Abstract
MR-guided focused ultrasound surgery (MRgFUS) is a quickly developing technology with potential applications across a spectrum of indications traditionally within the domain of radiation oncology. Especially for applications where focal treatment is the preferred technique (for example, radiosurgery), MRgFUS has the potential to be a disruptive technology that could shift traditional patterns of care. While currently cleared in the United States for the noninvasive treatment of uterine fibroids and bone metastases, a wide range of clinical trials are currently underway, and the number of publications describing advances in MRgFUS is increasing. However, for MRgFUS to make the transition from a research curiosity to a clinical standard of care, a variety of challenges, technical, financial, clinical, and practical, must be overcome. This installment of the Vision 20/20 series examines the current status of MRgFUS, focusing on the hurdles the technology faces before it can cross over from a research technique to a standard fixture in the clinic. It then reviews current and near-term technical developments which may overcome these hurdles and allow MRgFUS to break through into clinical practice.
Keywords: high-intensity ultrasound, focused ultrasound surgery, HIFU, minimally invasive therapy, thermal therapy, neuromodulation, ablative therapy
INTRODUCTION
Image-guided focused ultrasound surgery (FUS) is a quickly developing technology that uses high-intensity focused ultrasound (HIFU) along with MR image-guidance (MRgFUS;1, 2) or diagnostic ultrasound image guidance (USgFUS;3, 4, 5) for therapeutic purposes. Image-guided focused ultrasound surgery (also sometimes referred to as HIFU-therapy or HIFU-surgery) has potential applications across a wide range of indications that span benign and malignant tumors, pain, vascular problems, and others.
FUS has the potential to be a disruptive technology in the context of certain subspecialties within radiation oncology, particularly for focal techniques such as stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) which have similar focal treatment and minimally invasive characteristics. However in other settings, FUS also has the potential to be an effective approach alongside traditional Radiation Oncology techniques.
In the United States, MRgFUS has been deployed clinically for the noninvasive treatment of uterine fibroids and bone metastases. Clinical trials are currently underway for a variety of other applications both within and outside the realm of oncology. In most of these cases the intent of the therapy is to locally destroy targets using heat; the system is capable of conformally heating targets to ablation temperatures while largely sparing normal tissue. However, research is also focusing on uses of MRgFUS as an adjuvant therapy to traditional radiation or chemotherapy,6, 7 and for the targeted delivery of therapeutic agents8 that can be used by themselves or in tandem with radiation.
Recent symposiums and workshops and an increase in the number of focused ultrasound surgery publications demonstrate that the technology is viable and is likely to see significant development. MR-guided focused ultrasound surgery was also recently named by Time Magazine as one of the 50 most important inventions9 (the second time MRgFUS has appeared in Time magazine10).
This edition of the Vision 20/20 series examines the current state of MRgFUS and some likely paths for its future development. This paper is divided into two main parts: Part I reviews the basic physics and biology of MR-guided focused ultrasound as well as indications currently cleared in the United Stated; Part II describes the challenges that confront MRgFUS as it evolves toward a more widely available clinical tool, along with a review of current developments that should overcome these challenges in the near future.
Part I. Physics and Biology
MR-GUIDED FOCUSED ULTRASOUND—BASIC PRINCIPLES AND CURRENT CLINICAL STATUS
Technology overview
Basic components
Current focused ultrasound surgery systems are roughly classified into two types based on the technique used for image guidance. Ultrasound-guidance combines the therapeutic high-intensity ultrasound with diagnostic ultrasound imaging for target localization and postprocedure verification. MR-guidance allows for both target localization and in vivo real-time monitoring of temperature through a technique called MR thermometry,11 as well as postprocedure imaging (typically with contrast-enhancement) to verify tissue destruction.
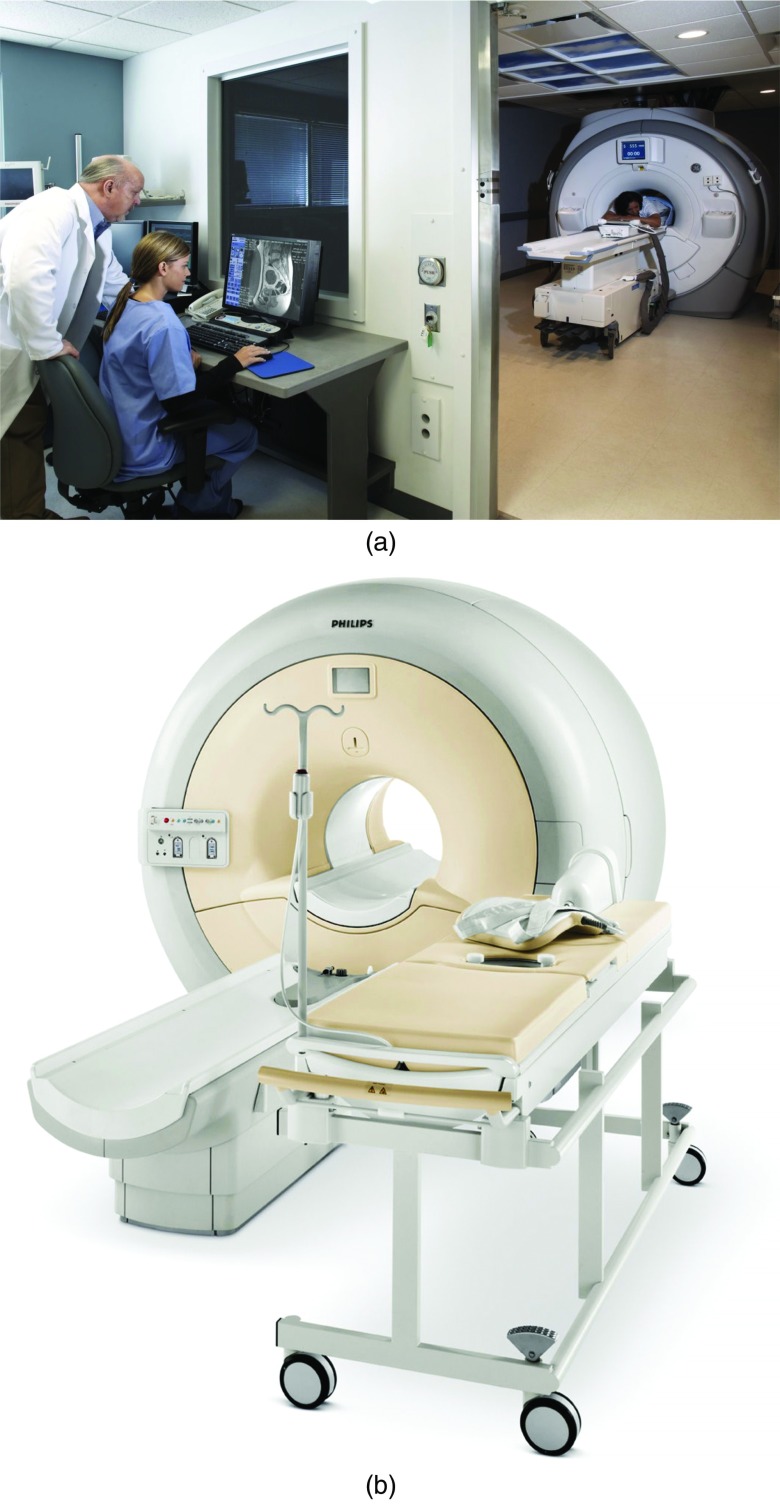
Figures 1a, 1b illustrate two of the MR-guided systems currently on the market or in late-stage development. Table 1 describes the components of a typical FUS system.
Figure 1.
Examples of currently available commercial MR-guided focused ultrasound systems. (a) Insightec OR MR-guided system (image courtesy of InSightec Ltd.). (b) Philips Sonalleve MR-guided system (image courtesy of Philips Medical Systems).
Table 1.
Major components of a clinical high-intensity focused ultrasound surgery system.
| Component | Purpose |
|---|---|
| Multielement phased array ultrasound transducer | Generate focused, high-intensity ultrasound beams. Usually electronically steerable. |
| Water bath | Creates an acoustic window between transducer and tissue, assists in surface cooling |
| MR (MRgFUS) or diagnostic ultrasound (USgFUS) unit | Target localization and tracking / real-time thermometry |
| Customized MR table (for MRgFUS) | Allows the ultrasound transducer and water bath to fit in table and interface with MR unit |
| Accessory immobilization devices (body molds, immobilization masks, stereotactic frames, etc.) | Target immobilization |
| Motion tracking and gating devices | Assists with targeting of moving targets |
| Treatment planning and delivery software | Allows imaging and treatment planning to be integrated, used to predict treatment effects |
The current commercially available MR-guided FUS systems use multielement phased array ultrasound transducers. These transducers are composed of a number of individual piezoelectric ultrasound elements. In most cases the transducers are constructed in a concave design to provide them with a natural focus. Beam steering and focusing is usually accomplished through a combination of mechanical translation and tilt (for gross positioning of the focal spot), and electronic steering (for fine control of the focal spot location). The degree to which electronic control of the transducers can adjust focal spot position and shape depends in part on the number of transducer elements and the overall geometry of the transducers.12
In most cases, for MR-guided FUS systems the transducer is built into the MR treatment couch, however intracorporal transducers13 and relocatable, strap-on transducers have also been developed and can be used for locations where fixed transducers in the treatment table cannot reach the intended treatment site.
Basic physics of MR-guided focused ultrasound
An ultrasound beam is composed of longitudinal mechanical waves with frequencies above the range of human auditory perception (i.e., greater than ∼20 kHz). As the waves traverse the medium, they apply pressure to the particles (atoms or molecules) in the medium, causing them to oscillate back and forth. While the particles move only a tiny distance, the collective motion of the particles creates wave fronts of compression and rarefraction which transmit the ultrasound energy.14
Acoustic impedance, reflection, and acoustic windows
As ultrasound passes through hetereogenous media such as exists in the body, the beam encounters tissue types with varying physical and acoustic properties. At each of these interfaces, a proportion of the ultrasound energy is reflected back from the interface, and the remaining energy is transmitted. For diagnostic applications, it is the detection of the reflections which forms the basis of image creation. For therapeutic applications such as FUS, the goal is to minimize reflection and transmit sufficient energy to the targeted tissue to cause a desired biological effect.
Every medium has a characteristic property called the acoustic impedance (Z) (units g cm−2 s−1), which is the product of the physical density and the speed of sound in the medium:
Maximum transmission through heterogeneous tissue interfaces occurs when the acoustic impedance of the tissue types on either side of the interface is equal. When the impedances are not equal, the fraction of ultrasound energy which is reflected increases in proportion to the difference between the acoustic impedances of the interfacing tissues. For example, due to large differences in acoustic impedance, ultrasound does not readily propagate from water or tissue into air (or vice versa).15
Thus, a critical component of every HIFU system is to have a robust acoustic window between the transducer and the target. A combination of materials including mineral oil, gel, and degassed water are used to create an interface between the transducer and the skin of the patient. Any air-filled organs (such as bowel) must be out of the path of the ultrasound beam. Bubbles, whether in the transducer-patient interface or within the patient's tissue can cause reflection and scattering of the ultrasound beam. Setup procedures for each treatment generally include instructions for removing bubbles at the transducer-patient interface, and treatment preparations can include techniques such as bladder and rectal filling to help move air-filled organs such as intestine out of the beam path.
Frequency vs depth vs sharpness
With HIFU, for a given transducer there is a direct tradeoff between the frequency of the ultrasound waves, the penetration depth, and the sharpness of the lesion. Ultrasound intensity for a plane-wave beam propagating in an absorbing medium attenuates (scatters and absorbs) exponentially
| (1) |
where I is the intensity at any point x and I0 is the intensity at the origin. The intensity attenuation coefficient, μ, describes the attenuation per unit length. μ is a power-law function of frequency,
| (2) |
where a and b are constants specific to the medium, and f is the frequency.16 This relationship means that increasing the ultrasound frequency increases the absorption, i.e., more heating occurs per unit length, and also decreases the possible depth of penetration. The intensity at the focus is reduced due to prefocal absorption. Likewise, a decrease in frequency increases the penetration of the beam, but makes it more difficult to create a sharply defined thermal focus.16
The attenuation coefficient also changes in tissue which has been coagulated, making it more difficult to ablate tissue which is located downstream of tissue which has already been ablated.17
Thus, the optimal frequency for HIFU varies by treatment and (for a given transducer radius of curvature) is always a balance between penetration depth and the ability to create a focus. It should also be noted that the sharpness of the focus also depends on geometric factors, such as the radius of curvature of the transducer, so there can be a complex set of engineering tradeoffs involved in developing a system.16 For most extracorpeal abdominal and pelvic applications, a frequency close to 1 MHz is employed as a best trade-off between focus and penetration. For intracavitary and interstitial systems that require smaller penetration depths, higher frequencies of between 3 and 12 MHz are often used. Extracorpeal systems use lower frequencies in order to penetrate deep within tissue, and compensate for the difficulty in creating a focus by employing higher power levels.16 For shallow applications such as cosmetic18, 19 and ocular20, 21, 22 indications, frequencies greater than 2 MHz have been employed.
Thermal energy transfer
Transfer of thermal energy to tissue occurs primarily through two mechanisms.15 The first, relaxation absorption, is described by the following relationship:
where fr,n are the various relaxation frequencies present in the tissue. For a homogeneous medium, the relaxation frequency corresponds to a relaxation time τ, which is the time required for elastic forces within the medium to return the medium to its original position after displacement from a pressure wave. If the frequency is such that the wave arrives at the same instant the medium is in its maximum elastic recoil (or relaxation), the amount of energy extracted from the pressure wave is maximized. In general, there is a linear relationship between frequency and the relaxation absorption coefficient.15
The second mechanism of thermal energy transfer, called classical absorption, is due to friction between particles in the medium, and is proportional to the square of the frequency:
The interparticle friction converts the ultrasound energy into heat. As such, classical absorption is proportional to the viscosity of the medium.15 For thermal applications of FUS, where temperature elevation is the desired endpoint, classical absorption is the primary mechanism for heating.16
Image guidance
Image guidance plays a critical role in focused ultrasound surgery. Imaging is required to identify and localize the therapeutic target and surrounding anatomy, to ensure the patient is correctly positioned with respect to the ultrasound transducer, and to verify that an appropriate acoustic interface exists between the transducer and the target. Real-time image guidance can provide targeting feedback during the treatment itself. For instance in thermal therapies a technique known as thermometry can be used to identify areas of tissue which have reached an appropriate ablation threshold and which areas remain to be treated.
Ultrasound-guided FUS (USgFUS) systems rely on diagnostic ultrasound to provide both anatomical and real-time treatment feedback. Feedback at real-time frame rates is possible by identifying areas of hyperechogenicity in treated tissue.4 However, this method of ultrasound guidance is not optimal for determining absolute temperature changes or for spatial resolution of the treated tissue.23 Ultrasound thermometry is possible with quite high temperature resolution, however the window of temperatures that can be mapped is small, and most methods depend on knowledge of tissue-specific parameters such as attenuation, speed of sound, and changes in backscattered energy with temperature, which are often poorly characterized at ablative temperatures.3
MR-guided systems (MRgFUS) use MR for diagnostic and planning imagery as well as for feedback during procedures in the form of MR-thermometry.
This is possible because a number of MR parameters including T1 and T2 relaxation, proton resonance frequency, and magnetization transfer coefficient have temperature dependencies which can be exploited. In proton-resonance frequency shift (PRF) thermometry, the most commonly used thermometry technique, temperature-dependent phase changes in gradient-recalled echo (GRE) pulse sequences are used to determine the temperature change.24 Images acquired during a sonication are subtracted from baseline images acquired prior to heating.
The change in temperature can be represented as
where Δφ is the phase change, γ is the gyromagnetic ratio, Bo is the main magnetic field, and TE is the time to echo, and α is the PRF change coefficient for aqueous tissue (−0.01 ppm/°C).25
MR-thermometry can provide quantitative temperature measurements over the range of temperatures used in ablative and subablative FUS techniques are generally independent of tissue type for most soft tissues,26 and can be acquired at near real-time framerates with customized pulse sequences.27
PRF-shift thermometry works best when the reference and sonication images are perfectly coregistered. Any misalignment, including that caused by patient shifts, internal organ movement, edema, and changes in tissue due to thermal coagulation, will result in artifacts in the resulting thermal map. This limits the use of PRF thermometry in situations of poor patient immobilization or respiratory organ motion.28, 29 Thermometry measurements within fatty tissue is also degraded25 as the phase dependence of lipids is almost independent of temperature.30 In situations of adipose tissue or mixed tissue types, fat suppression can be used to compensate.30, 31
Physical tissue effects
Current clinical MRgFUS systems are designed mainly with target ablation as an endpoint. However, ultrasound (and high-intensity ultrasound in particular) has several physical effects in tissue, all of which can theoretically be used for treatment advantage:
Thermal effects: ultrasound absorption in tissue causes microscopic-scale frictional heating of tissue generated by shearing caused by the longitudinal compression and rarefraction pressure variations of the ultrasound.16 The amount of heating is predictable and repeatable and can be measured with techniques such as MR-thermometry.32, 33, 34 It should be noted that when used for tissue ablation FUS thermal effects are significantly different from traditional hyperthermia therapies as used in oncologic settings.35 Ablative FUS achieves much higher temperatures in a much shorter amount of time, and in a more localized area of tissue. Thermal effects causing ablation of tissue are currently the primary effects used in FUS.
Mechanical affects: Rarefaction of the ultrasound wave can draw gas out of the surrounding tissue forming microbubbles. These bubbles will oscillate with the ultrasound waves and will grow in resonance to the waves until violently imploding.36, 37 The resulting mechanical action due to implosion shock waves and thermal effects due to broad-spectrum ultrasound emission is much greater than what is achieved with ultrasound waves alone.
Acoustic streaming: Ultrasound waves passing through a fluid can transfer momentum to the fluid.38, 39 This can cause a velocity gradient, which in turn causes shear stress.
Biological tissue effects
The physical effects outlined above can be used alone or in combination to achieve a variety of desirable biological effects. These are summarized below:
Local Ablation: At temperatures above a certain threshold (∼56°C for > 1 s, but varies for different tissue types) coagulative necrosis (ablation) occurs. The boundary between lethal and sublethal effects can be extremely sharp, on the order of a few cell-thicknesses. The area of necrosis is surrounded by a rim of damaged cells that typically die soon after exposure.12 Thus, the effect of FUS on tissue that is thermally ablated is immediate and complete if all cells have been raised to the ablation threshold. In some settings such as tumors, this may be advantageous as compared to ionizing radiation, where the probability of cell death is a stochastic function of dose, and cell death can be temporally delayed because of the dependence on cell-division.40
Thrombolysis: At low-frequency but high-intensity, with temperatures below that required for ablation, acoustic streaming can cause changes in cell membranes and the fibrin mesh that work to increase thrombolysis. This could play a role in the treatment of stroke or other thrombosis.41
Arterial occlusion: At ablation temperatures, thermal coagulation of blood vessels can occur. This could make possible new treatment options for arteriovenous malformations and highly vascular targets.42, 43
Radiosensitization: High-intensity ultrasound at subablation temperatures can generate hyperthermia which causes radiosensitization or chemosensitization of targeted tissue.44, 45 This effect could play an important role in combination FUS/radiation therapies.46
Soft tissue erosion (histotripsy): Short, high-intensity pulses of ultrasound can achieve mechanical erosion of soft tissues, especially at tissue/fluid interfaces. The boundary between damaged and interact tissue can be at a subcellular order of magnitude.47
Sonication in the presence of microbubbles has been shown to alter cell membrane permeability at subablation temperatures.48 Potential applications taking advantage of this effect include drug delivery, selective opening of the blood-brain-barrier (BBB),49 and gene therapy.50
Current clinical status
The modern history of focused ultrasound surgery can be traced back to its first mention by Wood and Loomis in 1927.51 In 1944, Lynn and Putnam proposed using ultrasound to destroy tissue.52 Soon after, the Fry brothers described the creation of focal lesions in the central nervous system with high-intensity ultrasound.53 Experiments soon demonstrated the ability to create lesions in deep-seated tissue in the brain,54 and later the ablation of tumor tissue.55 However, the ongoing development of FUS was inhibited by technical constraints including a lack of effective target visualization technologies, the inability to effectively refocus ultrasound after it has been distorted by tissue interfaces, large power requirements, and limits at the time on transducer design. The relative ease of delivery of focal ionizing radiation diverted efforts toward the development of techniques such as radiosurgery.56
Detailed studies of the acoustical properties of the human skull57 demonstrated that under certain conditions it might be possible to focus ultrasound energy through the skull.58 Methods were later developed that made this idea a practical reality,59 applying phase-compensation techniques similar to older methods developed for diagnostic imaging.60
In parallel with advances in the creation of focal ultrasonic lesions came advances in imaging technology. Parker et al., discovered that temperature variations can be mapped in an NMR image.61 This insight led directly to the idea of using MR as a temperature-feedback device, a technique which is now the cornerstone of MRgFUS.
