efficient way to achieve this goal was through a one-pot reduction of the nitro group and in situ reductive amination with aldehydes under hydrogen atmosphere in the presence of palladium on activated charcoal. Using this one-pot protocol, 3 was converted to 5 in high yield (Scheme 3). The NHEt group at the C7 position in 5 was protected as a tert-butyl carbamate (Boc) (6) to minimize the potential interference with the next N-alkylation on the N5 position. Subsequently, the SEM protecting group in 6 was selectively removed by treatment with TBAF to provide 7.
Scheme 3.
Introduction of Secondary Amine Groups at the C7 Position of Pyrrolopyrimidines.
We were also able to introduce tertiary amines and amide groups at the C7 position of pyrrolopyrimidines by step-wise approaches as illustrated in Scheme 4. First, the nitro group in 4g was reduced to the corresponding amine in 8. Next, alkylation or acylation of 8 provided 9 and 10, respectively. Although there are three NH groups in 8, the newly formed primary amine is more reactive likely due to the low electron density and/or steric hindrance on the other two basic nitrogen atoms. The yield for the alkylated 9 was 81% while those for acylated 10 with various acid chlorides were lower due to competing N-diacylation at the same position.
Scheme 4.
Introduction of Tertiary Amines and Amide Groups at the C7 Position of 8.
Finally, R3 substituents at the N5 position of pyrrolopyrimidines were introduced by N-alkylation (Table 2). Primary alkyl chlorides reacted with 7 at 150 °C for 15 min under microwave irradiation to provide the desired N-alkylated products (entries 1–3). For secondary chlorides (entry 4), a larger excess of halide (3 equivalents) and longer reaction time (30 min) were needed. Primary bromides (entries 5–8) also gave the desired N-alkylation products under the same reaction conditions as for primary chlorides. However, cyclohexyl bromide or iodide (entry 9) did not provide any desired alkylation product, presumably due to the elimination reaction of the bromide/iodide. The final products 11 were obtained by removal of the Boc group under acidic conditions.
Table 2.
N-Alkylation at the N5 Position of 7.
 | ||||
|---|---|---|---|---|
| Entry | R3X | Product | 11 | Yield (%) |
| 1 |  |
11a | 93a | |
| 2 |  |
11b | 74a | |
| 3 |  |
 |
11c | 72a, b |
| 4 |  |
11d | 92c | |
| 5 |  |
 |
11e | 74a |
| 6 |  |
 |
11f | 75a |
| 7 |  |
11g | 76a | |
| 8 |  |
11h | 65a | |
| 9 |  |
No desired productc | ||
1.5 equiv R3X, DMF, µW, 150 °C, 15 min;
In product 11c, the Boc group on the aniline nitrogen has been removed as well under the treatment of TFA;
3 equiv halide, DMF, µW, 150 °C, 30 min
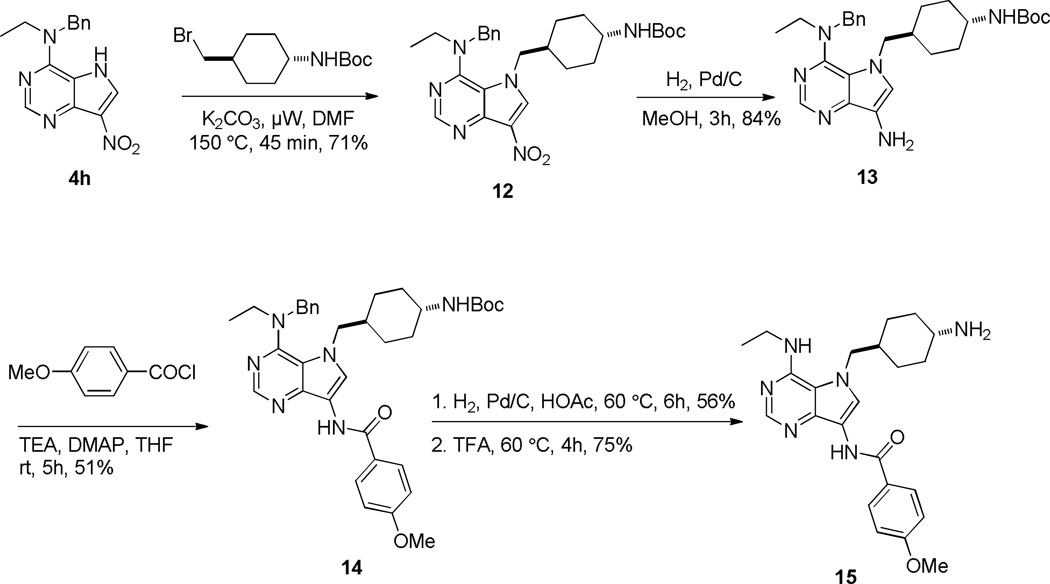
A selectivity issue arises for compounds with a NHR group at the C4 position such as 4g and 4i (Table 1) in the final N-alkylation reaction because the nitrogen at the C4 position of the pyrimidine demonstrated reactivity similar to the pyrrole nitrogen. Accordingly, the nitrogen at the C4 position had to be protected early in the synthesis. For example, compound 4h (Table 1) could serve as an intermediate for this purpose as the nitrogen at the C4 position was protected by a benzyl (Bn) group. N-alkylation reaction of 4h with trans-tert-butyl (4-(bromomethyl)cyclohexyl)carbamate proceeded smoothly in good yield (Scheme 5). After the reduction of the nitro group in 12 and N-acylation of 13, compound 14 was obtained. The Bn protecting group in 12 survived under the reductive hydrogenation conditions but could subsequently be removed under acidic conditions with higher temperature. The t-butoxycarbonyl (Boc) protecting group was removed by trifluoroacetic acid (TFA) after the removal of the Bn group in 14 to provide 15. Through this alternative route, the pyrrole nitrogen could be selectively alkylated.