Beginning in the early 1990s, the developments in ultrasound delivery technology, computer modeling of ultrasound beams, and imaging began to coalesce. In 1992, reports emerged describing MR-guided FUS on ex vivo muscle tissue,1 and the following year on in vivo tissue.2 Several reports using US-guided systems for treating benign prostatic hyperplasia and prostate cancer appeared in the early 1990s.62, 63, 64 By 1995, integrated systems for MRgFUS were being developed,65 and improvements in MR-thermometry were being reported.24 In 2000, large-scale arrays for trans-cranial FUS were developed that allowed the ultrasound energy to be spread out over a wide area, increasing the efficiency of ultrasound delivery to spots deep within the brain.66 Shortly thereafter, time-reversal algorithms that could simulate the effect of and correct for the effect of the skull on the ultrasound focus were reported.67
Parallel to these developments were a series of clinical trials conducted in China in the 2000s for patients with hepatocellular,68 renal,69 bone,70 pancreatic,71 and other malignancies72, 73, 74 using relatively simple US-assisted systems mechanically steered to their target. These early trials demonstrated the potential effectiveness of FUS for solid tumors with even manually guided devices.
In 2004, the first MR-guided ultrasound surgery system received FDA clearance for the treatment of uterine fibroids. In 2006, reports emerged discussing the use of microbubbles to enhance heating effects and thus reduce the power required to create a lesion.75, 76 Since 2004, the number of potential indications, clinical trials, and reports continues to expand. In Europe, several MR-guided and US-guided focused ultrasound systems have expanded beyond uterine fibroids in the clinical realm with CE marks for palliative treatment of bone metastases and treatments for prostate cancer. In 2012, the FDA announced clearance for the first time in the United States for a system for palliative treatment of bone metastases.
While approved clinical indications are still limited, MRgFUS is experiencing a wide-ranging surge of research and development. Several vendors are now marketing FUS devices, and there are a variety of new devices in the near-term development pipeline. A range of clinical trials are now in progress or are planned.
Clinical systems manufacturers and FUS centers of research
There are several current manufacturers of “commercially available” FUS systems summarized in Table 2. Most of these devices are still either under development or are considered investigational in the United States. At the time of this writing the InSightec Exablate has been cleared by the FDA for the treatment of uterine fibroids and for the palliative treatment of painful bone metastases.
Table 2.
Current manufacturers of USgFUS and MRgFUS systems.
| Company | Location | Device | Type |
|---|---|---|---|
| Chongqing Haifu (HIFU) Technology Company, Ltd. | Chongqing, China | Haifu System | USgFUS |
| Insightec, Inc. | Tirat Carmel, Israel | Exablate 2000, Exablate 4000, Exablate 2100 | MRgFUS |
| US Hifu, LLC. | Charlotte, NC, USA | Sonablate 500 (prostate cancer) | USgFUS |
| EDAP TMS | Vaulx-en-Velin, France | Ablatherm (prostate cancer) | USgFUS |
| Philips Healthcare, Inc. | Boston, MA, USA | Sonalleve | MRgFUS |
| Profound Medical, Inc. | Toronto, ON, Canada | Prostate system | MRgFUS |
| Image Guided Therapy, Inc. | Pessac, France | TargetedFUS | MRgFUS |
FUS research is currently conducted at academic institutions worldwide, and there are three primary research organizations devoted to its development and clinical adoption summarized in Table 3.
Table 3.
Scientific organizations promoting the adoption of focused ultrasound technology.
| Name | Year founded | Mission | URL |
|---|---|---|---|
| Focused Ultrasound Surgery Foundation | 2006 | To accelerate the development and clinical acceptance of MRgFUS | http://www.fusfoundation.org |
| International Society for Therapeutic Ultrasound | 2001 | Increase and diffuse knowledge of therapeutic ultrasound to the scientific and medical community | http://www.istu.org/ |
| Society for Thermal Medicine | 1986 | Fostering interaction and innovation in the study of biological, physical, and medical applications of thermal therapy for cancer and other diseases | http://psfebus.allenpress.com/eBusSFTM/ |
Current clinical indications for MRgFUS
Uterine fibroids
One of the most established current clinical indications for the use of FUS is in the treatment of uterine fibroids. Fibroids which are homogeneous and hypointense relative to skeletal muscle on pretreatment T2-weighted imaging seem to respond better than inhomogenous or higher intensity fibroids, presumably because the latter are more vascular and thus sink heat away from the treatment site. Clinical outcome also seems to correlate well with nonperfused volume (NPV) on contrast-enhanced T1-weighted imaging immediately posttreatment.77, 78
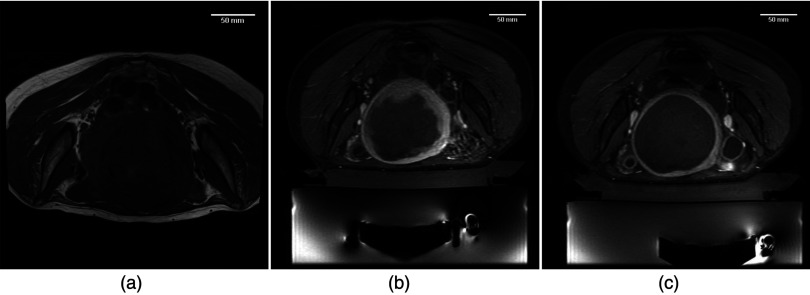
Figure 2 shows representative pretreatment and posttreatment imaging from a patient treated in two sessions with MRgFUS. Figure 2a shows the fibroid pretreatment. Figure 2b was acquired immediately after the first treatment session. The NPV of fibroid was calculated to be approximately 67%. Figure 2c was acquired after the second session, and the NPV percentage approached 100%.
Figure 2.
MR-guided focused ultrasound for uterine fibroids. Images document the treatment of a fibroid patient treated in two MRgFUS sessions approximately 1 week apart. (a) Preprocedure: axial T1 spin-echo image of a patient with a large (∼542 cm3) uterine fibroid. Image shows some hetereogeneity of the fibroid, as well as some possible necrotic areas. (b) Postprocedure 1: axial, postcontrast, fat suppressed, fast spoiled gradient echo pulse sequence. Image shows large nonperfused volume in the center of the fibroid. Volume calculations estimate that 63% of fibroid volume was nonperfused. (c) Postprocedure 2: axial, postcontrast, fat suppressed, fast spoiled gradient echo pulse sequence. Image shows the remaining volume of the lesion is now nonperfused. Approximately 100% of the fibroid volume was ablated. [Images courtesy of the University of Virginia Department of Radiology].
As of this writing, thousands of FUS uterine fibroid treatments have been carried out worldwide. The success rates in terms of symptom reduction for medium-sized fibroids are comparable to other therapies in this field, but with the advantage of a noninvasive, outpatient procedure.79, 80
Bone metastases
Several ongoing trials in Europe, Asia, and the United States continue to investigate FUS for palliative treatment of primary and metastatic bone tumors. Because bone preferentially absorbs ultrasound energy and converts it to heat, bone tends to heat faster than soft tissue. The objective in palliative bone treatments is not necessarily to ablate the tumor itself, rather it is to heat the bone cortex and destroy or inactivate the nerves innervating the periosteum which is the origin of the pain.81
One advantage of FUS over standard radiotherapy regimens is that pain relief can happen very quickly with FUS. A small 2007 study of 12 patients at Sheba Medical Center found that with a mean follow-up of 59 days, 10 patients out of 12 (two patients died from disease progression within 1 month of treatment) reported substantial pain relief on questionnaires and visual acuity scale (VAS).82 A 2008 study by Gianfelice et al. reported that 11 patients in the study with a mean pretreatment VAS score of 6.0 had a mean VAS score of 0.5 (92% decrease) at 3 months follow-up.83 A multicenter study with 3-month follow-up on 25 patients found 72% of patients reporting significant pain improvement, with mean VAS scores reduced to 1.8 from 5.9.84 In all three studies no adverse events were recorded.
Part II. Challenges and Solutions
The long history of focused ultrasound demonstrates that although many of the individual technologies have been used in place for some time, it is only recently that focused ultrasound has emerged as a serious clinical possibility. Even now, while there is much potential for focused ultrasound, the number of proven clinical indications for MRgFUS remains quite small. Many barriers to entry—clinical, technical, and regulatory, must be overcome before MRgFUS becomes a standard fixture in the clinic. Part II of this paper explores some of the challenges MRgFUS must overcome before it can be more generally accepted, followed by near-term developments that may help it meet these challenges.
PHYSICS AND ENGINEERING CHALLENGES
Challenge: Cavitation detection and control
The mechanical effects of ultrasound energy in FUS can cause small gas bubbles trapped in tissue to oscillate, a process called cavitation. In noninertial cavitation, these bubbles undergo repeated cycles of linear or nonlinear rarefraction and compression. The bubbles in turn scatter the ultrasound waves, with the amount of scatter proportional to the incident pressure. As the pressure continues to increase, the bubbles reach a threshold size at which they violently collapse during the compression part of the cycle. This phenomenon is termed inertial cavitation.37 The rapid heating characteristic of focused ultrasound can also cause boiling if the temperature in tissue reaches its boiling point (∼100 °C).85, 86
If boiling or cavitation occur where they are not wanted, they can produce enhanced heating,87 displacement of the lesion,86 and potentially significant damage to tissue. Noninertial cavitation can also have the opposite effect; microbubbles in the prefocal field cause an increase in attenuation of the ultrasound energy and can effectively shield the target from ablation.
Unfortunately, there are many parameters involved in the production of cavitation, and therefore cavitation prediction becomes quite complex and difficult to control. The threshold for cavitation depends on bubble size, bubble density, local temperature, incident acoustic pressure, and excitation frequency. These parameters (with the exception of excitation frequency) are all subject to change from moment to moment.37 Thus, efforts to either predict or monitor targeted tissue for cavitation effects are being implemented or under development and would be a significant development for FUS.
Challenge: Calcifications
Tissues within the body that contain macro- or microscopic calcifications (such as intracranial calcifications) pose a theoretical risk in focused ultrasound treatments. Calcifications absorb ultrasound energy in a manner similar to that of bone. The additional absorption of energy can cause unwanted excessive heating away from the focus, and can also act as a shield, blocking ultrasound energy from reaching its intended target. In at least one experiment reported at the 9th International Symposium on Therapeutic Ultrasound, a sonication was stopped because of suspected far-field heating of a calcification.88
Macroscopic calcifications can often be visualized on CT imaging and can be managed through careful beam targeting and by the algorithms used for refocusing the ultrasound beams through bone.89 However, microcalficiations that are too small to visualize on CT may be difficult to correct for.
Challenge: Standing waves and reflections
Like microscopic calcifications, the presence of standing waves in certain FUS indications, especially intracranial applications, presents a theoretical risk that has yet to be fully explored. Standing waves can occur when the ultrasound beam path between two tissue interfaces is an integer multiple of half-wavelengths. Standing waves can be dangerous because they can cause areas of undesired heating and damage outside of the focus. In the brain, where the effect has been most studied, this can occur within the skull or brain tissue.90 Targeting areas of tissue near interfaces (e.g., soft tissue/air, soft tissue/bone) can cause reflections that can shift the area of lesioning away from the planned location.91 This effect has been shown to be especially prevalent in preclinical studies using animal models, where the smaller skulls (and often lower frequencies) involved in the studies make standing waves common.92
The transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia (TRUMBI) trial employing nonfocused, low-frequency (300 kHz) ultrasound in addition to tissue plasminogen activator (tPA) for thrombolysis in ischemic stroke patients showed an increased hemorrhage risk as compared to tPA alone.93 Subsequent simulation found that standing waves could cause large increases in rarefractional pressure that exceeded the threshold for the development of inertial cavitation. The authors of the study theorized that standing waves could have been a factor in the increased hemorrhage risk.94
Challenge: Developing standards for exposure, “dose,” calibration, and clinical acceptance
There is currently no clear agreement on the most appropriate parameters to measure in order to characterize the focused ultrasound beam. Likewise, there is little agreement on how to describe exposure for FUS, or the analog of “absorbed dose” as it is understood in traditional radiation oncology.95 This creates several challenges for clinical focused ultrasound—it means there is no robust way to standardize treatment parameters for any given clinical situation. There is not necessarily any way to duplicate an experiment reported in a journal on a different treatment device. It also means that comparative treatments on different devices, or even different treatments on the same device, are quite difficult to execute.
There are currently no internationally accepted standards for calibration of high-intensity focused ultrasound machines,96 although efforts to this effect are in progress.95 One could argue that for purely thermal treatments being monitored by temperature imaging techniques such as MR-thermometry, precise calibration is not required as the temperature is being reported during the treatment. For purely ablative therapies, perhaps the only important consideration is that the time/temperature reached in the treatment crosses the threshold for ablation.
However, in most situations calibration will remain a critical component in the safety and effectiveness of FUS. FUS is used in situations where the target tissue may be surrounded by healthy tissue. Experiments thus far have shown that the effect relationship between CEM 43° and tissue damage is quite sensitive.97 Small changes in heating time (for a given temperature) or temperature (for a given time) can have a large effect on tissue damage. Thus, miscalibration of equipment can directly put healthy tissue at risk of the target is overtreated. Conversely, unintentional undertreatment of the target may result in the need for repeat treatments or even disease progression. When operating under nonthermal conditions or in the presence of cavitation or microbubbles, temperature monitoring via imaging may not be as effective, and accurate calibration will likely become even more critical.
More practically for the widespread adoption of FUS technology, standards for calibration would help to provide the confidence in FUS that is a component that regulatory bodies and insurance companies look for as they make clearance and reimbursement decisions.96
When the emerging field is viewed broadly, FUS in some ways is inherently more complicated than ionizing radiation. Whereas currently ionizing radiation is most commonly used to deliver a known absorbed dose to a volume of tissue in one or more sessions, with FUS there are a variety of possible treatment techniques (subablative, ablative, mechanical, mechanism for drug delivery, etc.). For FUS, this complicates the development of a standard set of calibration procedures. In addition, many of the measurement tools available for diagnostic ultrasound (force balances, hydrophones, etc.) can be damaged by the high-temperatures and pressures found in high-intensity focused ultrasound beams.96 However, without agreement on the most appropriate parameters and methods for quantifying them, there is great difficulty in comparing different FUS treatment techniques, or even comparing the same treatment technique on two different FUS systems.
Without standards for calibration, critical tasks such as acceptance testing of new clinical equipment become difficult. Gorny et al. describe acceptance tests they performed on a clinical FUS device for fibroid treatments. Their tests relied on MR-thermometry measurements of temperature and thermal spot location. No tests were performed on the actual calibration of the system, so there is no way to know if the power, frequency, and energy settings of the device were in fact accurate.98 The risk would be that a different machine operated with identical settings and environmental conditions could deliver different results. This could complicate the ability of a treating clinician to apply their experience across different devices.
PHYSICS AND ENGINEERING: SOLUTIONS AND FUTURE DEVELOPMENTS
Cavitation detection
Of the major causes of unwanted heating discussed above, cavitation detection and control has been the focus of the majority of research efforts. Current clinical FUS systems follow two basic techniques to detect cavitation. US-guided systems attempt to detect bubble formation using B-mode ultrasound to look for hyperechogenicity at the focus; however, these systems cannot be used during the actual therapeutic sonications because the HIFU signal interferes with the B-mode images.99 As enhanced temperature rise has correlated with bubble-formation,87 MR-guided systems can instead monitor temperature rise and look for unplanned temperature rise as a surrogate for cavitation. In either system, cavitation away from the immediate focus can evade detection.
Ongoing work on more sophisticated methods for cavitation detection takes advantage of the range of phenomena characteristic of cavitation. Sonoluminescence detectors can detect the light emission from bubble collapse. Laser scattering and laser interferometry can detect bubble clouds and individual bubbles, respectively.100 Hydrophone-based cavitation detectors look for characteristic frequency signatures of cavitating bubbles.101 A drawback to these methods is in localization of individual cavitation events and their efficacy in vivo. The use of dual receivers can allow for the localization of cavitation events to a fairly small detection volume.102 However to be useful for focused ultrasound these detectors may need to work faster and also capture the time-course of cavitation events. Combination methods are in development which may begin to address these shortcomings, such as a dual-method system that combines passive (i.e., receive-only) ultrasound detection with ultrafast subtraction of B-mode ultrasound images relative to presonication reference images that can detect individual cavitation events.103 Another promising method uses a passive ultrasound array combined with a beamforming algorithm to spatially and temporally map cavitation events.99
It may be that the required level of sophistication for cavitation detection is dependent on the clinical technique. For ablative techniques where a specific level of heating at a specifically planned location is the goal, inertial cavitation anywhere may be undesirable, so mapping the location of cavitation events may not be necessary. Conversely for cavitation enhanced techniques, histotripsy, and other techniques, the location of cavitation events becomes critical, and more sophisticated detection is likely to be required.37
Detecting and controlling standing waves and microcalcifications
Unexpected damage to healthy tissue attributed to standing waves has thus far been limited to lower frequency systems in the brain which have a good match between wavelength and skull dimensions. These systems operate at frequencies lower than those currently operated clinically; however, further studies will be required to determine safe operating envelopes in terms of anatomic areas of risk and operating characteristics in order to preclude standing wave formation. Techniques have been explored to prevent the formation of standing waves. One technique is to introduce small random modulations in ultrasound frequency to try to dampen standing waves within the areas of risk.90
Further studies will also be required in order to determine how best to image and detect microcalcifications, as well as to determine the minimum size a calcification needs to reach before it can cause clinically significant effects in terms of unwanted heating or beam distortion. Ultrashort echo time MR (UTE) imaging (discussed in more detail below) may be one method for detecting microcalcifications in tissue.104
Many tumors involve calcified tissue,105, 106, 107, 108, 109, 110 and this may become an important factor in determining whether FUS is indicated for clinical treatment in individual cases.
Standards for “absorbed dose” and calibration
Absorbed dose
As with ionizing radiation, “absorbed dose” cannot be directly measured for FUS. Exposure can be measured in terms of parameters such as acoustic power and pressure. A variety of proxies for absorbed dose have been proposed for FUS, including degree × minutes, specific absorption rate (SAR), power density, etc.
In the ablative systems currently on the market, the de facto standard for thermal dose is the thermal isoeffective dose:111
where t is the time in minutes, T is the average temperature during the time interval, and R = 2 for T > 43 °C. In the case of FUS, where the temperature is changing quickly with time, the equation is summed over small time intervals, assuming a constant T over each interval. The thermal isoeffective dose describes the relationship between heating and time. The constant R has been estimated from in vivo and in vitro studies on a variety of tissue types using Arrhenius analysis of cell survival. The cutoff temperature of 43 °C was chosen arbitrarily by the authors of the report; however, it is close to the temperature at which the slope of the Arrhenius plots change in human tissue.
The isoeffective dose concept is popular because it is simple to understand, and if tissue tolerances are well known at a single temperature/time point, the equation can be used to extrapolate to various temperature/time combinations. However, the values for R are not well known at very high temperatures (>57 °C) generated with HIFU, are not proven at the temperature change rates associated with HIFU for thermal ablation, and are not necessarily constant across different tissue types.97, 112 Isoeffective dose may be sufficient for temperatures up to 57 °C, and may be useful for describing the outer boundary of tissue damage. A direct Arrhenius relationship may be sufficient for describing thermal effects or damage within the higher temperature zone.113, 114 Further research on tissue response to various thermal treatments may provide methods to better characterize “thermal dose,” and may eventually be able to incorporate cavitation, FUS-mediated pharmokinetics, and other effects into some sort of “thermal dose equivalent” somewhat similar in idea to the “Effective Dose”115 used to describe the overall biological damage associated with an exposure to ionizing radiation.