Scheme 5.
Selective N-Alkylation at N5 Position in 4h.
We have also investigated the potential applications of the synthetic analogs as kinase inhibitors. Four structurally different compounds 9, 10b, 11a, 15 were selected for the initial study. Each of the four compounds was tested for its capacity to inhibit a panel of 48 kinases using the Profiler Pro Kit (Caliper Life Sciences) at the concentration of 10 µM. All the experiments were carried out in duplicates. To our delight, the fms-like receptor tyrosine kinase-3 (FLT3) was the only kinase that was inhibited by more than 50% and only by 9 (details in supplemental materials). FLT3 is a validated target for drug discovery for a variety of diseases.16 We thus further tested the inhibitory activity of 9 in a concentration-dependent manner. The inhibition of FLT3 kinase activity was measured at the ATP Km using a microfluidic capillary electrophoresis (MCE) assay17 in which phosphorylated and unphosphorylated substrate peptides were separated and analyzed through a LabChip EZ Reader. The IC50 of 9 in this assay was 1.92 µM. Further SAR exploration to improve the potency of 9 is currently on-going and will be reported in due course.
CONCLUSION
In summary, we have developed an efficient and robust route to prepare 4,5,7-trisubstituted pyrrolopyrimidines from the common intermediate 1. The synthesis features a SNAr substitution reaction, cross-coupling reaction, one-pot reduction/reductive amination and N-alkylation reaction. These reactions occur rapidly with high yields and have broad substrate scopes. A variety of R2 and R3 groups can be selectively introduced into 4,5,7-trisubstituted pyrrolopyrimidines at a late stage of the synthesis. This synthetic strategy is highly desirable when large numbers of 4,5,7-trisubstituented pyrrolopyrimidine analogues are needed in high yields and purity for SAR development via biological evaluation. In addition, a new and selective FLT3 inhibitor 9 has been identified from the synthesized pyrrolopyrimidine analogues.
EXPERIMENTAL SECTION
General Experimental Details
All reagents were commercially available and used without any further purification. Microwave reaction was carried out using a Discover-S reactor with a vertically-focused IR external temperature sensor and an Explorer 72 autosampler. The dynamic mode was used to set up the desired temperature and hold time with the following fixed parameters: PreStirring, 1 min; Pressure, 200 psi; Power, 200 W; PowerMax, off; Stirring, high. Flash chromatography was carried out on Teledyne ISCO Combi Flash Rf using pre-packed silica gel disposable columns. A gradient from 0% to 100% ethylacetate (100% to 0% hexane) for nonpolar compounds or to 20% methanol (100% to 80% CH2Cl2) for polar compounds was used as elutes. Analytical thin-layer chromatography (TLC) was performed with silica gel 60 F254, 0.25 mm pre-coated TLC plates. TLC plates were visualized using UV254 or phosphomolybdic acid with charring. All 1H NMR spectra were obtained with a 400 MHz spectrometer and 13C NMR spectra were obtained with a 100 MHz spectrometer. Preparative HPLC was performed with the UV detection at 220 or 254 nm. LC-MS was performed with the UV detection at 220 nm, 254 nm, and 280 nm, and a single quadrupole mass spectrometer using electrospray ionization (ESI) source. High-resolution (positive ion) mass spectra (HRMS) were acquired using a LCMS-TOF mass spectrometer.
4-Chloro-7-nitro-5H-pyrrolo[3,2-d]pyrimidine (1)
The solution of 7-nitro-3H-pyrrolo[3,2-d]pyrimidin-4(5H)-one (5.0 g, 27.8 mmol) in POCl3 (12.6 g, 83.3 mmol) was heated under reflux for 5.0 h. Then the mixture was cooled to room temperature and poured onto the chipped ice with vigorous stirring, then, basified with K2CO3 to PH 8~9. The resulting mixture was filtered and washed with water (2x) and EtOAc (3x) to provide the title compound (4.5 g, 82%) as yellow powder. 1H NMR (400 MHz, DMSO-d6) δ 9.11 (s, 1H), 8.90 (s, 1H); 13C NMR (101 MHz, DMSO-d6) δ 153.1, 144.7, 142.8, 137.1, 128.1, 124.6; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C6H4ClN4O2, 199.0023; found 198.9869
4-([1,1'-Biphenyl]-4-yloxy)-7-nitro-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[3,2-d]pyrimidine (3)
To a DMF (10 mL) solution of 1 (1.0 g, 5.0 mmol) and SEMCl (1.1 mL, 6.0 mmol) was added NaH (0.4 g, 60wt% in mineral oil, 10 mmol) at 0 oC. The resulting mixture was warmed to room temperature and stirred overnight. The reaction was quenched by water. The aqueous phase was extracted by ether (2x) and EtOAc (2x). The combined organic layers were dried (Na2SO4) and concentrated. The residue was passed a short silica pad to provide 4-chloro-7-nitro-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[3,2-d]pyrimidine (2) (1.6 g) which was used directly for the next step. A mixture of 2 (1.6 g, 4.88 mmol), [1,1'-biphenyl]-4-ol (1.66 g, 9.76 mmol), and K2CO3 (2.02 g, 14.64 mmol) in 25 mL DMF was stirred for 5.0 h at room temperature. The reaction was quenched by water and extracted with ether (3X). The combined organic layers were dried (Na2SO4) and concentrated. The residue was purified by column chromatography with ISCO system to provide the title compound 3 (2.1 g, 91% over 2 steps) as a light yellow solid. 1H NMR (400 MHz, CDCl3) δ 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 8.45 (s, 1H), 7.73–7.67 (m, 2H), 7.63–7.58 (m, 2H), 7.49–7.43 (m, 2H), 7.40–7.35 (m, 1H), 7.34–7.29 (m, 2H), 5.87 (s, 2H), 3.77–3.65 (m, 2H), 1.03–0.93 (m, 2H), −0.02 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 156.3, 154.0, 150.8, 144.1, 140.2, 139.8, 133.8, 129.1, 129.0, 128.7, 127.7, 127.3, 122.1, 115.2, 79.4, 67.8, 18.0, −1.3; LC-MS (ESI+): tR = 6.285 min, m/z 463.2 [M+1]+.