System calibration
There are a variety of candidate parameters that could be used in system calibrations. Ultrasound systems in similar frequency ranges are commonly characterized by radiation force measurements of the entire cross-section of the beam on a target using a radiation force balance, and measures of the spatial and temporal distribution of pressure using piezoelectric hydrophones. Intensity and ultrasound power are derived from these pressure and radiation force measurements.95
In high-intensity beams, these traditional measurements are quite difficult to perform. The high thermal temperatures generated in the focal ultrasound field can change the response of hydrophones, and can damage the radiation force targets and hydrophones. Induced cavitation can shield the instruments from the beam, and can also cause mechanical damage. Assumptions used to derive intensity and power from pressure measurements are not valid for focal fields. Nonlinear harmonics can change the response of the pressure measurements.95 Various groups are working on new techniques to allow measurements of these parameters in high-intensity fields and to allow direct measurement of intensity. For instance, Shaw and Hodnett propose a castor oil target and a buoyancy-based measurement to that can provide accurate power measurements in high-intensity focused fields.96
As discussed earlier, for thermal ablation treatments, it may be that all of these issues can be wrapped into a temperature measurement (performed in a phantom for calibration, and in vivo for actual treatments), since that is the ultimate endpoint of the treatment. This is the basic approach taken by the MR-thermometry community. However, questions remain regarding precision of the system to discern small changes in temperature and the spatial location of the temperature changes. For targets near critical normal tissues, this may become an issue. For procedures where ablation is not the primary endpoint temperature rise by itself is not a sufficient calibration parameter.96
CLINICAL AND FINANCIAL CHALLENGES
Challenge: Expanding clinical indications and gaining insurance coverage
As discussed above, in the Unites States at the time of this writing, treatment of uterine fibroids and bone metastases are the only FDA-cleared indications for commercial MRgFUS. While this would allow MRgFUS to become a niche treatment for a certain subset of patients, it is not likely to be sufficient to effect the widespread adoption of the technology or to displace existing alternative treatments. The number of clinical indications for MRgFUS must expand in the future if MRgFUS is to be more widely accepted.
Expanding clinical indications from the perspective of safety and effectiveness is only part of the challenge of gaining clinical acceptance of MRgFUS in an increasingly crowded treatment marketplace. Achieving insurance coverage and reimbursement for any new medical device is a tremendous barrier to entry. This is especially true when a device is new, and not simply an incremental improvement over an existing device. Even in the instance of uterine fibroids, very few insurance carriers reimburse for the procedure, and not in all parts of the country.
In the United States, insurance coverage decisions are driven primarily by Medicare and its Centers for Medicare and Medicaid Services (CMS). National coverage decision through the CMS are the preferred outcome, however local coverage decisions through Medicare local contractors are more common, leading to varying coverage rules in different areas of the country. Coverage decisions are not a completely transparent procedure, and the required data for efficacy, safety, and cost effectiveness are not standardized.116 Reimbursement is likewise a complicated endeavor, with payment terms generally set by CMS and used as a benchmark by private insurers. If new technologies are assigned to payment groups where the payment does not cover the cost of the procedure for the provider, the provider will not have any incentive to adopt the new technology. Vendors of new devices, and providers hoping to adopt these devices, must therefore work closely with both specialty societies and CMS in order to achieve adoption.117 It may be especially difficult for indications where there is a crowded field of existing treatment options. Thus, new indications for MRgFUS have an uphill battle to wage regarding insurance coverage and reimbursement. Without these, widespread acceptance will be difficult.
Challenge: Proving safety and effectiveness
Solving the reimbursement puzzle will be difficult unless the case can be made that MRgFUS is safe, and at least as effective as existing treatment options for any given indication. A recent paper in European Urology performed a systematic review of the literature looking at evidence for the use of MRgFUS for prostate cancer and applying a quasiobjective grading scheme for the quality of the evidence. They concluded the current evidence was of “very low quality, mainly due to study designs that lack control groups.” They also took issue with the particular survival and biochemical control endpoints used in many of the studies.118 This point of view was reiterated in a recent edition of the Point/Counterpoint series published in Medical Physics examining the evidence for MRgFUS vs radiation therapy for prostate cancer.119
MRgFUS is an emerging technology. By definition, it will not have a long track record of class 1 evidence supporting its use. However, as attempts to rein in healthcare costs increasingly gain a sense of urgency, proof of cost effectiveness will be vital for the success of MRgFUS.
CLINCIAL AND FINANCIAL SOLUTIONS AND FUTURE DEVELOPMENTS
The role of clinical and scientific societies
The focused ultrasound research and clinical community has organized around several clinical, and scientific organizations whose purposes are to accelerate basic and translational research with HIFU, facilitate collaboration among international centers of research, and perhaps most importantly, to build awareness of the technology and its potential with the public, with CMS and the insurance market. These societies are assisting the creation of interinstitutional collaborations, sharing of technical expertise, and acting as liaisons between industry and academic institutions and between industry and regulatory agencies. In this sense, MRgFUS is not trying to gain a foothold in the medical marketplace organically, but rather through a deliberate strategy, one that may be unique in medicine.
We have discussed how to prevent MRgFUS from simply becoming a niche treatment modality; it must expand its range of generally accepted indications. Likewise, for FUS to gain approval for reimbursement in the United States it cannot remain a one- or two-indication treatment option. Table 4 outlines clinical trials currently active or recruiting in the US clinical trials database (NCT) for breast cancer, functional neurosurgery, brain metastases, uterine fibroids, bone metastases, and prostate cancer. Much more work is taking place around the world on a wide range of indications and possibilities through preclinical and clinical trials, some which expand the possibilities for FUS by taking advantage of both ablative and nonablative techniques.
Table 4.
Trials currently active or recruiting in the US clinical trials database (NCT) for breast cancer, functional neurosurgery, brain metastases, uterine fibroids, bone metastases, and prostate cancer.
| NCT No. | Title | Recruiting, Not yet recruiting, Completed | Conditions | Device/intervention | Sponsors | Phase |
|---|---|---|---|---|---|---|
| NCT01141062 | Therapeutic MRI guided high intensity focused ultrasound ablation of uterine fibroids | Recruiting | Uterine fibroids | Device: Philips MR-guided HIFU | Philips Healthcare | Phase III |
| NCT00837161 | Pilot study of mri-guided high intensity focused ultrasound ablation of uterine fibroids | Completed | Uterine fibroids|uterine leiomyomata | Device: Philips MR guided HIFU system | Philips Healthcare|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | Phase I|Phase II |
| NCT01097239 | Examining the role of transrectal high intensity focused ultrasound (HIFU) in rectal pelvic cancer | Recruiting | Rectal cancer | Device: Sonablate 500 (High Intensity Focused Ultrasound (HIFU)) | Imperial College London|Imperial College Healthcare NHS Trust | Phase I|Phase II |
| NCT01064960 | Clinical trial protocol for therapeutic mri-guided high intensity focused ultrasound ablation of uterine fibroids in a 3T MRI scanner | Completed | Uterine leiomyomas | Device: Philips MR-guided HIFU system | Philips Healthcare|Philips Medical Systems | Phase III |
| NCT01422629 | High intensity focused ultrasound (HIFU) to treat breast fibroadenoma | Recruiting | Breast fibroadenoma | Device: Ultrasonic ablation device | Theraclion | |
| NCT00897897 | Therapeutic magnetic resonance imaging (MRI)-guided high intensity focused ultrasound (HIFU) ablation of uterine fibroids | Completed | Uterine fibroids | Procedure: HIFU | Philips Healthcare|Philips Medical Systems | |
| NCT01489787 | Study to evaluate a high intensity focused ultrasound (HIFU) procedure in patient with liver metastases | Recruiting | Neoplasm metastasis | Procedure: HIFU|Procedure: HIFU|Procedure: HIFU | Centre Leon Berard|National Cancer Institute, France|CLARA | Phase I|Phase II |
| NCT01504308 | Philips pivotal clinical trial for MRI-HIFU of uterine fibroids | Not yet recruiting | Uterine fibroids | Device: MR-HIFU treatment for ablation of uterine fibroids | Philips Healthcare | Phase II|Phase III |
| NCT01421407 | Efficacy and safety of high intensity focused ultrasound (HIFU) device to treat secondary hyperparathyroidism | Recruiting | Secondary hyperparathyroidism|end stage renal disease|parathyroid hyperplasia | Device: Ultrasonic ablation device | Theraclion | |
| NCT01291498 | High intensity focused ultrasound (HIFU) for parathyroid adenoma | Recruiting | Parathyroid adenomas | Device: High Intensity Focused Ultrasound | Oxford University Hospitals NHS Trust|Theraclion | |
| NCT01117246 | Pilot study for palliation of pain in bone metastases by MR-HIFU | Completed | Secondary malignant neoplasm of bone | Device: High Intensity Focused Ultrasound | Philips Healthcare | Phase I|Phase II |
| NCT01309048 | Magnetic resonance-guided high intensity focused ultrasound for palliation of painful skeletal metastases | Recruiting | Bone metastasis | Device: Philips MR-guided HIFU system | Philips Healthcare | Phase I|Phase II |
| NCT01060982 | Observation of histological changes in parathyroid adenomas following high intensity focused ultrasound (HIFU) treatment procedure | Recruiting | Primary parathyroid adenomas | Device: Ultrasonic ablation device | Theraclion | |
| NCT01194648 | High-intensity focused ultrasound in treating patients with localized prostate cancer | Recruiting | Male erectile disorder|prostate cancer|therapy-related toxicity|urinary incontinence | Procedure: high-intensity focused ultrasound ablation | University College London Hospitals | Phase II |
| NCT01239641 | High intensity focused ultrasound ablation virus myomectomy to treat uterine fibroids | Recruiting | Uterine fibroid | Procedure: High intensity focused ultrasound | Chongqing Medical University | Phase IV |
| NCT00987675 | High-intensity focused ultrasound ablation in treating patients with progressive prostate cancer | Recruiting | Prostate cancer | Procedure: high-intensity focused ultrasound ablation | University College London Hospitals | Phase II |
| NCT00295802 | Ablatherm integrated imaging high intensity focused ultrasound for the indication of low risk, localized prostate cancer | Active, not recruiting | Prostate cancer | Device: Integrated Imaging High Intensity Focused Ultrasound | EDAP TMS S.A. | Phase II|Phase III |
| NCT01331954 | Treatment of breast fibroadenoma with high intensity focused ultrasound (HIFU) | Recruiting | Breast fibroadenoma | Device: Ultrasonic ablation device | Theraclion | |
| NCT00988130 | High-intensity focused ultrasound focal ablation in treating patients with progressive prostate cancer | Recruiting | Prostate cancer|sexual dysfunction and infertility | Procedure: high-intensity focused ultrasound ablation | University College London Hospitals | Phase II |
| NCT00180739 | Safety trial of magnetic resonance (MR) guided focused ultrasound surgery (FUS) in women with uterine fibroids wishing to pursue pregnancy in the future | Completed | Uterine fibroids|pregnancy | Procedure: Magnetic Resonance Guided Focused Ultrasound | Imperial College London|InSightec | Phase IV |
| NCT00772317 | A multicenter clinical study of the sonablate®500 for the treatment of locally recurrent prostate cancer with HIFU | Recruiting | Recurrent prostate cancer | Device: High Intensity Focused Ultrasound | USHIFU, LLC | Phase III |
| NCT00770822 | Clinical study of the sonablate® 500 to treat localized (T1c/T2a) prostate cancer | Active, not recruiting | Prostate cancer | Device: HIFU (Sonablate® 500)|Device: Brachytherapy | USHIFU, LLC | |
| NCT01377519 | Magnetic resonance guided focused ultrasound for uterine fibroids | Recruiting | Uterine fibroids | Procedure: MR Guided Focused Ultrasound | University of California, San Francisco | |
| NCT00573586 | Treatment of localized prostate cancer with high intensity focused ultrasound using the sonablate® 500 system in Canada | Not yet recruiting | Prostate cancer | Device: Sonablate 500 (SB-500) | USHIFU, LLC | Phase IV |
| NCT01473485 | ExAblate (magnetic resonance-guided focused ultrasound surgery) treatment of brain tumors | Recruiting | Glioma|metastatic brain cancer | Device: ExAblate Transcranial System | InSightec | |
| NCT00030277 | High-intensity focused ultrasound in treating patients with locally recurrent prostate cancer | Completed | Prostate cancer | Procedure: high-intensity focused ultrasound ablation | Focus Surgery | Phase I |
| NCT01338467 | Glaucoma treatment by circular cyclocoagulation using high intensity focused ultrasound with the EYEOP medical device | Recruiting | Glaucoma | Device: EYEOP device | EyeTechCare | |
| NCT00656305 | ExAblate (magnetic resonance-guided focused ultrasound surgery) treatment of metastatic bone tumors for the palliation of pain | Recruiting | Bone metastases|multiple myeloma | Device: ExAblate 2000|Device: Sham | InSightec | Phase III |
| NCT00159328 | Efficacy study of magnetic resonance (MR) guided focused ultrasound in the treatment of large fibroids | Completed | Uterine fibroids | Procedure: Magnetic Resonance Guided Focused Ultrasound | Imperial College London|InSightec-TxSonics | Phase IV |
| NCT01104272 | Subcutaneous contouring using high intensity focused ultrasound | Completed | Body sculpting | Device: LipoSonix (Ultrasound treatment of Subcutaneous Adipose Tissue) | Medicis Technologies Corporation | |
| NCT00995878 | The FIRSTT: comparing MRgFUS(MR-guided focused ultrasound) versus UAE (uterine artery embolization)for uterine fibroids. | Recruiting | Symptomatic uterine leiomyomas|fibroid|myomas | Procedure: Focused ultrasound (MRgFUS)|Procedure: Uterine artery embolization (UAE) | Mayo Clinic|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | Phase IV |
| NCT00030290 | Ultrasound in treating patients with prostate cancer confined to the prostate | Completed | Prostate cancer | Procedure: high-intensity focused ultrasound ablation | Focus Surgery | Phase I |
| NCT00147108 | MR-guided focused ultrasound surgery in the treatment of breast fibroadenomas | Completed | Breast fibroadenoma | Device: ExAblate 2000 | InSightec | Phase III |
| NCT01091883 | Study comparing the safety and effectiveness of magnetic resonance guided focused ultrasound (MRgFUS) and external beam radiation (EBRT) for treatment of metastatic bone tumors and multiple myeloma | Recruiting | Bone cancer|secondary malignant neoplasm of bone|pain | Device: Exablate treatment|Radiation: Radiation | InSightec | Phase III |
| NCT01304758 | ExAblate transcranial MR-guided focused ultrasound in the treatment of essential tremor | Recruiting | Essential tremor | Device: ExAblate Transcranial MRgFUS System | InSightec | Phase I |
| NCT01226576 | Focal MR-guided focused ultrasound treatment of localized low-risk prostate cancer: feasibility study | Not yet recruiting | Localized low-risk prostate cancer | Device: MRgFUS Treatment | InSightec | Phase II |
| NCT01232582 | Safety and efficacy of MRgFUS for the treatment of low back pain | Recruiting | Lower back pain, facets joints osteoarthritis | Device: Exablate treatment | InSightec | Phase II |
| NCT01092988 | A clinical study to evaluate safety of the ExAblate 2100 UF V2 system in the treatment of symptomatic uterine fibroids | Recruiting | Uterine fibroids|bleeding|pain | Device: Exablate 2100 | InSightec | |
| NCT01142791 | Safety study of ExAblate for the treatment of uterine fibroids | Recruiting | Uterine fibroids | Device: ExAblate | InSightec | Phase IV |
| NCT00365989 | MR-guided focused ultrasound treatment of uterine fibroids with enhanced sonication | Completed | Uterine leiomyoma|uterine fibroids | Device: ExAblate 2000 | InSightec | Phase III |
| NCT01085565 | Focused ultrasound surgery in the treatment of pain resulting from metastatic bone tumors with the ExAblate 2100 conformal bone system | Recruiting | Bone cancer|secondary malignant neoplasm of bone|pain | Device: ExAblate 2100 | InSightec | Phase II |
| NCT00295217 | MR-guided focused ultrasound surgery in the treatment of uterine fibroids: Software V4.2 validation | Completed | Uterine fibroids|uterine leiomyomas | Device: ExAblate 2000 | InSightec | Phase III |
| NCT01285960 | ExAblate UF V2 system for the treatment of symptomatic uterine fibroids | Not yet recruiting | Uterine fibroid(s) | Device: ExAblate UF V2 | InSightec | Phase III |
| NCT00981578 | ExAblate conformal bone system treatment of metastatic bone tumors for the palliation of pain | Recruiting | Bone metastases | Device: ExAblate 2100 | InSightec | Phase I |
| NCT01229826 | Magnetic resonance elastography (MRE) of uterine fibroids | Recruiting | Uterine fibroids | Radiation: MR Elastography | Mayo Clinic | |
| NCT01328067 | Study to evaluate the safety and effectiveness of MRgFUS compared with myomectomy for the treatment of uterine fibroids | Not yet recruiting | Uterine fibroids|bleeding|pelvic pain | Device: Exablate 2100|Procedure: Myomectomy | InSightec | Phase IV |
New clinical indications: Ablative techniques
Breast cancer
Local treatment of breast tumors was an indication targeted early on by researchers in the field because of its favorable soft tissue interface and relative ease of transmitting the required ultrasound energy.120 Since this early feasibility study on breast fibroadenomas, several small feasibility trials have been completed investigating the use of FUS as a lumpectomy replacement. Gianfelice et al. showed that dynamic contrast-enhanced MRI could be used to evaluate the efficacy of a MRgFUS treatment for small breast lesions.121 Wu et al. reported on a clinical trial with 48 patients randomized to radical mastectomy or HIFU followed by radical mastectomy. Histopathology showed complete necrosis of the tumors after HIFU. In 2005, the same group reported on another 22 patients treated as part of a nonrandomized prospective trial of HIFU followed by radiation, tamoxifen, and chemotherapy. After a median 54.8 month follow-up period they report an 89% recurrence-free survival, with good cosmetic results.122 In 2007, Furasawa reported that after a mean of 14 months follow-up 20 out of 21 cases of ductal carcinoma remained recurrence-free, with 2 skin burns reported.123 However, other results have been less encouraging, with Zippel et al. reporting that eight of ten patients had at least some amount of residual tumor at lumpectomy 7–10 days after MRgFUS.124
The reported studies thus far are early, single-instruction studies with small patient cohorts that provide very limited evidence for more widespread adoption.125 Wider adoption is also limited by the technical issues including difficulty in measuring temperature via MR-thermometry due to the fat content of breast tissue,25, 31 extended treatment time and patient positioning requirements, as well as the politics of breast-cancer care which can have a significant impact on treatment options.126 A successful multi-institution clinical trial may help open up breast cancer as a common indication for MRgFUS.
Prostate cancer
FUS is an attractive therapy for localized prostate cancer because it is minimally invasive, can be delivered in a single session, has good acoustic accessibility, and can be repeated if needed or combined with more traditional therapies if required. FUS systems for prostate cancer commonly make use of transrectal transducers which place the ultrasound beams in close proximity to the prostate. A number of clinical trials on significant numbers of patients with early-stage prostate cancer have been completed. Early results are comparable and in some cases better than that reported for traditional radiotherapy or radical prostatectomy. In one multicenter series of 803 mainly low and intermediate-risk patients, the study reported overall and cancer-specific survival rates at 8 years of 89% and 99%, and metastasis-free 8-year survival rate of 97%. Five-year biochemical-free survival rates were reported as high as 84% for low-risk patients.127 Another series of 181 patients treated with USgFUS reported biochemical disease-free survival rates of 85%, 80%, and 78% at 1-, 3-, and 5-year follow-up. Complications included urethral stricture (22%), epididymitis (6%), rectourethral fistula (1%), transient incontinence (0.6%), erectile dysfunction (20%), and retrograde ejaculation (9%).128 FUS has also been proposed as a salvage therapy after failure with radiotherapy.129, 130 The technique may be limited by the accuracy of staging. For patients with micrometastases who ultimately fail FUS, options may include salvage radiotherapy131 or radical prostatectomy.132 Also, while these results are encouraging, FUS is an emerging technology, and the quality of evidence for FUS is still immature.118
Liver and other abdominal targets
Radiofrequency ablation of hepatic tumors has been shown to be an effective technique to gain local control.133 Focused ultrasound would provide a less-invasive improvement on this technique.134 Moore et al.135 and Yang et al.136, 137 conducted some early experiments on animal models. Chen looked at thermal lesion histopathology to confirm cell destruction within thermal lesions.138
In human patients, Kennedy et al. report on a safety and efficacy series using extracorporeal US-guided HIFU and finds transient pain and minor skin burns as the only complications.139 Li reports a normalization of clinical symptoms in 83% of a cohort of 100 patients.140 Leslie et al. reported on a phase II efficacy trial that showed MR-guided FUS to be feasible, and that postprocedure MR accurately predicts tumor ablation when compared to histology.141 One difficulty in using FUS for abdominal targets is the presence of intervening anatomy such as ribs and bowel. Preferential absorption of energy in the ribs can distort the ultrasound focus. The presence of gas in the bowel can cause reflections, unwanted heating, and effectively prevent ultrasound energy from reaching the target. Bowel between the transducer and the target is often a contraindication for uterine fibroid procedures.142 Quesson describes a method for identifying and turning off transducer elements that would result in beams passing through the ribs.143
Lesioning for neuropathic pain and functional disorders
Patients with chronic pain syndromes have for many years been treated with subthalamic lesioning using either radiofrequency lesioning144 or radiosurgery145 to interrupt pain pathways and improve symptoms. Stereotactic RF-lesioning conventionally requires a burr-hole through the skull, with the possibility of associated complications. Radiosurgical lesioning avoids this requirement, but necessitates large doses of ionizing radiation and has a delayed onset of symptomatic benefit. With FUS it may become possible to noninvasively place discrete lesions in the brain at very controlled target sites such as the subthalamic area in patients with chronic pain syndromes. Jeanmonod et al. describe a technique to treat neuropathic pain whereby 3–4 mm lesions are created in the posterior part of the central thalamic nucleus using MR-guided FUS. The study achieved an improvement in pain (by Visual Analog Scale) of 41% at one year. In all cases the patients reported somatosensory and vestibular effects during the treatment, and a hemorrhage was reported in one case.146 Another trial targeting the central lateral nucleus of the thalamus reported a mean 68% subjective pain reduction and no observed neurological deficits or side effects in a small cohort of nine patients.147
Trials at the author's (D.S.) institution have begun to investigate the use of focused ultrasound for movement disorders such as essential tremor148 and Parkinson's disease. Current remedies for essential tremor and Parkinson's disease include the long-term insertion of deep brain stimulators.149, 150 While effective, stimulators are associated with a non-negligible morbitity151, 152, 153 and occasionally require surgical revision.154 FUS treatments for movement disorders follow a technique similar to those described above, and thus avoid the creation of a burr-hole as is required for the current remedies such as the insertion of deep brain stimulators. More data and longer term followup will be required to learn if ablative lesioning via FUS will lead to better outcomes than stimulators.