7-Nitro-4-phenoxy-5H-pyrrolo[3,2-d]pyrimidine (4a) (General procedure A)
A 10 mL microwave tube was charged with 1 (92 mg, 0.46 mmol), K2CO3 (193 mg, 1.4 mmol), anhydrous DMF (2.5 mL) and phenol (66 mg, 0.70 mmol). The resulting mixture was heated at 175 °C for 50 minutes under microwave irradiation. After cooling to room temperature, the reaction was diluted with EtOAc and water. The aqueous phase was extracted with EtOAc (3x). The combined organic extracts were washed with brine and dried (Na2SO4), concentrated under reduced pressure. The residue was purified by column chromatography with ISCO system to provide the title compound 4a (88 mg, 75%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 13.83 (s, 1H), 8.94 (s, 1H), 8.56 (s, 1H), 7.52 – 7.45 (m, 2H), 7.35 – 7.28 (m, 3H); 13C NMR (101 MHz, DMSO-d6) δ 156.2, 153.0, 152.1, 143.3, 134.3, 130.3, 128.3, 126.4, 122.5, 115.1; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C12H9N4O3, 257.0675; found 257.0659.
7-Nitro-4-propoxy-5H-pyrrolo[3,2-d]pyrimidine (4f)
To a suspension of NaH (23.1 mg, 60% in mineral oil, 0.57 mmol) in anhydrous THF (2.0 mL) was added propanol (57.6 mg, 0.96 mmol) at 0 °C in a 10 mL microwave tube. After stirred for 30 min at 0 °C, 1 (95 mg, 0.48 mmol) was added and the resulting mixture was heated at 120 °C for 20 minutes under microwave irradiation. After cooling to room temperature, the reaction was diluted with EtOAc and water. The aqueous phase was extracted with EtOAc (3x). The combined organic extracts were dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by column chromatography with ISCO system to provide the title compound 4f (77 mg, 72%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 13.49 (s, 1H), 8.76 (s, 1H), 8.61 (s, 1H), 4.49 (t, J = 6.6 Hz, 2H), 1.86 – 1.76 (m, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ 156.6, 153.3, 142.2, 133.2, 128.2, 115.0, 68.4, 22.2, 10.7; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C9H11N4O3, 223.0831; found 223.0810.
N-Butyl-7-nitro-5H-pyrrolo[3,2-d]pyrimidin-4-amine (4g) (General procedure B)
To a solution of 1 (200 mg, 1.01 mmol) in anhydrous i-PrOH (15 mL) was added butylamine (89 mg, 1.21 mmol). The resulting mixture was heated at 80 °C for overnight under nitrogen atmosphere. After cooling to room temperature, the reaction was diluted with EtOAc and water. The aqueous phase was extracted with EtOAc (3x). The combined organic extracts were and washed with brine and dried (Na2SO4), concentrated under reduced pressure. The residue was purified by column chromatography with ISCO system to provide the title compound 4g (215 mg, 92%) as a yellow solid. 1H NMR (400 MHz, CDCl3+CD3OD) δ 8.21 (s, 1H), 8.05 (s, 1H), 3.55 (t, J = 7.1 Hz, 2H), 1.64 – 1.56 (m, 2H), 1.42 – 1.33 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3+CD3OD) δ 151.8, 147.4, 129.9, 127.3, 125.2, 112.8, 41.1, 30.6, 19.6, 12.6; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C10H14N5O2, 236.1147; found 236.1128.
7-Nitro-4-phenyl-5H-pyrrolo[3,2-d]pyrimidine (4j) (General procedure C)
A 10 mL microwave tube was charged with 1 (150 mg, 0.76 mmol), K2CO3 (262 mg, 1.90 mmol), phenylboronic acid (139 mg, 1.14 mmol), Pd(PPh3)4 (44 mg, 0.038 mmol), DMF (2.0 mL) and H2O (1.0 mL). The resulting mixture was stirred at room temperature for 3.0 min and then heated at 150 °C for 15 min. After cooling to room temperature, the mixture was partitioned in H2O and Et2O. The aqueous phase was extracted with Et2O (3x). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by column chromatography with ISCO system to provide the title compound 4j (159 mg, 87%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.14 (s, 1H), 9.00 (s, 1H), 8.05 – 8.01 (m, 2H), 7.64 – 7.60 (m, 3H); 13C NMR (101 MHz, DMSO-d6) δ 153.7, 151.0, 142.8, 136.8, 135.0, 131.4, 129.5, 129.4, 129.2, 127.7; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C12H9N4O2, 241.0726; found 241.0719.