New clinical indications: Nonablative techniques
Most research that has reached the stage of clinical investigation both in the United States and abroad deals with ablative techniques as described above. However, there are a number of potentially interesting applications of FUS that do not rely solely on direct tissue destruction. These applications are in preclinical development and will likely not find their way into the clinic in the near-term; however, they may have significant consequence when they do.
Targeted drug delivery
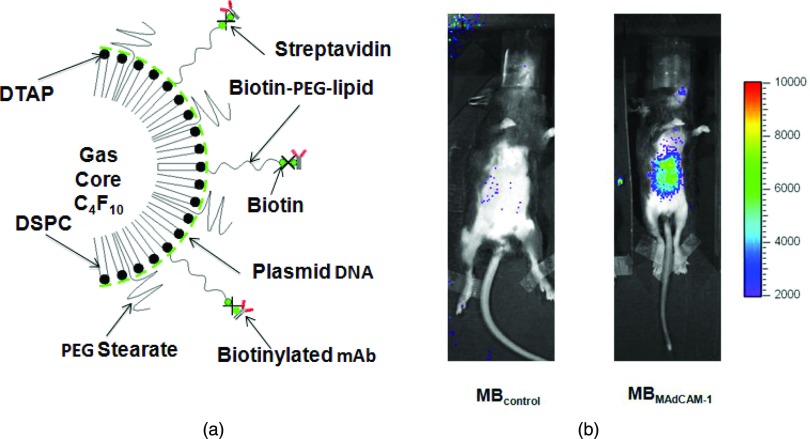
One of the more promising future applications for FUS involves targeted delivery of drugs to specific organ and/or tumor sites. The theoretical advantages to this approach would be to create a high therapeutic concentration of the drug at the desired treatment site while limiting systemic side-effects. Drugs can be packaged into carriers such as liposomes,155, 156, 157 microbubbles,158 or nanoparticles.159, 160 These can then be injected systemically, however activation would only occur upon sonication at the intended target. Mechanisms of drug release can be through heat-activation,161 lysis of the carrier, or by increasing the blood vessel permeability of the target162 Dromi et al. demonstrated that the use of liposomes created to be sensitive to temperature, combined with a pulsed high-intensity ultrasound exposure, resulted in faster drug delivery and higher in vivo drug concentration155 than the use of temperature-insensitive liposomes or the use of liposomes without ultrasound exposure.
The techniques for clinical drug delivery are being quickly refined.157 Klibanov et al. summarize a range of techniques for creating liposomes for a variety of drug delivery models.163 In one recent study by Eisenbrey et al., researchers were able to deliver doxorubicin to a liver tumor in a rabbit model. They achieved a 50% reduction in doxyrubicin concentration in nontargeted areas of the liver, and a 110% increase in levels near the periphery of the tumor.164 Klibanov et al. developed a construct of liposome coated microbubbles. The liposomes were loaded with calcein and thrombin. In an in vitro model consisting of canine blood, pulsed ultrasound resulted in destruction of the microbubbles, with significant release of calcein and a detectable increase in blood clotting.165
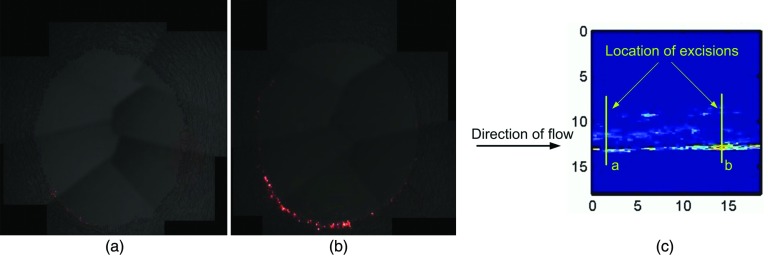
One technical challenge with drug delivery using microbubbles is ensuring the proximity of the microbubble to the intended target. Studies of the kinetics of microbubble transit through vessels show that they tend to travel along the central axis of the vessel.166 For drug delivery, it may be desirable for the bubbles to burst and release their payload close to the vessel endothelium. Patil et al. address this problem by using ultrasound radiation force to push the microbubbles closer to the vessel wall before destroying them and releasing their payloads. Simultaneous imaging allows for real-time imaging monitoring of the microbubble accumulation. A high pulse-repetition frequency pulse is then applied to destroy the bubbles (Fig. 3).167
Figure 3.
Fluorescence microphotographs of two fragments (control and ultrasound-treated) of a swine artery after intravenous administration of DiI fluorescent-dye impregnated microbubbles (Ref. 167) (a) Fluorescence observed in the control fragment of the artery after microbubble administration. (b) Fluorescence observed in the ultrasound-treated fragment after microbubble administration and insonation with radiation-force ultrasound, followed by a “destruction” pulse to locally destroy the microbubbles. (c) Ultrasound image of the artery at the end of the applied ultrasound sequence with the locations of the excised control (a) and ultrasound-treated (b) fragments. [Figures courtesy of Abhay Patil, Philips Healthcare, and John Hossack, University of Virginia] (Ref. 167).
Blood-brain-barrier opening
The efficacies of chemotherapeutic agents are severely restricted in the brain. One reason is the blood-brain-barrier (BBB); a permeability barrier which prevents large molecules from penetrating into the parenchyma from the brain vasculature.168 In many systemic chemotherapy treatments, it becomes almost impossible to deliver a therapeutic concentration of drug to the brain without an unacceptable risk of toxicity to the rest of the body. In the setting of brain tumors, the tumors themselves manifest aspects of the BBB (the blood tumor barrier or BTB), and in addition often have decreased density of capillaries, requiring elevated dosing for drugs to penetrate into tumor tissue in sufficient concentrations.169
FUS has been shown to have an ability to open the BBB,170 albeit with some evidence of damage to the surrounding brain tissue. Hynynen et al. improved on this approach, using lower ultrasound intensities with microbubble contrast agents to selectively and reversibly open the BBB in rabbits.171 Several researchers from the same group at Brigham and Women's Hospital have extended this work (Fig. 4). McDannold et al. looked at histological effects of the technique, finding little evidence that unwanted hypoxia or apoptosis would occur.49 Hynynen et al. used ultrasound frequencies more compatible with transcranial FUS procedures.171 Kinoshita et al. report on successful delivery of Herceptin (trastuzumab) through the BBB using the technique.162
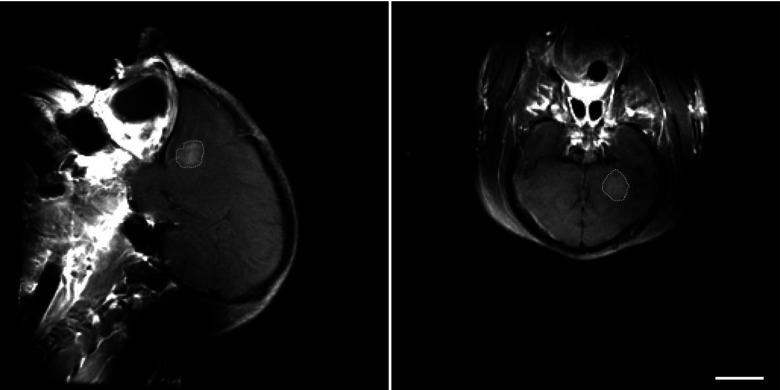
Figure 4.
Contrast-enhanced T1-weighted MRI showing blood-brain barrier disruption induced in a brain volume in a macaque by focused ultrasound and microbubbles. The disruption was produced in a 1 cm3 volume using low-energy focused ultrasound pulses combined with a circulating microbubble ultrasound contrast agent. The sonications were applied transcranially using a clinical prototype MRI-guided focused ultrasound system (ExAblate 4000, InSightec). Note the lack of contrast enhancement in the ultrasound beam path. This noninvasive technique is being investigated to target the delivery of drugs that normally do not reach the brain due to the presence of the blood-brain barrier. [Image courtesy of Dr. Nathan McDannold, Brigham & Women's Hospital, Boston, MA.]
While work to better understand the biological effects and refine the technique is ongoing, the early results suggest that FUS presents a unique capability for BBB-opening as it appears to be reversible, localizable, and noninvasive.
Sonothrombolysis
Although the thrombolytic potential of ultrasound has been known for many years,172 it is only recently that the possibility of using ultrasound alone or in combination with thrombolytic agents such as recombinant tissue plasminogen activator (rTPA) has become an important area of investigation for FUS.173 In the CLOTBUST phase I trial, stroke patients eligible for TPA therapy were randomized to either 2 h of monitoring with transcranial Doppler (TCD) ultrasound or placebo monitoring, along with a bolus of TPA.174 The group monitored by TCD showed stronger outcomes based 24 h (44% vs 40% dramatic clinical improvement, p = 0.7) and at 3 months (42% vs 29%, modified Rankin scale, p = 0.20).175 While not statistically significant, this trial showed the potential for FUS sonothrombolysis.
Success in this area would represent a major potential indication for FUS. Stroke remains a significant cause of long-term disability and a tremendous burden on the overall health-care system.176 The current state-of-the-art treatment for ischemic stroke is rTPA, however this is severely constrained in its eligibility criteria, with only 8% of patients generally able to benefit under current exclusion guidelines.177
Pain management
Studies as far back as the 1960s examined the effect of ultrasound on nerve conduction and the ability to create reversible nerve blocks.178, 179 The ability of FUS to create targeted, focal sonications makes it an attractive potential technique for the treatment of various pain pathologies such as facet rhizotomy and spasticity.180 Foley et al. have demonstrated FUS to be capable of creating sciatic nerve blocks in rabbits.181
Work at the author's unit (W.G., unpublished data) is currently directed at decreasing the pain associated with lumbar spine facet joint disease. Facet joint problems are commonly treated by local injections of local anaesthetics, steroids, or sclerosants, etc., or local radio frequency ablation all aimed at limiting the pain fibers around the facet joints which are believed to cause the patients pain in this condition. Focused ultrasound, using relatively low power sonications targeted at facet joints, can be used to try to destroy the neural fibers running over the facets to decrease the pain felt from these areas. Early pilot work suggests excellent symptom improvement and very good improvement of disability in these patients with chronic back pain up to 6 months posttreatment. Much more work is clearly required in this area but the potential of FUS as a noninvasive method of treating chronic back pain is very attractive. This early study also suggests that focused ultrasound may be a very effective way of producing local nerve blocks which could be applied in many other areas where currently pain specialists use percutaneous needle placement guided by fluoroscopy or CT.182
Combined therapies—focused ultrasound and radiation
Focused ultrasound surgery is by definition a localized treatment. While margins can be taken around a tumor to account for subclinical disease, as with traditional surgery these may be insufficient to ensure the adequate control of highly infiltrative disease. Oncology patients also often have micrometastases not evident on current imaging modalities; these patients are often treated with large field external-beam radiation and/or systemic chemotherapy. In these cases, focused ultrasound could be an effective primary treatment option with the goal of controlling or debulking the grossly evident disease and mitigating acute symptoms. In one study of patients with localized prostate cancer, salvage external beam radiation was used to treat patients with local recurrence after focused ultrasound. At a median followup of 37 months, the disease-free survival rate was 64%.131 Alternatively, FUS could be used as a salvage treatment after local failure with radiation, with one study reporting a 71% disease-free rate after whole gland ablation of the prostate after localized recurrence post- external-beam radiotherapy.129 It has been known for some time that heat can be a potent radiosensitizer,44, 45 likely because heat insult prevents the cells from efficiently repairing sublethal DNA damage from radiation.183 Hypoxic tissues do not show the same resistance to thermal insult as they do to ionizing radiation.184 Thus, there is a possibility for a synergistic effect by combining FUS-delivered thermal energy and ionizing radiation. One potentially attractive idea would be to use ablative FUS to debulk the hypoxic areas of large tumors which do not normally respond well to radiation and then treat the surrounding tissue with traditional radiation.
One issue to be worked out is the optimal sequencing of FUS and radiation. At subablative temperatures, studies have found that tumor control and normal tissue reactions are highest when delivering concomitant radiation and hyperthermia.185 Using high-intensity focused ultrasound, an increased reduction in cell survival has been found when radiation delivery follows ultrasound, but not if radiation precedes ultrasound.186
It is likely that in the future FUS will be used in combination with other chemo- and radiotherapies. Much work remains to determine the most effective combination treatments and the sequence in which they should be considered.
FUS-mediated gene therapy
Research has suggested that FUS can affect the efficacy of gene therapy, as well as provide a method for spatial and temporal control of gene expression.187 FUS used to achieve sublethal temperature increases can affect the regulation of heat-shock proteins such as hsp70, which can then be used as promoters for gene insertion.188 Also, in a method similar to that used for targeted drug delivery, pulsed HIFU-induced cavitation, often achieved through the use of microbubble contrast agents189 or nanoparticles,190 can cause an increase in the permeability of cellular membranes and result in more efficient uptake of DNA and subsequent gene expression.50 This temporary increase in sonoporation would then allow for localized insertion of genetic material at the target and minimize the risks of systematic effects.191 A variety of preclinical studies are reporting progress on in vitro and in vivo tissue models.192, 193, 194, 195, 196 Figure 5 illustrates a conceptual framework for gene transfection in a murine model for Crohn's disease using microbbubbles loaded with targeted antibodies and luciferase-encoding plasmids.197
Figure 5.
FUS/microbubble-mediated gene transcription (Ref. 197). (a) A positively charged microbubble is complexed with a luciferase-encoding plasmid, and carries an antibody against a marker for Crohn's disease. Control bubbles carry a nonspecific Immunoglobulin G (IgG) antibody. Bubbles are injected intravenously and left to circulate for 2 days, with the targeted bubbles accumulating in the target intestinal inflammation zone and attaching to the Crohn's disease marker on the vascular endothelium. After the circulating bubbles exit the bloodstream, ultrasound is performed. Two days later, luciferin is injected, and optical imaging of the induced bioluminescence is performed. (b) Control vs experimental results. The left-hand figure shows an animal injected with control bubbles. Right hand figure shows animal injected with targeted antibody bubbles. Note the accumulation bubbles in the targeted animal, demonstrating transfection. [Image courtesy of Alexander Klibanov, University of Virginia] (Ref. 197).
Cardiac disease
FUS has been explored for its potential to treat cardiac disease including arrhythmia, hypertrophic cardiac myopethy, and atrial fibrillation. Englel et al. reported on a preclinical study where they successfully created midmyocardial lesions in areas of the left and right ventricles of ex vivo canine hearts, and also in an in vivo, open-chest study where the ultrasound was gated to an electrocardiogram.198 However, Mezner et al. describe a clinical study using balloon catheters to deliver HIFU in order to achieve pulmonary vein isolation in 32 patients with paroxysmal atrial fibrillation. While the technique achieved an 87% isolation rate, and a 56% atrial-fibrillation-free rate after a median follow-up of 3.8 years, the trial had to be halted because of severe complications.199 FUS may one day be able to compete with similar techniques such as radiofrequency ablation, but much work remains to solve current technical hurdles.
Neuromodulation
One of the more exotic potential applications of FUS is to cause desired modifications in behavior by targeting various functional centers in the brain with low-power ultrasound. These treatments would be subablative; no tissue would be permanently damaged, but the neuromodulatory effects may nonetheless be durable.
The neuromodulation potential of ultrasound was first described by Fry et al. in the 1950s. They describe reversible reduction in visually evoked potentials in cats sonicated with high power ultrasound.200 Rinaldi et al. in 1991 reported an ability to modulate the evoked potentials of an in vitro hippocampus by sonicating to a power of 80 W/cm2 using 750 KHz ultrasound with a pulse repetition of 150 KHz and a duration similar to electrical signals found naturally to evoke potentials in the brain. Field potentials were found to decrease during ultrasound exposure, and were at least partially reversible.201 Tyler et al. demonstrated that low-intensity, low-frequency ultrasound could be used to stimulate hippocampal circuits in ex vivo mouse brains.202 This work was later extended to intact mice, and was used to disrupt seizure activity in epileptic mouse models.203
Work in neuromodulation is in a very preliminary stage, however it holds potential for a new class of clinical applications for focused ultrasound techniques.
TREATMENT DELIVERY CHALLENGES
Challenge: Time of treatment
Perhaps the most daunting technical limitation of current MRgFUS systems has to do with time of treatment. Current ablative treatments are slow; often several hours are required to ablate a midsized (50–100 cm3) tumor such as a uterine fibroid. Some current devices use a large number of individual sonication spots to ablate a target. During each sonication, tissue in the near-field absorbs some amount of ultrasound energy, albeit not as much as in the focal area. Each sonication is therefore followed by a cooling period to prevent thermal-buildup that would result in unwanted near-field heating if the sonications were performed continuously. This reduces the effect of thermal buildup, but with the tradeoff of time for cooling and energy lost through outward diffusion.204 Each sonication additionally requires MR prescan time for gradient and RF calibration as well as MR-thermometry scan time. A single sonication is, therefore, a several minute process, and there can be upwards of a hundred individual sonications in a given treatment. But because of locally variable heat diffusion of ultrasound energy away from each focus, some sonications fail to cause ablation temperature in tissue and these locations must be repeated.204
The extended treatment times mean the patient is required to lie motionless on the treatment table for the duration of the treatment. And, since the most efficient way to get ultrasound energy to the target is through the shortest beam path length, it means that often the patient is asked to place their body weight on the tumor; often this is quite a painful request. It means that the treating physician, be it radiation oncologist, radiologist, surgeon, etc., is tied up performing the procedure for the duration, along with the rest of the treatment team, which can include anesthesiology, nursing, physics, etc. In the case of MR-guided FUS, it also goes without saying that the MR unit (which is so far not typically dedicated to FUS) is also allocated for the duration of the treatment; an expensive proposition for a device in extremely high demand.205, 206
Challenge: Presence of bone, implants, or intervening bowel
Bone absorbs ultrasound with an efficiency almost 90 times greater than that of soft tissue.207 Thus, tumors lying in the shadow of bone require much greater energy to ablate than targets not shadowed by bone. Bone also defocuses the ultrasound focus, often requiring a corrective refocusing in order to achieve a focal spot.208 When the bone absorbs this ultrasound energy, it heats much more efficiently than soft tissue.209 This can lead to damage to the bone matrix, as well as unwanted burns to surrounding tissue.210, 211 Similar issues may come into play when treating patients with metal implants,212 patients with embedded metal fragments, or any nontissue debris that may be in the near-field of the focused ultrasound beams.
Similarly, tumors that lie near bone or implants, such as meningiomas in the brain, may be difficult to treat with focused ultrasound. The acoustic window available to reach the tumor is often constrained by bone, and the resulting defocusing and bone heating may be difficult or impossible to compensate for.
As with bone, gas that exists between the transducer and the focus can cause difficulties for treatment. Segments of bowel anterior to uterine fibroids is often a contraindication for treatment.213 Gas in the bowel will effectively stop ultrasound energy from propagating further in the tissue and can reflect the energy back toward the transducer, potentially leading to unwanted near-field heating and burns.
Challenge: Protecting sensitive structures
In many situations where focused ultrasound might be considered a treatment option, the target lies close to critical structures that constrain the geometric pathways for energy delivery. Currently, commercial treatment planning systems for FUS provide functionality to create “no-pass zones” that effectively shield sensitive structures by restricting the available sonication locations and restricting the ultrasound beam directions. In some cases, for instance where a critical structure lies in the postfocus ultrasound field, this technique may contraindicate FUS because there remains no path that can adequately cover the target. Another current solution is to restrict the minimum allowed distance between the target and nearby critical structures in order to minimize the energy that reaches the critical areas,214 however this can exclude some otherwise indicated patients.
TREATMENT DELIVERY SOLUTIONS AND FUTURE DEVELOPMENTS
Treating through and around obstacles
Some methods for avoiding obstacles such as intervening bone, bowel, or metal implants are already in clinical practice. Beam paths can be adjusted to avoid the obstruction, and beam no-pass zones can be defined in the clinical treatment planning software to help optimize these beam paths.213
As described earlier, targets for focused ultrasound often involve interference from the ribs. Two strategies have emerged to deal with this problem. The first follows directly from the idea of refocusing energy through the skull; researchers are developing methods to correct for focal beam distortion caused by the ribs.215, 216 A second strategy is to detect the location of the ribs and then selectively turn off individual transducers in the phased array, effectively refocusing the beam through the intercostal space.217, 218 This latter method is in early clinical testing by some MRgFUS manufacturers.
For the case of intervening bowl, techniques such as filling the bladder with saline can in some cases push the bowel out of the beam path. Degassed water balloons used to compress the abdomen can also be helpful.219
Volumetric sonication
Most work in reducing treatment time has focused on methods to increase the sonicated volume. Early results focused on the use of phased array transducers220 or temporal switching among predefined patterns of focal spots221 to increase the ablated volume of each individual sonication. More recent work involves optimizing the locations of the individual focal spots into concentric circular patterns so that outward heat diffusion is effectively captured in the volumetric ablation. In effect, the heat already diffusing out of one location is used to minimize the energy required at the subsequent location.204 Binary feedback can be used during sonication to optimize the sonication duration of each concentric layer.222 These techniques are able to increase the volume of necrosed tissue per unit of applied energy, reducing overall power requirements and decreasing the treatment time. They also increase the uniformity of thermal dose within the targeted tissue, reducing the likelihood of undertreatment of parts of the volume as compared to the individual sonication spot technique.204 Another possibility is to use transducers designed specifically to ablate larger volumes. Melodelima et al. report an eight-element toric transducer that can create a 19.5 mm diameter lesion in as little as 40 s.223
While large volume sonication remains a challenge in current clinical systems, there are few technical hurdles that would prevent larger sonication volumes from being included. This should help to reduce the overall time of treatment.220
Microbubbles for protecting critical structures and enhancing heating
Methods to directly shield a critical structure in the postfocus field would still allow the target to be treated without risk to the critical structure. One such method proposes to use bubbles (generated via cavitation or boiling) to shield postfocal tissue from unwanted temperature rise.224
Bubbles created in the focal zone can absorb, scatter, and reflect ultrasound energy, preventing unwanted temperature rise in postfocal tissue, especially at tissue/air or tissue/bone interfaces.225, 226 While work remains to control the density, location, and extent of the bubbles, this technique could form the basis of a method for shielding of critical structures that is more flexible than the current avoidance techniques.214
For certain applications, cavitation or injected microbubbles might be a theoretical asset. Cavitation has been shown to have the potential to locally enhance heating in the focal region.37 Likewise, the presence of injected microbubbles in tissue has been shown to reduce the power and temperature requirements for soft tissue lesioning.87, 227, 228, 229 In locations where it is difficult to deliver sufficient energy for a lesion via standard techniques, enhanced techniques using cavitation or microbubbles may sufficiently increase the treatment envelope.
Indication-specific transducer designs
High intensity ultrasound technology has also been developed for delivering thermal therapy from directly within or adjacent to a deep target volume via intraluminal, endocavity, endoscopic, laparoscopic, or percutaneous approaches. Due to the enhanced spatial control and energy penetration afforded by ultrasound, these technologies may have significant advantages over the RF, MW, laser, and cryotherapy technology as currently applied for tumor ablation and hyperthermia therapy.13, 230, 231 These ultrasound devices can direct or conform the heating volume to the target area while protecting or avoiding other tissues, and potentially treat larger volumes in shorter times. Although more invasive than external or extracorporeal HIFU devices, these are still considered minimally invasive surgical approaches. These technologies may be preferable for sites where bone or bowel are intervening or external acoustic windows are too narrow for treating deep seated targets with extracorporeal HIFU; or where localization of all power and energy propagation within the target tissue is critical; or a less complex and shorter duration procedure—albeit more invasive—is desired. Due to the size restrictions inherent to a catheter-based approach and the proximity of the particular applicator to the target region, many devices need not be geometrically or electronically focused, further reducing device and procedure complexity. Typically MRI, US, CT, or fluoroscopy techniques can be used to guide the placement of these devices. Furthermore, many of these applicator configurations include MR compatible versions that can be used and monitored in real-time using MR temperature feedback. Many of these technologies are commercially available or are in the final stages of development and testing in clinical pilot studies. Some examples of this technology as currently under development or implemented in clinical studies are reviewed (transrectal HIFU systems, either MR or US directed, and cardiac interventions are covered in other sections).
Intraductal or intraluminal high-intensity ultrasound devices have been configured with a rotating planar transducer segment at the distal end of a flexible catheter,232 which can be positioned under endoscopy and fluoroscopic guidance within tumor obstruction of the bile duct; varying applied power levels and rotation position were used to shape the thermal lesion over 360° at the site of treatment233 in human pilot studies. Makin et al. created a 32-element array intrastitial transducer integrated into a complete assembly including a coupling balloon and piercing tip. This design was shown to be capable of complete liver tumor ablation in vivo in rabbits.234, 235 There is a possibility of using dual-mode arrays to both deliver and monitor the conformal thermal ablation.236 Larger diameter (10 mm) transesophageal applicators with rotating planar transducer segments have been devised and used in pilot studies for ablation of tumor volumes of the esophagus;237 currently improved versions with phased arrays and MR compatible devices suitable for MR-guided procedures with fast MR temperature monitoring are under evaluation238 and indicate potential for precision MR directed procedures.
Transurethral ultrasound devices have been devised for fast targeted ablation of prostate cancer and BPH. Device configurations for this application include linear arrays of planar239, 240, 241 or curvilinear transducers242 with dynamic rotation or stationary multisectored tubular arrays13, 243 that can be used under MR guidance and control for accurate therapy delivery.244 Dual frequency selection or modulation has been demonstrated an effective approach for controlling rate and depth of power absorption245 to produce conformal target ablation. Recent human pilot studies demonstrate feasibility of very precise treatment of targets within the prostate using a rotating planar configuration with proven accurate MR feedback control.246
An endorectal ultrasound applicator, consisting of a multisectored tubular array, has been used for hyperthermia treatment of prostate cancer combined with external beam radiation therapy.247 Delivering hyperthermia to the whole prostate gland is achievable, with no rectal toxicity, and shown to improve survival when delivered with external beam radiation.248 MR compatible versions of this device and control algorithms for feedback control have been investigated demonstrate MR directed hyperthermia with this approach is feasible.249 There is considerable potential for this technology to be implemented for thermal targeted drug delivery to the prostate.
Catheter-based ultrasound applicators based on arrays of multisectored tubular transducer segments have been applied for interstitial and endocervical delivery of hyperthermia in conjunction with HDR brachytherapy250, 251 for treatment of prostate and cervical cancer; clinical pilot studies have demonstrated enhanced thermal penetration and spatial control compared to alternative modalities for applying interstitial hyperthermia as an adjunct to radiation or chemotherapy. High-power configurations of these 13–14 g percutaneous applicators have been evaluated in vivo for fast and large volume ablations, shaped in length and angle by adjusting power delivered to each array element and sector activation pattern, and can be directional to direct energy toward the target volume while avoiding nontargeted adjacent regions.252 Single or multiple applicators can be used under MRI guidance and thermal monitoring to target specific regions of tissue including brain253 and prostate,254 and generate conformal therapeutic heating (ablation or hyperthermia) over large and targeted volumes, with superior spatial control, target localization, and fast treatment.
As advances in endocavity and catheter-based devices coalesce with improvements in MR monitoring techniques, MR-guided minimally invasive high-intensity ultrasound is poised for clinical implementation in many sites either difficult to reach with external HIFU approaches or sites where ultrasound rivals current ablative technology as currently within the interventional radiology and surgery armamentarium. When considering drug activation or radiosensitization using heat, these minimally invasive techniques should provide an ideal modality for generating and maintaining localized temperature distributions 40 °C–43 °C for 30–60 min interval within a large volume, which can be difficult with external HIFU sources due to prefocal heating.255 In consideration of MR-guided thermal ablation, the fast treatment times and conformal target localization will make these minimally invasive high-intensity ultrasound technologies more acceptable.
IMAGE GUIDANCE AND TREATMENT PLANNING CHALLENGES
Challenge: Internal organ movement
Internal organ movement, such as that caused by respiratory motion, is a well-known problem in radiation therapy.256 With focused ultrasound, patient motion or internal organ motion can cause problems in targeting the focal spots, and can also cause artifacts in the thermometry used for real-time evaluation of the treatment.257 FUS treatments also assume a very rapid temperature gradient outside of the targeted tissue, and thus rely on precise targeting to achieve a desired effect. Just as with stereotactic radiosurgery, accurate targeting in the setting of respiratory motion becomes an issue of vital importance.258
Challenge: Treatment planning
FUS treatments proceed through several general phases. Treatment planning is a set of procedures that result in a general treatment strategy and treatment parameters. Treatment monitoring during the procedure ensures that the target is being appropriately treated and nontarget tissue remains untreated. Postsonication evaluation involves a determination of what tissue received optimal treatment and what remains to be treated.
The thermal spots generated by current FUS systems are often smaller in dimension than the targets they are used to treat. Complete target coverage may be achieved through the use of a collection of individual thermal spots arranged to cover the intended target,259 or by using a volumetric sonication method.222, 260 However, there are often constraints that can restrict the number and/or orientation of these individual thermal spots such as nerves, bone, bowel, or other anatomy that can be damaged if ultrasound passes through them. In many cases these constraints require that the transducer be positioned in particular orientations relative to the patient's anatomy. In other cases, techniques that can reorient patient anatomy may be required (one example being filling the bladder with saline in order to push the intestines out of the beam path in fibroid treatments). It may be that certain regions of a tumor remain inaccessible due to normal tissue constraints. Finally, parameters driving the ultrasound beam must be optimized for depth of focus, lesion size, angle of the transducer relative to the target, etc., in some cases requiring a correction for beams passing through bony structures such as skull.261, 262 The treatment planning results in a treatment strategy that attempts to optimize the treatment to meet its objectives while respecting these constraints.
Figure 6 shows screen captures from the treatment planning systems of two manufacturers of MRgFUS systems. Each system has functionality to localize targets, define beam paths, visualize real-time thermal data, and monitor treatments for undesirable heating away from the target areas. During treatment planning, the target is defined, along with ultrasound no-pass zones intended to protect critical structures. The system will create a proposed targeting solution for the target, and will indicate areas of the target which cannot be treated with the current targeting plan due to constraints on the treatment.
Figure 6.
Examples of treatment planning systems for MRgFUS. (a) Treatment planning for the InSightec Exablate Neuro. Planning screens allow the operator to set treatment parameters, monitor beam paths per transducer, thermal lesion location, time/temperature graphs, and ultrasound frequency spectrum. [Image courtesy of the InSightec Ltd.] (b) Treatment planning for the Philips Sonalleve MRgFUS system. This system allows the operator to monitor real-time temperature rise at the target, as well as in near-field and far-field regions [Image courtesy of Philips Healthcare].
Treatment planning on current clinical systems can take up a significant fraction of total treatment time, requiring a significant commitment from the treating physician to contour targets, define critical structures, and optimize beam directions.263 In addition, not all current clinical treatment planning can automatically optimize sonication parameters. Nonlinear propagation can have a significant effect on temperature deposition patterns, but this is not always modeled in current treatment systems.264, 265 Instead, parameters generally useful for each indication are predefined and must be optimized by hand on a sonication-by-sonication basis. Physicians not familiar with ultrasound biophysics266 may not be prepared to perform these optimization tasks.
IMAGE GUIDANCE SOLUTIONS AND FUTURE DEVELOPMENTS
Strategies to account for internal organ motion
As was discussed previously, classic PRF MR-thermometry techniques are suboptimal in the setting of patient or organ motion. Several strategies have been developed which attempt to overcome this limitation through the inclusion of a priori information regarding anatomical movement in the image.
Referenceless thermometry techniques attempt to overcome the limitations of PRF thermometry by using areas of the image distant from the intended thermal focus as a surrogate for the baseline phase of the image. The technique is still subtraction-based. A region-of-interest (ROI) outside of the heated area of the treatment is created (usually by the operator). Surface fitting is then applied to identify and remove background variations not related to temperature.267, 268 Referenceless techniques are sensitive to the selection of ROIs, and to local susceptibility effects.28 Recent work has refined the basic technique to include using the difference in PRF-thermal coefficients between fat and water to achieve a better temperature estimation at tissue boundaries.269
Multibaseline thermometry techniques trade the ROI-creation step for a preparatory imaging stage where a series of baseline thermometry images are acquired over the periodic movement cycle of the tissue of interest. These multiple baseline images are stored in a lookup table and are matched to incoming thermometry images taken during the subsequent sonication stage.270, 271 Multibaseline methods tend to work well for periodic motion, but fail in the presence of spontaneous motion that is not well represented by the presonication baseline acquisitions.28
Hybrid methods attempt to combine the advantages of both the referenceless and multibaseline techniques. This method models the total phase shift in an image as separate anatomical-induced shifts, temperature-induced shifts, and shifts due to respiratory motion, bowel filling, etc. The temperature of a given voxel is then determined by fitting the observed data to the model through minimization of a cost function. The hybrid method was shown to perform well in the setting of liver and heart, in situations of anatomical motion, and requires a smaller database of baseline images. Another strategy to account for organ motion and allow for continuous sonication is to use rapid imaging techniques combined with trajectory prediction models to update ultrasound targeting to track moving targets. One example reported by Ries et al.272 makes use of high-spatial frequency (10 Hz) 2D MR image acquisitions. The inplane position of the target is determined using optical flow registration techniques. The out-of-plane position of the target is tracked using pencil beam navigators.273 Artifacts induced in the MR-thermometry images are corrected by assuming a periodic motion profile for the targeted tissue and looking up the appropriate phase correction from a table generated in an initial training step. Figure 7 illustrates the method on an in vivo porcine kidney. Figure 7a shows the heat deposition without the motion correction system. Figure 7b shows the increased heating and steeper heating profile achieved with the motion tracking system.
Figure 7.
Color-coded temperature map overlaid on T2* weighted anatomical MR images of porcine kidneys demonstrating a real-time motion compensation technique (Ref. 272). (a) Heating deposition without motion compensation. (b) Heating deposition with motion compensation. Notice the increase in heating magnitude and sharper temperature falloff. [Images courtesy of Mario Ries, Ph.D., Laboratory for Functional and Molecular Imaging, Bordeaux, France].
MR-ARFI: MR acoustic radiation force imaging
Thermometry is not the only method for monitoring changes in tissue due to FUS. For some time elastography techniques have existed which can measure changes in mechanical tissue properties.274 It was soon determined that the acoustic radiation force exerted on tissue by ultrasound could be used as a basis for measuring these viscoelastic changes.275 More recently, MR acoustic radiation force imaging (MR-ARFI) techniques have been developed which can measure tissue displacements caused by the acoustic radiation force and create a map of local mechanical tissue properties in the form of an image.276, 277
MR-ARFI uses an ultrasound beam impinging on tissue to cause a small longitudinal displacement of the tissue. Through a technique similar to PRF-shift thermometry, the displacement in tissue can be described by
where Δφ is the phase change, γ is the gyromagnetic ratio, Ge is the displacement encoding gradient strength, and τ is the displacement encoding gradient length. The displacing ultrasound pulse is synchronized with the displacement encoding gradients and assumes the tissue has reached a steady-state displacement while the encoding gradients are turned on.276 Figure 8 illustrates the correspondence between ARFI images and MR-thermometry images during FUS ablation of an in vivo porcine liver.
Figure 8.
MR-ARFI: (a) MR-ARFI and (b) MR-thermometry images acquired in the in vivo porcine liver. Both images are small FOV EPI acquisitions superimposed on a larger FOV gradient echo image acquired a few minutes before. After visualization of the displacement focus on MR-ARFI to verify the target location (Ref. 291), a steered HIFU ablation was performed with thermal monitoring, shown in the reduced FOV image on the right (Ref. 27). MR-ARFI images require only 3 J of energy, whereas a low temperature rise test ablation would require upwards of 800 J of energy. [Images of courtesy of Dr. Andrew B. Holbrook and Dr. Kim Butts Pauly, Stanford University].
MR-ARFI-based monitoring techniques provide a second method that can be used for monitoring treatments and can be correlated with thermometry techniques to provide more confidence in the results. ARFI techniques may also be quite important in monitoring some of the nonthermal applications for FUS outlined in this paper, including drug delivery and BBB-opening.
It should be noted that ultrasound-only variants of ARFI are being developed. One such technique, harmonic motion imaging (HMI) (Refs. 5 and 278) uses both therapy and diagnostic ultrasound transducers (sometimes combined). One transducer induces an oscillating displacement of tissue in the focal zone using the radiation-force property of ultrasound. The pulse-echo transducer then detects the RF echoes from the tissue and determines the tissue displacement. Investigations have shown that this technique can discern tumor inclusions from surrounding tissue in phantoms,279 and measure increases in stiffness in tissues that have been heated to the level of coagulative necrosis.280
Ultrashort TE (UTE) MR imaging of bone
Certain indications for FUS require a refocusing of the ultrasound beams to correct for the absorption and scatter of the beams through bone.262 This in turn requires information about the structure of the bone. Cortical bone has extremely short T2* decay times (<500 μs). Current clinical MR protocols have echo times that are much longer, such that the signal from cortical bone decays and little or no signal is recovered.281 The bone therefore appears dark on the resulting images, and the bone structure is lost.
Because of this, imaging used to correct the ultrasound focus is typically provided by CT imaging which shows good bone to soft-tissue contrast. However, CT acquisition of bone information has some drawbacks; it exposes the patient to a small dose of ionizing radiation;282 it can be inefficient in some centers to send a patient for a CT when the bulk of the procedure takes place in the setting of a MR, and the patient is not necessarily in treatment position at CT, therefore requiring a coregistration step to align the CT and treatment planning MR (or ultrasound) images.
A method to image bone on MR would mitigate many of these drawbacks, and especially for MR-guided FUS provides a convenient method for recovering the required bone structure information. Several groups have been investigating the use of ultrashort TE (UTE) imaging to recover signal from bone.281, 283, 284, 285, 286, 287 UTE imaging may also help detect other short T2* components in tissue, such as calcifications in the brain.104 While technically feasible, these sequences rely on extremely fast gradient systems that are not yet widely available in the clinic. However, in the future it may become feasible to acquire the required bone structure directly from MR-imaging, eliminating the need for a separate imaging step.
Treatment planning
One potential advantage for the control of FUS treatments as compared to ionizing radiation treatments is that FUS provides real-time feedback of tissue temperature. This means it may be possible to eliminate the manual optimization of operating parameters required of the physician in current workflows. Arora et al. have developed a control system for FUS that automatically monitors the treatment against constraints for maximum target tissue temperature, avoidance structure tissue temperature, and power levels to prevent unwanted cavitation.288, 289, 290 These sorts of closed-loop, automated control systems will likely further the goal of creating a “physician-friendly” device that is more likely to be accepted in the clinical community.
SUMMARY AND CONCLUSION
The idea to use ultrasound energy for therapeutic purposes is not a new idea; it has been explored and rejected several times over the years since it was first mentioned as a possibility. However, the confluence of imaging, computing, and ultrasound technology advancement over the last 10 years has revitalized the idea and brought it close to reality in the clinic.
This paper has explored some significant challenges that MRgFUS must overcome before it can find wide acceptance in the clinic. However, as we have tried to show, most of the challenges—technical, practical, and financial, are currently being addressed and seem likely to be overcome. MRgFUS is already a clinical reality. Whether it will become a more common clinical reality depends on whether the data accumulating about its clinical and cost effectiveness will allow it to compete and win in an ever more crowded marketplace of therapeutic techniques.