4-Benzyl-7-nitro-5H-pyrrolo[3,2-d]pyrimidine (4o) (General procedure D)
To a solution of 1 (96 mg, 0.48 mmol) and Pd(PPh3)4 (112 mg, 0.097 mmol) in anhydrous toluene (12 mL) was added a 0.5 M solution of benzylzinc (II) bromide in THF (2.0 mL) at room temperature. The resulting mixture was heated at 110 °C for 12 h and quenched by water (4.0 mL). The solvent was removed under the reduced pressure. The residue was dissolved in water and extracted with EtOAc (3x). The combined organic layers were dried (Na2SO4), and concentrated under reduced pressure. The residue was purified by column chromatography with ISCO system to give the title compound 4o (78 mg, 64%) as a yellow solid. 1H NMR (400 MHz, CD3OD+CDCl3) δ 9.00 (s, 1H), 8.43 (s, 1H), 7.25 – 7.11 (m, 5H), 4.35 (s, 2H); 13C NMR (101 MHz, CD3OD+CDCl3) δ 154.5, 153.4, 141.3, 136.1, 133.8, 128.7, 128.7, 127.1, 125.7, 39.2; LC-MS (ESI+): tR = 4.558 min, m/z 255.10 [M+1]+; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C13H11N4O2, 255.0882; found 255.0879.
tert-Butyl (4-([1,1'-biphenyl]-4-yloxy)-5H-pyrrolo[3,2-d]pyrimidin-7-yl)(ethyl)carbamate (7)
A mixture of 3 (46 mg, 0.10 mmol) and Pd/C (4.6 mg, 10 wt% Pd/C) in MeOH (5.0 mL) was added a solution of acetaldehyde (4.4 mg, 0.10 mmol) in MeOH (0.5 mL) drop-wisely under hydrogen atmosphere. After 4.0 h, the resulting mixture was filtered over a celite pad and washed with MeOH. After removal of MeOH solvent, the crude product was dissolved in 2.0 mL CH2Cl2. To this solution was added Et3N (20 mg, 0.20 mmol) and Boc2O (65 mg, 0.3 mmol). The reaction was stirred 6.0 h at room temperature and quenched with a saturated aqueous NH4Cl solution. After extraction with CH2Cl2 (3x), the combined organic layers were concentrated. The resulting residue was purified by a short silica column to provide 6 (50 mg) which was used directly for the next step. To a solution of 6 (50 mg, 0.088 mmol) in THF (0.5 mL) was added a solution of TBAF (0.22 mL, 1.0 M in THF, 0.22 mmol) and ethylenediamine (6.6 mg, 0.11 mmol) at room temperature. After refluxing for 1.0 h, the reaction mixture was diluted with EtOAc and washed with water. The combined organic layers are dried (Na2SO4) and concentrated. The residue was purified by column chromatography with ISCO system to provide the title compound 7 (25 mg, 58% over 3 steps) as a light yellow solid. 1H NMR (400 MHz, CD3OD) δ 8.50 (bs, 1H), 7.86 (bs, 1H), 7.77 – 7.70 (m, 2H), 7.68 – 7.62 (m, 2H), 7.49 – 7.43 (m, 2H), 7.42 – 7.33 (m, 3H), 3.74 (q, J = 7.1 Hz, 2H), 1.46 (bd, 9H), 1.20 (bs, 3H); LC-MS (ESI+): tR = 5.927 min, m/z 431.2 [M+1]+.
N4-butyl-5H-pyrrolo[3,2-d]pyrimidine-4,7-diamine (8)
The solution of 4g (85 mg, 0.36 mmol) and 10% palladium on actived carbon (53.9 mg, 0.30 mmol) in MeOH (15.0 mL) was stirred under hydrogen atmosphere at room temperature for 3.0 h. Then the reaction mixture was filtered though a pad of celite to afford the title compound 8 (68 mg, 92%) as a brown oil. 1H NMR (400 MHz, CD3OD) δ 8.20 (s, 1H), 7.03 (s, 1H), 3.58 (t, J = 7.1 Hz, 2H), 1.75 – 1.60 (m, 3H), 1.56 – 1.43 (m, 2H), 0.99 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 150.0, 147.5, 135.2, 121.6, 114.9, 112.6, 40.1, 31.2, 19.7, 12.7; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C10H16N5, 206.1406; found 206.1396.