Medical Physicists and Radiation Oncologists would do well to pay attention to MRgFUS and its progress. If it succeeds, it is possible MRgFUS will have a disruptive effect on current practice in certain aspects of radiation oncology, and could one day even replace ionization radiation for the subset of indications where focal treatments have been shown to be efficacious (for instance, single-fraction radiosurgery for benign tumors, functional disorders, and small numbers of metastases). However, for most indications (especially oncologic indications) it is likely that MRgFUS will be used in conjunction with radiotherapy, just as radiotherapy is often used concomitantly or adjuvantly with surgery. MRgFUS will be one of a variety of tools the treatment team will have at its disposal, helping to promote an effective, multidisciplinary, multimodality treatment strategy.
ACKNOWLEDGMENTS
Alexander Klibanov's research in FUS is supported by a grant from the National Institutes of Health (NIH CA102880).
The authors would like to thank Matt Eames, John Snell, and Arik Hananel from the Focused Ultrasound Surgery Foundation; Keyvan Farahani from the National Institutes of Health; Sham Sokka, Joy Polefrone, and Abhay Patil from Philips Healthcare; Jacob Chen and Eyal Zadicario from InSightec Ltd.; and John Hossack and Alan Matsumoto from the University of Virginia for their assistance in gathering materials for the paper. Keyvan Farahani additionally assisted with valuable suggestions on the content and structure of the paper. The authors would also like to thank Dr. Neal Kassell of the University of Virginia and the Focused Ultrasound Surgery Foundation for his “20/20 vision” regarding the clinical potential for high-intensity focused ultrasound which has motivated researchers throughout the world.
Finally, the authors would like to acknowledge the diligent efforts of the anonymous referees of this paper who have provided valuable perspective and who have positively shaped the final work.
References
- Cline H. E., Schenck J. F., Hynynen K., Watkins R. D., Souza S. P., and Jolesz F. A., “MR-guided focused ultrasound surgery,” J. Comput. Assist. Tomogr. 16, 956–965 (1992). 10.1097/00004728-199211000-00024 [DOI] [PubMed] [Google Scholar]
- Hynynen K., Darkazanli A., Unger E., and Schenck J. F., “MRI-guided noninvasive ultrasound surgery,” Med. Phys. 20, 107–115 (1993). 10.1118/1.597093 [DOI] [PubMed] [Google Scholar]
- Arthur R. M., Straube W. L., Trobaugh J. W., and Moros E. G., “Non-invasive estimation of hyperthermia temperatures with ultrasound,” Int. J. Hyperthermia 21, 589–600 (2005). 10.1080/02656730500159103 [DOI] [PubMed] [Google Scholar]
- Vaezy S., Shi X., Martin R. W., Chi E., Nelson P. I., Bailey M. R., and Crum L. A., “Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging,” Ultrasound Med. Biol. 27, 33–42 (2001). 10.1016/S0301-5629(00)00279-9 [DOI] [PubMed] [Google Scholar]
- Maleke C. and Konofagou E. E., “An all-ultrasound-based system for real-time monitoring and sonication of temperature change and ablation,” Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 164–167 (2006). 10.1109/IEMBS.2006.260845 [DOI] [PubMed] [Google Scholar]
- Guthkelch A. N. et al. , “Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial,” J. Neurooncol. 10, 271–284 (1991). 10.1007/BF00177540 [DOI] [PubMed] [Google Scholar]
- Uchida T., Shoji S., Nakano M., Hongo S., Nitta M., Usui Y., and Nagata Y., “High-intensity focused ultrasound as salvage therapy for patients with recurrent prostate cancer after external beam radiation, brachytherapy or proton therapy,” BJU Int. 107, 378–382 (2011). 10.1111/j.1464-410X.2010.09518.x [DOI] [PubMed] [Google Scholar]
- Kinoshita M., McDannold N., Jolesz F. A., and Hynynen K., “Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound,” Biochem. Biophys. Res. Commun. 340, 1085–1090 (2006). 10.1016/j.bbrc.2005.12.112 [DOI] [PubMed] [Google Scholar]
- Grossman L., Brock-Abraham C., Carbone N., Dodds E., Kluger J., Park A., Rawlings N., Suddath C., Sun F., Thompson M., Walsh B., and Webley K., “The 50 best inventions,” Time (Time, Inc., New York, 2011). [Google Scholar]
- Whitaker L., “Body & mind: Giving fibroids the heat,” Time (Time, Inc., New York, 2006). [Google Scholar]
- Hynynen K., Damianou C., Darkazanli A., Unger E., and Schenck J. F., “The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery,” Ultrasound Med. Biol. 19, 91–92 (1993). 10.1016/0301-5629(93)90022-G [DOI] [PubMed] [Google Scholar]
- Jolesz F. A. and McDannold N., “Current status and future potential of MRI-guided focused ultrasound surgery,” J. Magn. Reson. Imaging 27, 391–399 (2008). 10.1002/jmri.21261 [DOI] [PubMed] [Google Scholar]
- Diederich C. J., Stafford R. J., Nau W. H., Burdette E. C., Price R. E., and Hazle J. D., “Transurethral ultrasound applicators with directional heating patterns for prostate thermal therapy: In vivo evaluation using magnetic resonance thermometry,” Med. Phys. 31, 405–413 (2004). 10.1118/1.1639959 [DOI] [PubMed] [Google Scholar]
- Curry T. S., Dowdey J. E., and Murray R. C., Christensen's Physics of Diagnostic Radiology, 4th ed. (Lippincott Williams & Wilkins, Philadelphia, PA, 1990). [Google Scholar]
- Smith N. B. and Webb A., Introduction to Medical Imaging: Physics, Engineering, and Clinical Applications. (Cambridge University Press, New York, 2011). [Google Scholar]
- Haar G. T. and Coussios C., “High intensity focused ultrasound: Physical principles and devices,” Int. J. Hyperthermia 23, 89–104 (2007). 10.1080/02656730601186138 [DOI] [PubMed] [Google Scholar]
- Zderic V., Keshavarzi A., Andrew M. A., Vaezy S., and Martin R. W., “Attenuation of porcine tissues in vivo after high-intensity ultrasound treatment,” Ultrasound Med. Biol. 30, 61–66 (2004). 10.1016/j.ultrasmedbio.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Ferraro G. A., De Francesco F., Nicoletti G., Rossano F., and D’Andrea F., “Histologic effects of external ultrasound-assisted lipectomy on adipose tissue,” Aesthetic Plast. Surg. 32, 111–115 (2008). 10.1007/s00266-007-9031-8 [DOI] [PubMed] [Google Scholar]
- Jewell M. L., Solish N. J., and Desilets C. S., “Noninvasive body sculpting technologies with an emphasis on high-intensity focused ultrasound,” Aesthetic Plast. Surg. 35, 901–912 (2011). 10.1007/s00266-011-9700-5 [DOI] [PubMed] [Google Scholar]
- Burgess S. E., Iwamoto T., Coleman D. J., Lizzi F. L., Driller J., and Rosado A., “Histologic changes in porcine eyes treated with high-intensity focused ultrasound,” Ann. Ophthalmol. 19, 133–138 (1987). [PubMed] [Google Scholar]
- Coleman D. J., Lizzi F. L., Torpey J. H., Burgess S. E., Driller J., Rosado A., and Nguyen H. T., “Treatment of experimental lens capsular tears with intense focused ultrasound,” Br. J. Ophthalmol. 69, 645–649 (1985). 10.1136/bjo.69.9.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. H., Vogelsang B., Rondeau M. J., and Coleman D. J., “Therapeutic ultrasound for the treatment of glaucoma,” Am. J. Ophthalmol. 111, 327–337 (1991). [DOI] [PubMed] [Google Scholar]
- de Senneville B. D., Mougenot C., Quesson B., Dragonu I., Grenier N., and Moonen C. T., “MR thermometry for monitoring tumor ablation,” Eur. Radiol. 17, 2401–2410 (2007). 10.1007/s00330-007-0646-6 [DOI] [PubMed] [Google Scholar]
- Ishihara Y., Calderon A., Watanabe H., Okamoto K., Suzuki Y., and Kuroda K., “A precise and fast temperature mapping using water proton chemical shift,” Magn. Reson. Med. 34, 814–823 (1995). 10.1002/mrm.1910340606 [DOI] [PubMed] [Google Scholar]
- Rieke V. and Butts Pauly K., “MR thermometry,” J. Magn. Reson. Imaging 27, 376–390 (2008). 10.1002/jmri.21265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. D., Hinks R. S., and Henkelman R. M., “Ex vivo tissue-type independence in proton-resonance frequency shift MR thermometry,” Magn. Reson. Med. 40, 454–459 (1998). 10.1002/mrm.1910400316 [DOI] [PubMed] [Google Scholar]
- Holbrook A. B., Santos J. M., Kaye E., Rieke V., and Pauly K. B., “Real-time MR thermometry for monitoring HIFU ablations of the liver,” Magn. Reson. Med. 63, 365–373 (2010). 10.1002/mrm.22206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Senneville B. D., Roujol S., Moonen C., and Ries M., “Motion correction in MR thermometry of abdominal organs: A comparison of the referenceless vs. the multibaseline approach,” Magn. Reson. Med. 64, 1373–1381 (2010). 10.1002/mrm.22514 [DOI] [PubMed] [Google Scholar]
- Quesson B., Laurent C., Maclair G., de Senneville B. D., Mougenot C., Ries M., Carteret T., Rullier A., and Moonen C. T., “Real-time volumetric MRI thermometry of focused ultrasound ablation in vivo: a feasibility study in pig liver and kidney,” NMR Biomed. 24, 145–153 (2011). 10.1002/nbm.1563 [DOI] [PubMed] [Google Scholar]
- Kuroda K., Oshio K., Mulkern R. V., and Jolesz F. A., “Optimization of chemical shift selective suppression of fat,” Magn. Reson. Med. 40, 505–510 (1998). 10.1002/mrm.1910400402 [DOI] [PubMed] [Google Scholar]
- de Zwart J. A., Vimeux F. C., Delalande C., Canioni P., and Moonen C. T., “Fast lipid-suppressed MR temperature mapping with echo-shifted gradient-echo imaging and spectral-spatial excitation,” Magn. Reson. Med. 42, 53–59 (1999). [DOI] [PubMed] [Google Scholar]
- Cline H. E., Hynynen K., Hardy C. J., Watkins R. D., Schenck J. F., and Jolesz F. A., “MR temperature mapping of focused ultrasound surgery,” Magn. Reson. Med. 31, 628–636 (1994). 10.1002/mrm.1910310608 [DOI] [PubMed] [Google Scholar]
- Hynynen K., Vykhodtseva N. I., Chung A. H., Sorrentino V., Colucci V., and Jolesz F. A., “Thermal effects of focused ultrasound on the brain: Determination with MR imaging,” Radiology 204, 247–253 (1997). [DOI] [PubMed] [Google Scholar]
- McDannold N., Tempany C., Jolesz F., and Hynynen K., “Evaluation of referenceless thermometry in MRI-guided focused ultrasound surgery of uterine fibroids,” J. Magn. Reson. Imaging 28, 1026–1032 (2008). 10.1002/jmri.21506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J., “Historical perspectives of hyperthermia,” in Introduction to Hyperthermic Oncology, edited by Overgaard J. (Taylor & Francis, London, 1984), Vol. 2. [Google Scholar]
- Chen H., Li X., and Wan M., “The inception of cavitation bubble clouds induced by high-intensity focused ultrasound,” Ultrasonics 44(1), e427–e429 (2006). 10.1016/j.ultras.2006.05.021 [DOI] [PubMed] [Google Scholar]
- Coussios C. C., Farny C. H., Ter Haar G., and Roy R. A., “Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU),” Int. J. Hyperthermia 23, 105–120 (2007). 10.1080/02656730701194131 [DOI] [PubMed] [Google Scholar]
- Hariharan P., Myers M. R., Robinson R. A., Maruvada S. H., Sliwa J., and Banerjee R. K., “Characterization of high intensity focused ultrasound transducers using acoustic streaming,” J. Acoust. Soc. Am. 123, 1706–1719 (2008). 10.1121/1.2835662 [DOI] [PubMed] [Google Scholar]
- Myers M. R., Hariharan P., and Banerjee R. K., “Direct methods for characterizing high-intensity focused ultrasound transducers using acoustic streaming,” J. Acoust. Soc. Am. 124, 1790–1802 (2008). 10.1121/1.2957937 [DOI] [PubMed] [Google Scholar]
- Hall E. J. and Giaccia A. J., Radiobiology for the Radiologist (Lippincott Williams & Wilkins, Philadelphia, PA, 2006). [Google Scholar]
- Frenkel V., Oberoi J., Stone M. J., Park M., Deng C., Wood B. J., Neeman Z., M.HorneIII, and Li K. C., “Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model,” Radiology 239, 86–93 (2006). 10.1148/radiol.2391042181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K., Colucci V., Chung A., and Jolesz F., “Noninvasive arterial occlusion using MRI-guided focused ultrasound,” Ultrasound Med. Biol. 22, 1071–1077 (1996). 10.1016/S0301-5629(96)00143-3 [DOI] [PubMed] [Google Scholar]
- Vaezy S. and Zderic V., “Hemorrhage control using high intensity focused ultrasound,” Int. J. Hyperthermia 23, 203–211 (2007). 10.1080/02656730601169779 [DOI] [PubMed] [Google Scholar]
- Petin V. G., Komarov V. P., and Skvortzov V. G., “Combined action of ultrasound and ionizing radiation on yeast cells,” Radiat. Environ. Biophys. 18, 45–55 (1980). 10.1007/BF01324373 [DOI] [PubMed] [Google Scholar]
- Overgaard J., “The biological bases for the clinical application of hyperthermia as an adjuvant to radiotherapy,” Prog. Clin. Biol. Res. 132D, 205–216 (1983). [PubMed] [Google Scholar]
- Hurwitz M. D., “Today's thermal therapy: Not your father's hyperthermia: Challenges and opportunities in application of hyperthermia for the 21st century cancer patient,” Am. J. Clin. Oncol. 33, 96–100 (2010). 10.1097/COC.0b013e3181817a75 [DOI] [PubMed] [Google Scholar]
- Zhou Y. F., “High intensity focused ultrasound in clinical tumor ablation,” World J. Clin. Oncol. 2, 8–27 (2011). 10.5306/wjco.v2.i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M., Wu J., and Chiu J. F., “Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents,” J. Acoust. Soc. Am. 105, 2951–2957 (1999). 10.1121/1.426908 [DOI] [PubMed] [Google Scholar]
- McDannold N., Vykhodtseva N., Raymond S., Jolesz F. A., and Hynynen K., “MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits,” Ultrasound Med. Biol. 31, 1527–1537 (2005). 10.1016/j.ultrasmedbio.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Frenkel V. and Li K. C., “Potential role of pulsed-high intensity focused ultrasound in gene therapy,” Future Oncol. 2, 111–119 (2006). 10.2217/14796694.2.1.111 [DOI] [PubMed] [Google Scholar]
- Wood R. and Loomis A., “The physical and biological effects of high frequency sound waves of great intensity,” London Edinburgh Dublin Philos. Mag. J. Sci. 1927, 417–436 (1927). [Google Scholar]
- Lynn J. G. and Putnam T. J., “Histology of cerebral lesions produced by focused ultrasound,” Am. J. Pathol. 20, 637–649 (1944). [PMC free article] [PubMed] [Google Scholar]
- Fry W. J., W. H.MosbergJr., Barnard J. W., and Fry F. J., “Production of focal destructive lesions in the central nervous system with ultrasound,” J, Neurosurg 11, 471–478 (1954). 10.3171/jns.1954.11.5.0471 [DOI] [PubMed] [Google Scholar]
- Lele P. P., “Production of deep focal lesions by focused ultrasound–Current status,” Ultrasonics 5, 105–112 (1967). 10.1016/S0041-624X(67)80011-8 [DOI] [PubMed] [Google Scholar]
- Fry F. J. and Johnson L. K., “Tumor irradiation with intense ultrasound,” Ultrasound Med. Biol. 4, 337–341 (1978). 10.1016/0301-5629(78)90022-4 [DOI] [PubMed] [Google Scholar]
- Leksell L., “The stereotaxic method and radiosurgery of the brain,” Acta Chir. Scand. 102, 316–319 (1951). [PubMed] [Google Scholar]
- Fry F. J. and Barger J. E., “Acoustical properties of the human skull,” J. Acoust. Soc. Am. 63, 1576–1590 (1978). 10.1121/1.381852 [DOI] [PubMed] [Google Scholar]
- Fry F. J., Goss S. A., and Patrick J. T., “Transkull focal lesions in cat brain produced by ultrasound,” J. Neurosurg. 54, 659–663 (1981). 10.3171/jns.1981.54.5.0659 [DOI] [PubMed] [Google Scholar]
- Hynynen K. and Jolesz F. A., “Demonstration of potential noninvasive ultrasound brain therapy through an intact skull,” Ultrasound Med. Biol. 24, 275–283 (1998). 10.1016/S0301-5629(97)00269-X [DOI] [PubMed] [Google Scholar]
- Phillips D. J., Smith S. W., Ramm O. T., and Thurstone F. L., “A phase compensation technique for B-mode echoencephalography,” in Ultrasound in Medicine, edited by White D. (Plenum Press, New York, 1975), pp. 395–404. [Google Scholar]
- Parker D. L., Smith V., Sheldon P., Crooks L. E., and Fussell L., “Temperature distribution measurements in two-dimensional NMR imaging,” Med. Phys. 10, 321–325 (1983). 10.1118/1.595307 [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Susani M., and Marberger M., “Thermal ablation of BPH with transrectal high-intensity focused ultrasound,” Prog. Clin. Biol. Res. 386, 473–478 (1994). [PubMed] [Google Scholar]
- Gelet A., Chapelon J. Y., Bouvier R., Souchon R., Pangaud C., Abdelrahim A. F., Cathignol D., and Dubernard J. M., “Treatment of prostate cancer with transrectal focused ultrasound: Early clinical experience,” Eur. Urol. 29, 174–183 (1996). [PubMed] [Google Scholar]
- Chapelon J. Y., Ribault M., Vernier F., Souchon R., and Gelet A., “Treatment of localised prostate cancer with transrectal high intensity focused ultrasound,” Eur. J. Ultrasound 9, 31–38 (1999). 10.1016/S0929-8266(99)00005-1 [DOI] [PubMed] [Google Scholar]
- Cline H. E., Hynynen K., Watkins R. D., Adams W. J., Schenck J. F., Ettinger R. H., Freund W. R., Vetro J. P., and Jolesz F. A., “Focused US system for MR imaging-guided tumor ablation,” Radiology 194, 731–737 (1995). [DOI] [PubMed] [Google Scholar]
- Clement G. T., White J., and Hynynen K., “Investigation of a large-area phased array for focused ultrasound surgery through the skull,” Phys. Med. Biol. 45, 1071–1083 (2000). 10.1088/0031-9155/45/4/319 [DOI] [PubMed] [Google Scholar]
- Aubry J. F., Tanter M., Gerber J., Thomas J. L., and Fink M., “Optimal focusing by spatio-temporal inverse filter. II. Experiments. Application to focusing through absorbing and reverberating media,” J. Acoust. Soc. Am. 110, 48–58 (2001). 10.1121/1.1377052 [DOI] [PubMed] [Google Scholar]
- Wu F., Wang Z. B., Chen W. Z., Zhu H., Bai J., Zou J. Z., Li K. Q., Jin C. B., Xie F. L., and Su H. B., “Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma,” Ann. Surg. Oncol. 11, 1061–1069 (2004). 10.1245/ASO.2004.02.026 [DOI] [PubMed] [Google Scholar]
- Wu F., Wang Z. B., Chen W. Z., Bai J., Zhu H., and Qiao T. Y., “Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy,” J. Urol. 170, 2237–2240 (2003). 10.1097/01.ju.0000097123.34790.70 [DOI] [PubMed] [Google Scholar]
- Chen W., Wang Z., Wu F., Zhu H., Zou J., Bai J., Li K., and Xie F., “High intensity focused ultrasound in the treatment of primary malignant bone tumor,” Zhonghua Zhong Liu Za Zhi 24, 612–615 (2002). [PubMed] [Google Scholar]
- Wu F., Wang Z. B., Zhu H., Chen W. Z., Zou J. Z., Bai J., Li K. Q., Jin C. B., Xie F. L., and Su H. B., “Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: Initial experience,” Radiology 236, 1034–1040 (2005). 10.1148/radiol.2362041105 [DOI] [PubMed] [Google Scholar]
- Wu F., Chen W. Z., Bai J., Zou J. Z., Wang Z. L., Zhu H., and Wang Z. B., “Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound,” Ultrasound Med. Biol. 27, 1099–1106 (2001). 10.1016/S0301-5629(01)00389-1 [DOI] [PubMed] [Google Scholar]
- Chen W., Wang Z., Wu F., Bai J., Zhu H., Zou J., Li K., and Xie F., “High intensity focused ultrasound alone for malignant solid tumors,” Zhonghua Zhong Liu Za Zhi 24, 278–281 (2002). [PubMed] [Google Scholar]