N-butyl-7-(piperazin-1-yl)-5H-pyrrolo[3,2-d]pyrimidin-4-amine (9)
Bis(2-chloroethyl)amine (95.6 mg, 0.54 mmol) was added into the solution of 8 (110.1 mg, 0.54 mmol) in i-PrOH (10.0 mL) at room temperature. Then Na2CO3 (114.5 mg, 1.1 mmol) was added, the resulting reaction mixture was stirred for overnight at reflux. Then water (20 mL) was added and extracted with dichloromethane (3 X). The combined organic layers were dried over Na2SO4, filtered and condensed. The residue was purified by column chromatography with ISCO system to provide the title compound 9 (118.9 mg, 81%) as red oil. 1H NMR (400 MHz, CD3OD) δ 8.13 (s, 1H), 6.98 (s, 1H), 3.52 (t, J = 7.1 Hz, 2H), 3.17 – 3.08 (m, 4H), 3.09 – 2.99 (m, 4H), 1.72 – 1.62 (m, 2H), 1.51 – 1.42 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CD3OD) δ 150.0, 148.4, 137.9, 130.8, 113.8, 113.5, 51.7, 44.7, 40.0, 31.2, 19.8, 12.7; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C14H23N6, 275.1984; found 275.1978.
N-(4-(Butylamino)-5H-pyrrolo[3,2-d]pyrimidin-7-yl)benzamide (10a): (General procedure E)
To the solution of 8 (0.30 mmol, 1.0 eq), DIEA (0.45 mmol, 1.5 eq) and dichloromethane (3.0 mL) was added benzoyl chloride (13 mg, 0.090 mmol) at room temperature. After stirred for 8.0 h at room temperature, the reaction was quenched with a sat. NaHCO3 solution (5.0 mL) and extracted with dichloromethane (3x). The combined organic phases were dried (Na2SO4), filtered and concentrated under the reduced pressure. The residue was purified through prep-HPLC to provide the title compound 10a (10 mg, 39%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 12.78 (s, 1H), 10.45 (s, 1H), 8.85 (d, J = 5.5 Hz, 1H), 8.23 (s, 1H), 8.02 – 7.92 (m, 2H), 7.81 (d, J = 2.9 Hz, 1H), 7.49 (t, J = 7.4 Hz, 1H), 7.38 (t, J = 7.7 Hz, 2H), 3.64 (dd, J = 13.0, 7.2 Hz, 2H), 1.69 – 1.62 (m, 2H), 1.40 (dq, J = 14.6, 7.3 Hz, 2H), 0.93 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.5, 151.6, 142.9, 132.4, 132.3, 128.6, 127.7, 124.3, 120.9, 112.7, 112.5, 41.5, 30.9, 19.9, 13.6; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C17H20N5O, 310.1668; found 310.1664.
4-([1,1'-Biphenyl]-4-yloxy)-N-ethyl-5-(3-morpholinopropyl)-5H-pyrrolo[3,2-d]pyrimidin-7-amine (11a) (General procedure F)
A mixture of 7 (65 mg, 0.15 mmol), K2CO3 (62 mg, 0.45 mmol), 4-(3-chloropropyl)morpholine (40 mg, 0.23 mmol), and DMF (2 mL) in a microwave tube was heated under microwave irradiation at 150 oC for 10 min. After cool to room temperature, the mixture was washed with brine and extracted with EtOAc (3x). The combined organic layers was dried (over Na2SO4) and concentrated. The residue was purified by column chromatography with ISCO system to provide the desired intermediate. This intermediate was dissolved in a mixture of 2.0 mL of CH2Cl2 and 0.5 mL of TFA. After stirring at room temperature for 2.0 h, the solution was concentrated and purified through prep-HPLC to provide the title compound 11a (64 mg, 93%) as a yellow oil. 1H NMR (400 MHz, CD3OD) δ 8.43 (s, 1H), 8.08 (s, 1H), 7.77–7.70 (m, 2H), 7.67–7.60 (m, 2H), 7.48–7.32 (m, 5H), 4.67 (t, J = 7.0 Hz, 2H), 3.97 (bs, 2H), 3.76 (bs, 2H), 3.65 (q, J = 7.3 Hz, 2H), 3.48 (bs, 2H), 3.30–3.24 (m, 2H), 3.11 (bs, 2H), 2.54–2.39 (m, 2H), 1.40 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CD3OD) δ 157.0, 152.6, 151.2, 145.23, 141.4, 140.6, 130.0, 129.4, 128.6, 128.0, 123.5, 116.6, 116.0, 113.6, 64.9, 55.4, 53.1, 47.9, 47.1, 27.1, 11.7; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C27H32N5O2, 458.2556; found 458.2536.
tert-Butyl ((1r,4r)-4-((4-(benzyl(ethyl)amino)-7-nitro-5H-pyrrolo[3,2-d]pyrimidin-5-yl)methyl)cyclohexyl)carbamate (12)
A 10 mL microwave tube was charged with 4h (297 mg, 1.0 mmol), K2CO3 (345 mg, 2.5 mmol), DMF (2.0 mL), and trans-tert-butyl (4-(bromomethyl)cyclohexyl)carbamate (436 mg, 1.5 mmol). The resulting mixture was heated at 150 °C for 45 minutes under microwave irradiation. After cooling to room temperature, the reaction was diluted with EtOAc and washed with brine. The aqueous phase was extracted with EtOAc (3x). The combined organic extracts were dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by column chromatography with ISCO system to provide the title compound 12 (361 mg, 71%) as a white solid. 1H NMR (400 MHz, CD3OD) δ 8.50 (s, 1H), 7.44 – 7.22 (m, 6H), 4.61 (d, J = 6.9 Hz, 2H), 3.34 (s, 5H), 1.91 (d, J = 11.9 Hz, 2H), 1.69 – 1.60 (m, 3H), 1.41 (s, 9H), 1.34 – 1.29 (m, 3H), 1.20 – 1.08 (m, 4H); 13C NMR (101 MHz, CD3OD) δ 156.3, 151.9, 147.3, 138.8, 136.3, 128.5, 128.2, 127.5, 127.4, 125.8, 110.0, 78.6, 59.5, 52.1, 49.3, 48.7, 44.5, 37.1, 31.8, 28.1, 27.6; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C27H37N6O4, 509.2876; found 509.2869.
tert-Butyl ((1r,4r)-4-((7-amino-4-(benzyl(ethyl)amino)-5H-pyrrolo[3,2-d]pyrimidin-5-yl)methyl)cyclohexyl)carbamate (13)
To a suspension of 10% palladium on active carbon (13 mg, 0.013 mmol) in methanol (8.0 mL) was added 12 (125 mg, 0.25 mmol) at room temperature. The resulting mixture was stirred under hydrogen atmosphere for 3.0 h. Then the mixture was diluted with EtOAc and filtered through a pad of celite and condensed. The residue was purified by column chromatography with ISCO system to provide the title compound 13 (100 mg, 84%) as a green oil. 1H NMR (400 MHz, CD3OD) δ 8.35 (s, 1H), 7.41 (d, J = 4.6 Hz, 1H), 7.37 – 7.26 (m, 5H), 5.17 (s, 2H), 4.44 (d, J = 7.3 Hz, 2H), 3.93 – 3.84 (m, 2H), 3.34 (s, 3H), 1.93 (d, J = 10.7 Hz, 2H), 1.69 (d, J = 11.9 Hz, 2H), 1.42 (s, 9H), 1.32 (t, J = 7.2 Hz, 3H), 1.25 – 1.13 (m, 4H); 13C NMR (101 MHz, CD3OD) δ 156.4, 150.3, 145.7, 136.1, 128.8, 128.5, 127.5, 127.2, 123.9, 118.8, 111.1, 78.5, 56.2, 52.0, 49.4, 48.4, 44.5, 39.5, 37.8, 31.7, 28.2, 27.4, 12.4; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C27H39N6O2, 479.3134; found 479.3128.
tert-Butyl ((1r,4r)-4-((4-(ethyl(phenethyl)amino)-7-(4-methoxybenzamido)-5H-pyrrolo[3,2-d]pyrimidin-5-yl)methyl)cyclohexyl)carbamate (14)
To a solution of 13 (80 mg, 0.17 mmol), triethyl amine (25.3 mg, 0.25 mmol), catalytic amount of DMAP in anhydrous THF (3.0 mL) was added 4-methoxybenzoyl chloride (28.4 mg, 0.17 mmol) at room temperature. The resulting mixture was stirred for 5.0 h and quenched with water and diluted with EtOAc (20 mL). The aqueous phase was extracted with EtOAc (3x). The combined organic phase was dried (Na2SO4), filtered and condensed. The residue was purified by column chromatography with ISCO system to give the title compound 14 (53 mg, 51% yield) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 7.8 Hz, 2H), 7.65 (s, 1H), 7.56 (s, 1H), 7.35 – 7.19 (m, 5H), 6.94 (d, J = 8.7 Hz, 2H), 5.29 (s, 2H), 5.13 – 5.00 (m, 1H), 4.44 (s, 1H), 3.96 (d, J = 6.9 Hz, 2H), 3.86 (s, 3H), 3.37 – 3.25 (m, 1H), 1.94 – 1.81 (m, 3H), 1.51 – 1.45 (m, 2H), 1.41 (s, 9H), 1.35 – 1.18 (m, 3H), 0.96 (t, J = 9.7 Hz, 4H); 13C NMR (101 MHz, CD3OD) δ 169.1, 163.0, 156.4, 151.3, 142.8, 137.9, 131.0, 129.2, 128.1, 127.3, 127.0, 125.6, 113.5, 105.7, 78.5, 56.3, 54.6, 36.7, 31.6, 28.6, 27.3; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C35H45N6O4, 613.3502; found 613.3498.