- Wu F., Wang Z. B., Chen W. Z., Wang W., Gui Y., Zhang M., Zheng G., Zhou Y., Xu G., Li M., Zhang C., Ye H., and Feng R., “Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview,” Ultrason. Sonochem. 11, 149–154 (2004). 10.1016/j.ultsonch.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Fujishiro S., Mitsumori M., Nishimura Y., Okuno Y., Nagata Y., Hiraoka M., Sano T., Marume T., and Takayama N., “Increased heating efficiency of hyperthermia using an ultrasound contrast agent: A phantom study,” Int. J. Hyperthermia 14, 495–502 (1998). 10.3109/02656739809018250 [DOI] [PubMed] [Google Scholar]
- Yu T., Wang G., Hu K., Ma P., Bai J., and Wang Z., “A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: A rabbit kidney study,” Urol. Res. 32, 14–19 (2004). 10.1007/s00240-003-0362-x [DOI] [PubMed] [Google Scholar]
- Lenard Z. M., McDannold N. J., Fennessy F. M., Stewart E. A., Jolesz F. A., Hynynen K., and Tempany C. M., “Uterine leiomyomas: MR imaging-guided focused ultrasound surgery–imaging predictors of success,” Radiology 249, 187–194 (2008). 10.1148/radiol.2491071600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaki K., Fukunishi H., Funaki T., Sawada K., Kaji Y., and Maruo T., “Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: Relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images,” Am. J. Obstet. Gynecol. 196, 184.e1–184.e6 (2007). 10.1016/j.ajog.2006.08.030 [DOI] [PubMed] [Google Scholar]
- Taran F. A., Tempany C. M., Regan L., Inbar Y., Revel A., and Stewart E. A., “Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas,” Ultrasound Obstet. Gynecol. 34, 572–578 (2009). 10.1002/uog.7435 [DOI] [PubMed] [Google Scholar]
- Zaher S., Gedroyc W. M., and Regan L., “Patient suitability for magnetic resonance guided focused ultrasound surgery of uterine fibroids,” Eur. J. Obstet. Gynecol. Reprod. Biol. 143, 98–102 (2009). 10.1016/j.ejogrb.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Ripamonti C. and Fulfaro F., “Malignant bone pain: Pathophysiology and treatments,” Curr. Rev. Pain. 4, 187–196 (2000). 10.1007/s11916-000-0078-3 [DOI] [PubMed] [Google Scholar]
- Catane R., Beck A., Inbar Y., Rabin T., Shabshin N., Hengst S., Pfeffer R. M., Hanannel A., Dogadkin O., Liberman B., and Kopelman D., “MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases–Preliminary clinical experience,” Ann. Oncol. 18, 163–167 (2007). 10.1093/annonc/mdl335 [DOI] [PubMed] [Google Scholar]
- Gianfelice D., Gupta C., Kucharczyk W., Bret P., Havill D., and Clemons M., “Palliative treatment of painful bone metastases with MR imaging–Guided focused ultrasound,” Radiology 249, 355–363 (2008). 10.1148/radiol.2491071523 [DOI] [PubMed] [Google Scholar]
- Liberman B., Gianfelice D., Inbar Y., Beck A., Rabin T., Shabshin N., Chander G., Hengst S., Pfeffer R., Chechick A., Hanannel A., Dogadkin O., and Catane R., “Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: A multicenter study,” Ann. Surg. Oncol. 16, 140–146 (2009). 10.1245/s10434-008-0011-2 [DOI] [PubMed] [Google Scholar]
- Khokhlova T. D., Canney M. S., Lee D., Marro K. I., Crum L. A., Khokhlova V. A., and Bailey M. R., “Magnetic resonance imaging of boiling induced by high intensity focused ultrasound,” J. Acoust. Soc. Am. 125, 2420–2431 (2009). 10.1121/1.3081393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova V. A., Bailey M. R., Reed J. A., Cunitz B. W., Kaczkowski P. J., and Crum L. A., “Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom,” J. Acoust. Soc. Am. 119, 1834–1848 (2006). 10.1121/1.2161440 [DOI] [PubMed] [Google Scholar]
- Sokka S. D., King R., and Hynynen K., “MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh,” Phys. Med. Biol. 48, 223–241 (2003). 10.1088/0031-9155/48/2/306 [DOI] [PubMed] [Google Scholar]
- Werner B., Morel A., Zadicario E., Jeanmonod D., and Martin E., “Transcranial MR-guided high intensity focused ultrasound for non-invasive functional neurosurgery,” AIP Conf. Proc. 1215, 101–104 (2010). 10.1063/1.3367106 [DOI] [Google Scholar]
- Makariou E. and Patsalides A. D., “Intracranial calcifiations,” Appl. Radiol. 38, 48–50 (2009). [Google Scholar]
- Tang S. C. and Clement G. T., “Standing-wave suppression for transcranial ultrasound by random modulation,” IEEE Trans. Biomed. Eng. 57, 203–205 (2010). 10.1109/TBME.2009.2028653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp E., Partanen A., Karczmar G. S., and Fan X., “Safety limitations of MR-HIFU treatment near interfaces: a phantom validation,” J. Appl. Clin. Med. Phys. 13, 168–175 (2012). 10.1120/jacmp.v13i2.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M. A., Huang Y., and Hynynen K., “The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model,” Phys. Med. Biol. 55, 5251–5267 (2010). 10.1088/0031-9155/55/18/001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffertshofer M., Gass A., Ringleb P., Sitzer M., Sliwka U., Els T., Sedlaczek O., Koroshetz W. J., and Hennerici M. G., “Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: Results of a phase II clinical trial,” Stroke 36, 1441–1446 (2005). 10.1161/01.STR.0000170707.86793.1a [DOI] [PubMed] [Google Scholar]
- Baron C., Aubry J. F., Tanter M., Meairs S., and Fink M., “Simulation of intracranial acoustic fields in clinical trials of sonothrombolysis,” Ultrasound Med. Biol. 35, 1148–1158 (2009). 10.1016/j.ultrasmedbio.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Shaw A. and ter Haar G., “Requirements for measurement standards in high intensity focused ultrasound (HIFU) fields,” National Physics Laboratory, Report DQL AC 015, 7–71 (2006).
- Shaw A. and Hodnett M., “Calibration and measurement issues for therapeutic ultrasound,” Ultrasonics 48, 234–252 (2008). 10.1016/j.ultras.2007.10.010 [DOI] [PubMed] [Google Scholar]
- Dewhirst M. W., Viglianti B. L., Lora-Michiels M., Hanson M., and Hoopes P. J., “Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia,” Int. J. Hyperthermia 19, 267–294 (2003). 10.1080/0265673031000119006 [DOI] [PubMed] [Google Scholar]
- Gorny K. R., Hangiandreou N. J., Hesley G. K., Gostout B. S., McGee K. P., and Felmlee J. P., “MR guided focused ultrasound: Technical acceptance measures for a clinical system,” Phys. Med. Biol. 51, 3155–3173 (2006). 10.1088/0031-9155/51/12/011 [DOI] [PubMed] [Google Scholar]
- Gyongy M. and Coussios C. C., “Passive spatial mapping of inertial cavitation during HIFU exposure,” IEEE Trans. Biomed. Eng. 57, 48–56 (2010). 10.1109/TBME.2009.2026907 [DOI] [PubMed] [Google Scholar]
- Burdin F., Tsochatzidis N. A., Guiraud P., Wilhelm A. M., and Delmas H., “Characterisation of the acoustic cavitation cloud by two laser techniques,” Ultrason. Sonochem. 6, 43–51 (1999). 10.1016/S1350-4177(98)00035-2 [DOI] [PubMed] [Google Scholar]
- Coleman A. J., Choi M. J., and Saunders J. E., “Detection of acoustic emission from cavitation in tissue during clinical extracorporeal lithotripsy,” Ultrasound Med. Biol. 22, 1079–1087 (1996). 10.1016/S0301-5629(96)00118-4 [DOI] [PubMed] [Google Scholar]
- Cleveland R. O., Sapozhnikov O. A., Bailey M. R., and Crum L. A., “A dual passive cavitation detector for localized detection of lithotripsy-induced cavitation in vitro,” J. Acoust. Soc. Am. 107, 1745–1758 (2000). 10.1121/1.428572 [DOI] [PubMed] [Google Scholar]
- Gateau J., Aubry J. F., Pernot M., Fink M., and Tanter M., “Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 517–532 (2011). 10.1109/TUFFC.2011.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A., Rees J. H., Brock C. S., Robson M. D., Gatehouse P. D., and Bydder G. M., “MRI of the brain with ultra-short echo-time pulse sequences,” Neuroradiology 45, 887–892 (2003). 10.1007/s00234-003-1076-z [DOI] [PubMed] [Google Scholar]
- Okada S., Ohaki Y., Inoue K., Kawamura T., Hayashi T., Kato T., and Kumazaki T., “Calcifications in mucinous and serous cystic ovarian tumors,” J. Nihon Med. Sch. 72, 29–33 (2005). 10.1272/jnms.72.29 [DOI] [PubMed] [Google Scholar]
- Ribeiro S. M., Ajzen S. A., and Trindade J. C., “Comparison of imaging methods for diagnosis of renal tumors and their calcifications,” Rev. Assoc. Med. Bras. 50, 403–412 (2004). 10.1590/S0104-42302004000400031 [DOI] [PubMed] [Google Scholar]
- Imhof H. and Frank P., “Pancreatic calcifications in malignant islet cell tumors,” Radiology 122, 333–337 (1977). [DOI] [PubMed] [Google Scholar]
- Dziukowa J., “Importance of radiologically detectable calcifications in the diagnosis of thyroid tumors,” Pol. Med. J. 11, 890–897 (1972). [PubMed] [Google Scholar]
- Schiffer D., Sibour F., and Vesco C., “Pathogenesis and significance of calcifications in cerebral tumors,” Minerva Neurochir. 5, 129–130 (1961). [PubMed] [Google Scholar]
- Leborgne R., “Diagnosis of tumors of the breast by simple roentgenography; calcifications in carcinomas,” Am. J. Roentgenol. Radium. Ther 65, 1–11 (1951). [PubMed] [Google Scholar]
- Sapareto S. A., “Thermal isoeffect dose: Addressing the problem of thermotolerance,” Int. J. Hyperthermia 3, 297–305 (1987). 10.3109/02656738709140400 [DOI] [PubMed] [Google Scholar]
- Borrelli M. J., Thompson L. L., Cain C. A., and Dewey W. C., “Time-temperature analysis of cell killing of BHK cells heated at temperatures in the range of 43.5 degrees C to 57.0 degrees C,” Int. J. Radiat. Oncol. Biol. Phys. 19, 389–399 (1990). 10.1016/0360-3016(90)90548-X [DOI] [PubMed] [Google Scholar]
- Pearce J., “Mathematical models of laser-induced tissue thermal damage,” Int. J. Hyperthermia 27, 741–750 (2011). 10.3109/02656736.2011.580822 [DOI] [PubMed] [Google Scholar]
- Pearce J. A., “Relationship between Arrhenius models of thermal damage and the CEM 43 thermal dose,” Proc. SPIE 7181, 718104 (2009). 10.1117/12.807999 [DOI] [Google Scholar]
- ICRP, “1990 Recommendations of the international commission on radiological protection. ICRP Publication 60,” Ann. ICRP 21, 1–201 (1991). [PubMed] [Google Scholar]
- Raab G. G. and Parr D. H., “From medical invention to clinical practice: The reimbursement challenge facing new device procedures and technology–Part 2: Coverage,” J. Am. Coll. Radiol. 3, 772–777 (2006). 10.1016/j.jacr.2006.02.028 [DOI] [PubMed] [Google Scholar]
- Raab G. G. and Parr D. H., “From medical invention to clinical practice: the reimbursement challenge facing new device procedures and technology–part 3: Payment,” J. Am. Coll. Radiol. 3, 842–850 (2006). 10.1016/j.jacr.2006.02.027 [DOI] [PubMed] [Google Scholar]
- Warmuth M., Johansson T., and Mad P., “Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer,” Eur. Urol. 58, 803–815 (2010). 10.1016/j.eururo.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Benedict S. H., De Meerleer G., Orton C. G., and Stancanello J., “Point/counterpoint. High intensity focused ultrasound may be superior to radiation therapy for the treatment of early stage prostate cancer,” Med. Phys. 38, 3909–3912 (2011). 10.1118/1.3561500 [DOI] [PubMed] [Google Scholar]
- Hynynen K., Pomeroy O., Smith D. N., Huber P. E., McDannold N. J., Kettenbach J., Baum J., Singer S., and Jolesz F. A., “MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study,” Radiology 219, 176–185 (2001). [DOI] [PubMed] [Google Scholar]
- Gianfelice D., Khiat A., Amara M., Belblidia A., and Boulanger Y., “MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings,” Breast Cancer Res. Treat. 82, 93–101 (2003). 10.1023/B:BREA.0000003956.11376.5b [DOI] [PubMed] [Google Scholar]
- Wu F., Wang Z. B., Zhu H., Chen W. Z., Zou J. Z., Bai J., Li K. Q., Jin C. B., Xie F. L., and Su H. B., “Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer,” Breast Cancer Res. Treat. 92, 51–60 (2005). 10.1007/s10549-004-5778-7 [DOI] [PubMed] [Google Scholar]
- Furusawa H., Namba K., Nakahara H., Tanaka C., Yasuda Y., Hirabara E., Imahariyama M., and Komaki K., “The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS),” Breast Cancer 14, 55–58 (2007). 10.2325/jbcs.14.55 [DOI] [PubMed] [Google Scholar]
- Zippel D. B. and Papa M. Z., “The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review,” Breast Cancer 12, 32–38 (2005). 10.2325/jbcs.12.32 [DOI] [PubMed] [Google Scholar]
- Brenin D. R., “Focused ultrasound ablation for the treatment of breast cancer,” Ann. Surg. Oncol. 18, 3088–3094 (2011). 10.1245/s10434-011-2011-x [DOI] [PubMed] [Google Scholar]
- McCormick B. C., “The politics and the ethics of breast cancer,” Brachytherapy 2, 119–120 (2003). 10.1016/S1538-4721(03)00102-8 [DOI] [PubMed] [Google Scholar]
- Crouzet S., Rebillard X., Chevallier D., Rischmann P., Pasticier G., Garcia G., Rouviere O., Chapelon J. Y., and Gelet A., “Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients,” Eur. Urol. 58, 559–566 (2010). 10.1016/j.eururo.2010.06.037 [DOI] [PubMed] [Google Scholar]
- Uchida T., Ohkusa H., Yamashita H., Shoji S., Nagata Y., Hyodo T., and Satoh T., “Five years experience of transrectal high-intensity focused ultrasound using the Sonablate device in the treatment of localized prostate cancer,” Int. J. Urol. 13, 228–233 (2006). 10.1111/j.1442-2042.2006.01272.x [DOI] [PubMed] [Google Scholar]
- Zacharakis E., Ahmed H. U., Ishaq A., Scott R., Illing R., Freeman A., Allen C., and Emberton M., “The feasibility and safety of high-intensity focused ultrasound as salvage therapy for recurrent prostate cancer following external beam radiotherapy,” BJU Int. 102, 786–792 (2008). 10.1111/j.1464-410X.2008.07775.x [DOI] [PubMed] [Google Scholar]
- Chalasani V., Martinez C. H., Lim D., and Chin J., “Salvage HIFU for recurrent prostate cancer after radiotherapy,” Prostate Cancer Prostatic Dis. 12, 124–129 (2009). 10.1038/pcan.2008.53 [DOI] [PubMed] [Google Scholar]
- Pasticier G., Chapet O., Badet L., Ardiet J. M., Poissonnier L., Murat F. J., Martin X., and Gelet A., “Salvage radiotherapy after high-intensity focused ultrasound for localized prostate cancer: early clinical results,” Urology 72, 1305–1309 (2008). 10.1016/j.urology.2008.02.064 [DOI] [PubMed] [Google Scholar]
- Liatsikos E., Bynens B., Rabenalt R., Kallidonis P., Do M., and Stolzenburg J. U., “Treatment of patients after failed high intensity focused ultrasound and radiotherapy for localized prostate cancer: salvage laparoscopic extraperitoneal radical prostatectomy,” J. Endourol. 22, 2295–2298 (2008). 10.1089/end.2008.9713 [DOI] [PubMed] [Google Scholar]
- de Meijer V. E., Verhoef C., Kuiper J. W., Alwayn I. P., Kazemier G., and Ijzermans J. N., “Radiofrequency ablation in patients with primary and secondary hepatic malignancies,” J. Gastrointest. Surg. 10, 960–973 (2006). 10.1016/j.gassur.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Haar G. T., Sinnett D., and Rivens I., “High intensity focused ultrasound–a surgical technique for the treatment of discrete liver tumours,” Phys. Med. Biol. 34, 1743–1750 (1989). 10.1088/0031-9155/34/11/021 [DOI] [PubMed] [Google Scholar]
- Moore W. E., Lopez R. M., Matthews D. E., Sheets P. W., Etchison M. R., Hurwitz A. S., Chalian A. A., Fry F. J., Vane D. W., and Grosfeld J. L., “Evaluation of high-intensity therapeutic ultrasound irradiation in the treatment of experimental hepatoma,” J. Pediatr. Surg. 24, 30–33 (1989). 10.1016/S0022-3468(89)80295-7 [DOI] [PubMed] [Google Scholar]
- Yang R., Reilly C. R., Rescorla F. J., Faught P. R., Sanghvi N. T., Fry F. J., T. D.FranklinJr., Lumeng L., and Grosfeld J. L., “High-intensity focused ultrasound in the treatment of experimental liver cancer,” Arch. Surg. 126, 1002–1009 (1991). 10.1001/archsurg.1991.01410320088012 [DOI] [PubMed] [Google Scholar]
- Yang R., Sanghvi N. T., Rescorla F. J., Galliani C. A., Fry F. J., Griffith S. L., and Grosfeld J. L., “Extracorporeal liver ablation using sonography-guided high-intensity focused ultrasound,” Invest. Radiol. 27, 796–803 (1992). 10.1097/00004424-199210000-00009 [DOI] [PubMed] [Google Scholar]
- Chen L., Rivens I., ter Haar G., Riddler S., Hill C. R., and Bensted J. P., “Histological changes in rat liver tumours treated with high-intensity focused ultrasound,” Ultrasound Med. Biol. 19, 67–74 (1993). 10.1016/0301-5629(93)90019-K [DOI] [PubMed] [Google Scholar]
- Kennedy J. E., Wu F., ter Haar G. R., Gleeson F. V., Phillips R. R., Middleton M. R., and Cranston D., “High-intensity focused ultrasound for the treatment of liver tumours,” Ultrasonics 42, 931–935 (2004). 10.1016/j.ultras.2004.01.089 [DOI] [PubMed] [Google Scholar]
- Li C. X., Xu G. L., Jiang Z. Y., Li J. J., Luo G. Y., Shan H. B., Zhang R., and Li Y., “Analysis of clinical effect of high-intensity focused ultrasound on liver cancer,” World J. Gastroenterol. 10, 2201–2204 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie T. A., Kennedy J. E., Illing R. O., Ter Haar G. R., Wu F., Phillips R. R., Friend P. J., Roberts I. S., Cranston D. W., and Middleton M. R., “High-intensity focused ultrasound ablation of liver tumours: Can radiological assessment predict the histological response?,” Br. J. Radiol. 81, 564–571 (2008). 10.1259/bjr/27118953 [DOI] [PubMed] [Google Scholar]
- Stewart E. A., Gedroyc W. M., Tempany C. M., Quade B. J., Inbar Y., Ehrenstein T., Shushan A., Hindley J. T., Goldin R. D., David M., Sklair M., and Rabinovici J., “Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique,” Am. J. Obstet. Gynecol. 189, 48–54 (2003). 10.1067/mob.2003.345 [DOI] [PubMed] [Google Scholar]
- Quesson B., Merle M., Kohler M. O., Mougenot C., Roujol S., de Senneville B. D., and Moonen C. T., “A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver,” Med. Phys. 37, 2533–2540 (2010). 10.1118/1.3413996 [DOI] [PubMed] [Google Scholar]
- Jeanmonod D., Magnin M., and Morel A., “Chronic neurogenic pain and the medial thalamotomy,” Schweiz Rundsch Med. Prax 83, 702–707 (1994). [PubMed] [Google Scholar]
- Steiner L., Forster D., Leksell L., Meyerson B. A., and Boethius J., “Gammathalamotomy in intractable pain,” Acta Neurochir. Suppl. (Wien) 52, 173–184 (1980). 10.1007/BF01402072 [DOI] [PubMed] [Google Scholar]
- Jeanmonod D., Werner B., Morel A., Michels L., Zadicario E., Schiff G., and Martin E., “Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain,” Neurosurg. Focus 32, E1 (2012). 10.3171/2011.10.FOCUS11248 [DOI] [PubMed] [Google Scholar]
- Martin E., Jeanmonod D., Morel A., Zadicario E., and Werner B., “High-intensity focused ultrasound for noninvasive functional neurosurgery,” Ann. Neurol. 66, 858–861 (2009). 10.1002/ana.21801 [DOI] [PubMed] [Google Scholar]
- Elias J., Huss D., Khaled M., Monteith S., and Frysinger R., paper presented at the a new paradigm for noninvasive lesioning and neuromodulation. Congress of Neurological Surgeons 2011 Annual Meeting Abstract, 2011. (unpublished).