N-(5-(((1r,4r)-4-Aminocyclohexyl)methyl)-4-(ethylamino)-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-4-methoxybenzamide (15)
To a solution of compound 14 (46 mg, 0.075 mmol) in methanol (5.0 mL) was added 10% palladium on activated carbon (5 mg, 0.0047 mmol) at room temperature. The resulting mixture was added 3 drops of acetic acid and was then heat to 60 °C under H2 atmosphere for 6.0 h. The reaction mixture was then diluted with EtOAc (20 mL) and filtered though a pad of celite. The solvent was removed and the residue was purified by column chromatography with ISCO system to give the debenzylated intermediate (22 mg, 56%) as a white solid. 1H NMR (400 MHz, CD3OD) δ 8.47 (s, 1H), 8.02 (d, J = 8.9 Hz, 2H), 7.82 (s, 1H), 7.09 (d, J = 8.9 Hz, 2H), 4.28 (d, J = 7.2 Hz, 2H), 3.89 (s, 3H), 3.80 (q, J = 7.2 Hz, 2H), 2.97 (s, 1H), 1.79-1.76 (m, 4H), 1.50 (s, 2H), 1.37 (t, J = 7.3 Hz, 3H), 1.24 – 1.07 (m, 5H); 13C NMR (101 MHz, CD3OD) δ 169.1, 163.5, 150.7, 148.1, 129.8, 129.3, 124.6, 113.7, 112.8, 109.1, 56.6, 54.7, 37.1, 36.1, 31.5, 28.4, 27.3, 13.0. To a solution of the debenzylated intermediate (15 mg, 0.029 mmol) in dichloromethane (5.0 mL) was added trifluoroacetic acid (32.7 mg, 0.29 mmol) at room temperature. The resulting mixture was heated at 60°C for 4.0 h. Then the reaction was diluted with dichloromethane (22.0 mL) and washed with NaHCO3 (sat.), water and brine sequentially. The organic phase was dried (Na2SO4), filtered and condensed. The residue was purified through preparative HPLC to give the title compound 15 (9.2 mg, 75% yield) as a clear oil. 1H NMR (400 MHz, CD3OD) δ 8.47 (s, 1H), 8.02 (d, J = 8.9 Hz, 2H), 7.82 (s, 1H), 7.09 (d, J = 8.9 Hz, 2H), 4.28 (d, J = 7.2 Hz, 2H), 3.89 (s, 3H), 3.80 (q, J = 7.2 Hz, 2H), 2.97 (s, 1H), 1.95-1.75 (m, 4H), 1.50 (s, 2H), 1.53-1.46 (m, 3H), 1.24 – 1.07 (m, 5H); 13C NMR (101 MHz, CD3OD) δ 168.9, 163.5, 150.8, 148.0, 130.1, 129.8, 129.3, 124.5, 113.8, 112.8, 108.9, 56.1, 54.7, 49.5, 36.5, 36.1, 29.3, 27.4, 13.0; HRMS (TOF, ESI+) m/z: [M+H]+ calculated for C23H31N6O2, 423.2508; found 423.2518.
Selectivity Profiling
The selected compounds were screened against 48 kinases using the Profiler Pro Kit (Caliper Life Sciences). Briefly, pre-plated enzyme stocks were reconstituted with 15 µL of supplied Reconstitution Buffer containing DTT and Protease Inhibitor Cocktail. 1 µL of compound at a concentration of 260 µM in 100% DMSO was transferred to the plate and the reaction was initiated by the addition of 10 µL substrate solution containing ATP and cofactors specific for each enzyme. The reaction was incubated at room temperature for 90 min and 45 µL of termination buffer was then added to stop the reaction. The plates were read in a Caliper EZ Reader.
Microfluidic Capillary Electrophoresis (MCE) Assay
The activity assays were performed in a 384 well, polypropylene microtiterplate, using 0.3 nM FLT-3 (Life Technologies cat# PHC9415), in a final volume of 50 uL of 50 mM Hepes pH 7.4 containing 10 mM MgCl2 , 1mM DTT, 0.01% Triton X-100, 0.1% Bovine Serum Albumin (BSA), 1uM fluorescent peptide substrate (5-FAM-KKKKEEIYFFF-CONH2) and 275 µM ATP (at Km for Flt3). All reactions were terminated following a 60-minute incubation, by addition of 20 uL of 70 mM EDTA. Phosphorylated and unphosphorylated substrate peptides were separated on a LabChip EZ Reader equipped with a 12-sipper chip in separation buffer supplemented with CR-8 and analyzed using EZ Reader software.
Supplementary Material
ACKNOWLEDGMENT
We thank Professor Stephen Frye for comments on manuscript and Professor K. H. Lee’s lab for HRMS support.
Funding Sources
This work was supported by the University Cancer Research Fund, School of Pharmacy, and Federal Funds from the National Cancer institute, National Institute of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental procedures, characterization of all compounds and biological methods, and copies of 1H and 13C spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
X. Wang, W. Zhang and J. Liu conceived and designed the experiments, W. Zhang and J. Liu performed the experiments, X. Wang, W. Zhang and J. Liu wrote the manuscript and Supporting Information.
REFERENCES
- 1.Imamura S, Oguro Y. Preparation of fused heterocyclic derivatives, particularly pyrrolo[3,2-d]pyrimidines and pyrrolo[3,2-b]pyridines, and their use for treating cancer. WO2007004749A1. 2007
- 2.Oguro Y, Miyamoto N, Takagi T, Okada K, Awazu Y, Miki H, Hori A, Kamiyama K, Imamura S. N-Phenyl-N′-[4-(5H-pyrrolo[3,2-d]pyrimidin-4-yloxy)phenyl]ureas as novel inhibitors of VEGFR and FGFR kinases. Bioorganic & Medicinal Chemistry. 2010;18(20):7150–7163. doi: 10.1016/j.bmc.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Saavedra OM, Claridge SW, Zhan L, Raeppel F, Vaisburg A, Raeppel S, Deziel R, Mannion M, Zhou NZ, Isakovic L. Thienopyridine and thienopyrimidine derivatives and their preparation, pharmaceutical compositions, and use as inhibitors of VEGF receptor and HGF receptor signaling for treatment of proliferative diseases. WO2007054831A2. 2007
- 4.Blake JF, Kallan NC, Xiao D, Xu R, Bencsik JR, Skelton NJ, Spencer KL, Mitchell IS, Woessner RD, Gloor SL, Risom T, Gross SD, Martinson M, Morales TH, Vigers GPA, Brandhuber BJ. Discovery of pyrrolopyrimidine inhibitors of Akt. Bioorg. Med. Chem. Lett. 2010;20:5607–5612. doi: 10.1016/j.bmcl.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 5.Bluhm H, Hochguertel M, Kroth H, Essers M, Gege C, Richter F, Taveras A. Heterobicyclic matrix metalloprotease inhibitors, their preparation, pharmaceutical compositions, and use in therapy. WO2008063669A1. 2008
- 6.Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Chang S-P, Doty JL, Elliott EA, Fisher MB, Hines M, Kent C, Kudlacz EM, Lillie BM, Magnuson KS, McCurdy SP, Munchhof MJ, Perry BD, Sawyer PS, Strelevitz TJ, Subramanyam C, Sun J, Whipple DA, Changelian PS. Discovery of CP-690,550: A Potent and Selective Janus Kinase (JAK) Inhibitor for the Treatment of Autoimmune Diseases and Organ Transplant Rejection. J. Med. Chem. 2010;53:8468–8484. doi: 10.1021/jm1004286. [DOI] [PubMed] [Google Scholar]
- 7.Calderwood DJ, Johnston DN, Munschauer R, Rafferty P. Pyrrolo[2,3-d]pyrimidines containing diverse N-7 substituents as potent inhibitors of Lck. Bioorg Med Chem Lett. 2002;12(12):1683–1686. doi: 10.1016/s0960-894x(02)00195-6. [DOI] [PubMed] [Google Scholar]
- 8.Evans GB, Furneaux RH, Lenz DH, Painter GF, Schramm VL, Singh V, Tyler PC. Second Generation Transition State Analogue Inhibitors of Human 5'-Methylthioadenosine Phosphorylase. J. Med. Chem. 2005;48:4679–4689. doi: 10.1021/jm050269z. [DOI] [PubMed] [Google Scholar]
- 9.Morris PE, Jr, Elliott AJ, Walton SP, Williams CH, Montgomery JA. Synthesis and biological activity of a novel class of purine nucleoside phosphorylase inhibitors. Nucleosides, Nucleotides Nucleic Acids. 2000;19:379–404. doi: 10.1080/15257770008033016. [DOI] [PubMed] [Google Scholar]
- 10.Girardet JL, Koh Y-H, Shaw S, Kin HW. Preparation of purines, azapurines, and deazapurines as non-nucleoside reverse transcriptase inhibitors for treatment of HIV infection. WO2006122003A2. 2006
- 11.(a) Zhao L, Tao K, Li H, Zhang J. Practical one-pot protocol for the syntheses of 2-chloro-pyrrolo[3,2-d]pyrimidines. Tetrahedron. 2011;67:2803–2806. [Google Scholar]; (b) Norman MH, Chen N, Chen Z, Fotsch C, Hale C, Han N, Hurt R, Jenkins T, Kincaid J, Liu L, Lu Y, Moreno O, Santora VJ, Sonnenberg JD, Karbon W. Structure-Activity Relationships of a Series of Pyrrolo[3,2-d]pyrimidine Derivatives and Related Compounds as Neuropeptide Y5 Receptor Antagonists. J. Med. Chem. 2000;43:4288–4312. doi: 10.1021/jm000269t. [DOI] [PubMed] [Google Scholar]
- 12.(a) Cherng Y-J. Synthesis of substituted pyridines by the reactions of halopyridines with sulfur, oxygen and carbon nucleophiles under focused microwave irradiation. Tetrahedron. 2002;58(24):4931–4935. [Google Scholar]; (b) Luo G, Chen L, Poindexter GS. Microwave-assisted synthesis of aminopyrimidines. Tetrahedron Letters. 2002;43(33):5739–5742. [Google Scholar]
- 13.Miyaura N, Yamada K, Suzuki A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979:3437–3440. [Google Scholar]
- 14.King AO, Okukado N, Negishi E. Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the palladium-catalyzed reaction of alkynylzinc reagents with alkenyl halides. J. Chem. Soc.. Chem. Commun. 1977:683–684. [Google Scholar]
- 15.Bambuch V, Otmar M, Pohl R, Masojidkova M, Holy A. C-Functionalization of 9-deazapurines by cross-coupling reactions. Tetrahedron. 2007;63:1589–1601. [Google Scholar]
- 16.Wiernik PH. FLT3 inhibitors for the treatment of acute myeloid leukemia. Clinical advances in hematology & oncology : H&O. 2010;8(6):429–436. 444. [PubMed] [Google Scholar]
- 17.(a) Pommereau A, Pap E, Kannt A. Two simple and generic antibody-independent kinase assays: comparison of a bioluminescent and a microfluidic assay format. Journal of biomolecular screening. 2004;9(5):409–416. doi: 10.1177/1087057104264175. [DOI] [PubMed] [Google Scholar]; (b) Dunne J, Reardon H, Trinh V, Li E, Farinas J. Comparison of on-chip and off-chip microfluidic kinase assay formats. Assay Drug Dev Technol. 2004;2(2):121–129. doi: 10.1089/154065804323056468. [DOI] [PubMed] [Google Scholar]; (c) Bernasconi P, Chen M, Galasinski S, Popa-Burke I, Bobasheva A, Coudurier L, Birkos S, Hallam R, Janzen WP. A chemogenomic analysis of the human proteome: application to enzyme families. Journal of biomolecular screening. 2007;12(7):972–982. doi: 10.1177/1087057107306759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.