- Boockvar J. A., Telfeian A., Baltuch G. H., Skolnick B., Simuni T., Stern M., Schmidt M. L., and Trojanowski J. Q., “Long-term deep brain stimulation in a patient with essential tremor: clinical response and postmortem correlation with stimulator termination sites in ventral thalamus. Case report,” J. Neurosurg. 93, 140–144 (2000). 10.3171/jns.2000.93.1.0140 [DOI] [PubMed] [Google Scholar]
- Deiner S. and Hagen J., “Parkinson's disease and deep brain stimulator placement,” Anesthesiol. Clin. 27, 391–415 (2009). 10.1016/j.anclin.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Binder D. K., Rau G. M., and Starr P. A., “Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders,” Neurosurgery 56, 722–732 (2005). 10.1227/01.NEU.0000156473.57196.7E [DOI] [PubMed] [Google Scholar]
- Kondziolka D., Whiting D., Germanwala A., and Oh M., “Hardware-related complications after placement of thalamic deep brain stimulator systems,” Stereotact. Funct. Neurosurg. 79, 228–233 (2002). 10.1159/000070836 [DOI] [PubMed] [Google Scholar]
- Sillay K. A., Larson P. S., and Starr P. A., “Deep brain stimulator hardware-related infections: incidence and management in a large series,” Neurosurgery 62, 360–366 366–367 (2008). 10.1227/01.neu.0000316002.03765.33 [DOI] [PubMed] [Google Scholar]
- Schwalb J. M., Riina H. A., Skolnick B., Jaggi J. L., Simuni T., and Baltuch G. H., “Revision of deep brain stimulator for tremor. Technical note,” J. Neurosurg. 94, 1010–1012 (2001). 10.3171/jns.2001.94.6.1010 [DOI] [PubMed] [Google Scholar]
- Dromi S., Frenkel V., Luk A., Traughber B., Angstadt M., Bur M., Poff J., Xie J., Libutti S. K., Li K. C., and Wood B. J., “Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect,” Clin. Cancer Res. 13, 2722–2727 (2007). 10.1158/1078-0432.CCR-06-2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian J. A., Dewhirst M. W., Needham D., and Viglianti B. L., “Rationale for and measurement of liposomal drug delivery with hyperthermia using non-invasive imaging techniques,” Int. J. Hyperthermia 24, 79–90 (2008). 10.1080/02656730701840147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce A. M., Vujaskovic Z., Yuan F., Needham D., and Dewhirst M. W., “Hyperthermia mediated liposomal drug delivery,” Int. J. Hyperthermia 22, 205–213 (2006). 10.1080/02656730600582956 [DOI] [PubMed] [Google Scholar]
- Kooiman K., Emmer M., Foppen-Harteveld M., van Wamel A., and de Jong N., “Increasing the endothelial layer permeability through ultrasound-activated microbubbles,” IEEE Trans. Biomed. Eng. 57, 29–32 (2010). 10.1109/TBME.2009.2030335 [DOI] [PubMed] [Google Scholar]
- Seip R., Chin C. T., Hall C. S., Raju B. I., Ghanem A., and Tiemann K., “Targeted ultrasound-mediated delivery of nanoparticles: On the development of a new HIFU-based therapy and imaging device,” IEEE Trans. Biomed. Eng. 57, 61–70 (2010). 10.1109/TBME.2009.2028874 [DOI] [PubMed] [Google Scholar]
- Dayton P. A., Zhao S., Bloch S. H., Schumann P., Penrose K., Matsunaga T. O., Zutshi R., Doinikov A., and Ferrara K. W., “Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy,” Mol. Imaging. 5, 160–174 (2006). [PMC free article] [PubMed] [Google Scholar]
- Needham D. and Dewhirst M. W., “The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors,” Adv. Drug. Deliv. Rev. 53, 285–305 (2001). 10.1016/S0169-409X(01)00233-2 [DOI] [PubMed] [Google Scholar]
- Kinoshita M., McDannold N., Jolesz F. A., and Hynynen K., “Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption,” Proc. Natl. Acad. Sci. U.S.A. 103, 11719–11723 (2006). 10.1073/pnas.0604318103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov A. L., “Preparation of targeted microbubbles: Ultrasound contrast agents for molecular imaging,” Med. Biol. Eng. Comput. 47, 875–882 (2009). 10.1007/s11517-009-0498-0 [DOI] [PubMed] [Google Scholar]
- Eisenbrey J. R., Soulen M. C., and Wheatley M. A., “Delivery of encapsulated Doxorubicin by ultrasound-mediated size reduction of drug-loaded polymer contrast agents,” IEEE Trans. Biomed. Eng. 57, 24–28 (2010). 10.1109/TBME.2009.2030497 [DOI] [PubMed] [Google Scholar]
- Klibanov A. L., Shevchenko T. I., Raju B. I., Seip R., and Chin C. T., “Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: A tool for targeted drug delivery,” J. Controlled Release 148, 13–17 (2010). 10.1016/j.jconrel.2010.07.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaweera A. R., Edwards N., Glasheen W. P., Villanueva F. S., Abbott R. D., and Kaul S., “In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells,” Circ. Res. 74, 1157–1165 (1994). 10.1161/01.RES.74.6.1157 [DOI] [PubMed] [Google Scholar]
- Patil A. V., Rychak J. J., Klibanov A. L., and Hossack J. A., “Real-time technique for improving molecular imaging and guiding drug delivery in large blood vessels: In vitro and ex vivo results,” Mol. Imaging 10, 238–247 (2011). 10.2310/7290.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N. J. and Romero I. A., “Transporting therapeutics across the blood-brain barrier,” Mol. Med. Today 2, 106–113 (1996). 10.1016/1357-4310(96)88720-X [DOI] [PubMed] [Google Scholar]
- Misra A., Ganesh S., Shahiwala A., and Shah S. P., “Drug delivery to the central nervous system: A review,” J. Pharm. Pharm. Sci. 6, 252–273 (2003). [PubMed] [Google Scholar]
- Patrick J. T., Nolting M. N., Goss S. A., Dines K. A., Clendenon J. L., Rea M. A., and Heimburger R. F., “Ultrasound and the blood-brain barrier,” Adv. Exp. Med. Biol. 267, 369–381 (1990). 10.1007/978-1-4684-5766-7_36 [DOI] [PubMed] [Google Scholar]
- Hynynen K., McDannold N., Vykhodtseva N., and Jolesz F. A., “Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits,” Radiology 220, 640–646 (2001). 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- Trubestein G., Engel C., Etzel F., Sobbe A., Cremer H., and Stumpff U., “Thrombolysis by ultrasound,” Clin. Sci. Mol. Med. Suppl. 3, 697s–698s (1976). [DOI] [PubMed] [Google Scholar]
- Medel R., Crowley R. W., McKisic M. S., Dumont A. S., and Kassell N. F., “Sonothrombolysis: An emerging modality for the management of stroke,” Neurosurgery 65, 979–993 (2009). 10.1227/01.NEU.0000350226.30382.98 [DOI] [PubMed] [Google Scholar]
- Alexandrov A. V., Wojner A. W., and Grotta J. C., “CLOTBUST: Design of a randomized trial of ultrasound-enhanced thrombolysis for acute ischemic stroke,” J. Neuroimaging 14, 108–112 (2004). [PubMed] [Google Scholar]
- Alexandrov A. V., Molina C. A., Grotta J. C., Garami Z., Ford S. R., Alvarez-Sabin J., Montaner J., Saqqur M., Demchuk A. M., Moye L. A., Hill M. D., and Wojner A. W., “Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke,” N. Engl. J. Med. 351, 2170–2178 (2004). 10.1056/NEJMoa041175 [DOI] [PubMed] [Google Scholar]
- “Prevalence of disabilities and associated health conditions among adults–United States, 1999,” MMWR Morb Mortal Wkly Rep50, 120–125 (2001). [PubMed]
- Rosamond W., Flegal K., Furie K., Go A., Greenlund K., Haase N., Hailpern S. M., Ho M., Howard V., Kissela B., Kittner S., Lloyd-Jones D., McDermott M., Meigs J., Moy C., Nichol G., O’Donnell C., Roger V., Sorlie P., Steinberger J., Thom T., Wilson M., and Hong Y., “Heart disease and stroke statistics–2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee,” Circulation 117, e25–e146 (2008). 10.1161/CIRCULATIONAHA.107.187998 [DOI] [PubMed] [Google Scholar]
- P. W.MadsenJr. and Gersten J. W., “The effect of ultrasound on conduction velocity of peripheral nerve,” Arch. Phys. Med. Rehabil. 42, 645–649 (1961). [PubMed] [Google Scholar]
- Young R. R. and Henneman E., “Reversible block of nerve conduction by ultrasound,” Arch. Neurol. 4, 83–89 (1961). 10.1001/archneur.1961.00450070085009 [DOI] [PubMed] [Google Scholar]
- Foley J. L., Little J. W., F. L.StarrIII, Frantz C., and Vaezy S., “Image-guided HIFU neurolysis of peripheral nerves to treat spasticity and pain,” Ultrasound Med. Biol. 30, 1199–1207 (2004). 10.1016/j.ultrasmedbio.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Foley J. L., Little J. W., and Vaezy S., “Image-guided high-intensity focused ultrasound for conduction block of peripheral nerves,” Ann. Biomed. Eng. 35, 109–119 (2007). 10.1007/s10439-006-9162-0 [DOI] [PubMed] [Google Scholar]
- Van Zundert J., Vanelderen P., Kessels A., and van Kleef M., “Radiofrequency treatment of facet-related pain: Evidence and controversies,” Curr. Pain Headache Rep. 16, 19–25 (2012). 10.1007/s11916-011-0237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga H. H. and Dikomey E., “Hyperthermic radiosensitization: Mode of action and clinical relevance,” Int. J. Radiat. Biol. 77, 399–408 (2001). 10.1080/09553000010024687 [DOI] [PubMed] [Google Scholar]
- Nielsen O. S., “Effect of fractionated hyperthermia on hypoxic cells in vitro,” Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 39, 73–82 (1981). 10.1080/09553008114550091 [DOI] [PubMed] [Google Scholar]
- Arcangeli G., Nervi C., Cividalli A., and Lovisolo G. A., “Problem of sequence and fractionation in the clinical application of combined heat and radiation,” Cancer Res. 44, 4857s–4863s (1984). [PubMed] [Google Scholar]
- Jernberg A., Edgren M. R., Lewensohn R., Wiksell H., and Brahme A., “Cellular effects of high-intensity focused continuous wave ultrasound alone and in combination with x-rays,” Int. J. Radiat. Biol. 77, 127–135 (2001). 10.1080/0955300010000791 [DOI] [PubMed] [Google Scholar]
- Moonen C. T., “Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound,” Clin. Cancer Res. 13, 3482–3489 (2007). 10.1158/1078-0432.CCR-07-0204 [DOI] [PubMed] [Google Scholar]
- Madio D. P., van Gelderen P., DesPres D., Olson A. W., de Zwart J. A., Fawcett T. W., Holbrook N. J., Mandel M., and Moonen C. T., “On the feasibility of MRI-guided focused ultrasound for local induction of gene expression,” J. Magn. Reson. Imaging 8, 101–104 (1998). 10.1002/jmri.1880080120 [DOI] [PubMed] [Google Scholar]
- Bekeredjian R., Grayburn P. A., and Shohet R. V., “Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine,” J. Am. Coll. Cardiol. 45, 329–335 (2005). 10.1016/j.jacc.2004.08.067 [DOI] [PubMed] [Google Scholar]
- Bekeredjian R., Behrens S., Ruef J., Dinjus E., Unger E., Baum M., and Kuecherer H. F., “Potential of gold-bound microtubules as a new ultrasound contrast agent,” Ultrasound Med. Biol. 28, 691–695 (2002). 10.1016/S0301-5629(02)00502-1 [DOI] [PubMed] [Google Scholar]
- Rahim A. A., Taylor S. L., Bush N. L., ter Haar G. R., Bamber J. C., and Porter C. D., “Spatial and acoustic pressure dependence of microbubble-mediated gene delivery targeted using focused ultrasound,” J. Gene Med. 8, 1347–1357 (2006). 10.1002/jgm.962 [DOI] [PubMed] [Google Scholar]
- Huber P. E., Mann M. J., Melo L. G., Ehsan A., Kong D., Zhang L., Rezvani M., Peschke P., Jolesz F., Dzau V. J., and Hynynen K., “Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid artery,” Gene Ther. 10, 1600–1607 (2003). 10.1038/sj.gt.3302045 [DOI] [PubMed] [Google Scholar]
- Liu Y., Kon T., Li C., and Zhong P., “High intensity focused ultrasound-induced gene activation in sublethally injured tumor cells in vitro,” J. Acoust. Soc. Am 118, 3328–3336 (2005). 10.1121/1.2041247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kon T., Li C., and Zhong P., “High intensity focused ultrasound-induced gene activation in solid tumors,” J. Acoust. Soc. Am. 120, 492–501 (2006). 10.1121/1.2205129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. C., Klibanov A. L., Wamhoff B. R., and Hossack J. A., “Targeted gene transfection from microbubbles into vascular smooth muscle cells using focused, ultrasound-mediated delivery,” Ultrasound Med. Biol. 36, 1470–1480 (2010). 10.1016/j.ultrasmedbio.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plathow C., Lohr F., Divkovic G., Rademaker G., Farhan N., Peschke P., Zuna I., Debus J., Claussen C. D., Kauczor H. U., Li C. Y., Jenne J., and Huber P., “Focal gene induction in the liver of rats by a heat-inducible promoter using focused ultrasound hyperthermia: preliminary results,” Invest. Radiol. 40, 729–735 (2005). 10.1097/01.rli.0000184763.62578.06 [DOI] [PubMed] [Google Scholar]
- Tlaxca J. L., Rychak J. J., Ernst P. B., Konkalmatt P. R., Shevchenko T. I., Pizzaro T. T., Rivera-Nieves J., Klibanov A. L., and Lawrence M. B., “Ultrasound-based molecular imaging and specific gene delivery to mesenteric vasculature by endothelial adhesion molecule targeted microbubbles in a mouse model of Crohn's disease,” J. Controlled Release 165, 216–225 (2013). 10.1016/j.jconrel.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D. J., Muratore R., Hirata K., Otsuka R., Fujikura K., Sugioka K., Marboe C., Lizzi F. L., and Homma S., “Myocardial lesion formation using high-intensity focused ultrasound,” J. Am. Soc. Echocardiogr. 19, 932–937 (2006). 10.1016/j.echo.2006.02.012 [DOI] [PubMed] [Google Scholar]
- Metzner A., Chun K. R., Neven K., Fuernkranz A., Ouyang F., Antz M., Tilz R., Zerm T., Koektuerk B., Wissner E., Koester I., Ernst S., Boczor S., Kuck K. H., and Schmidt B., “Long-term clinical outcome following pulmonary vein isolation with high-intensity focused ultrasound balloon catheters in patients with paroxysmal atrial fibrillation,” Europace 12, 188–193 (2010). 10.1093/europace/eup416 [DOI] [PubMed] [Google Scholar]
- Fry F. J., Ades H. W., and Fry W. J., “Production of reversible changes in the central nervous system by ultrasound,” Science 127, 83–84 (1958). 10.1126/science.127.3289.83 [DOI] [PubMed] [Google Scholar]
- Rinaldi P. C., Jones J. P., Reines F., and Price L. R., “Modification by focused ultrasound pulses of electrically evoked responses from an in vitro hippocampal preparation,” Brain Res. 558, 36–42 (1991). 10.1016/0006-8993(91)90711-4 [DOI] [PubMed] [Google Scholar]
- Tyler W. J., Tufail Y., Finsterwald M., Tauchmann M. L., Olson E. J., and Majestic C., “Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound,” PLoS One 3, e3511 (2008). 10.1371/journal.pone.0003511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y., Yoshihiro A., Pati S., Li M. M., and Tyler W. J., “Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound,” Nat. Protoc. 6, 1453–1470 (2011). 10.1038/nprot.2011.371 [DOI] [PubMed] [Google Scholar]
- Kohler M. O., Mougenot C., Quesson B., Enholm J., Le Bail B., Laurent C., Moonen C. T., and Ehnholm G. J., “Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry,” Med. Phys. 36, 3521–3535 (2009). 10.1118/1.3152112 [DOI] [PubMed] [Google Scholar]
- Agarwal R., Bergey M., Sonnad S., Butowsky H., Bhargavan M., and Bleshman M. H., “Inpatient CT and MRI utilization: Trends in the academic hospital setting,” J. Am. Coll. Radiol. 7, 949–955 (2010). 10.1016/j.jacr.2010.08.015 [DOI] [PubMed] [Google Scholar]
- Korogi Y. and Takahashi M., “Cost containment and diffusion of MRI: oil and water?. Japanese experience,” Eur. Radiol. 7(5), 256–258 (1997). 10.1007/PL00006904 [DOI] [PubMed] [Google Scholar]
- Hendee W. R. and Ritenour E. R., Medical Imaging Physics, 4th ed. (Wiley-Liss, New York, 2002). [Google Scholar]
- Aubry J. F., Tanter M., Pernot M., Thomas J. L., and Fink M., “Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans,” J. Acoust. Soc. Am. 113, 84–93 (2003). 10.1121/1.1529663 [DOI] [PubMed] [Google Scholar]
- Hynynen K. and DeYoung D., “Temperature elevation at muscle-bone interface during scanned, focused ultrasound hyperthermia,” Int. J. Hyperthermia 4, 267–279 (1988). 10.3109/02656738809051103 [DOI] [PubMed] [Google Scholar]
- Connor C. W. and Hynynen K., “Patterns of thermal deposition in the skull during transcranial focused ultrasound surgery,” IEEE Trans. Biomed. Eng. 51, 1693–1706 (2004). 10.1109/TBME.2004.831516 [DOI] [PubMed] [Google Scholar]
- Smith N. B., Temkin J. M., Shapiro F., and Hynynen K., “Thermal effects of focused ultrasound energy on bone tissue,” Ultrasound Med. Biol. 27, 1427–1433 (2001). 10.1016/S0301-5629(01)00454-9 [DOI] [PubMed] [Google Scholar]
- Lehmann J. F., Brunne G. D., Martinis A. J., and McMillan J. A., “Ultrasonic effects as demonstrated in live pigs with surgical metallic implants,” Arch. Phys. Med. Rehabil. 40, 483–488 (1959). [PubMed] [Google Scholar]
- Yoon S. W., Lee C., Cha S. H., Yu J. S., Na Y. J., Kim K. A., Jung S. G., and Kim S. J., “Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: A pictorial guide to relevant findings in screening pelvic MRI,” Eur. Radiol. 18, 2997–3006 (2008). 10.1007/s00330-008-1086-7 [DOI] [PubMed] [Google Scholar]
- Hindley J., Gedroyc W. M., Regan L., Stewart E., Tempany C., Hynyen K., McDannold N., Inbar Y., Itzchak Y., Rabinovici J., Kim H. S., Geschwind J. F., Hesley G., Gostout B., Ehrenstein T., Hengst S., Sklair-Levy M., Shushan A., and Jolesz F., “MRI guidance of focused ultrasound therapy of uterine fibroids: Early results,” AJR Am. J. Roentgenol. 183, 1713–1719 (2004). 10.2214/ajr.183.6.01831713 [DOI] [PubMed] [Google Scholar]
- Liu H. L., McDannold N., and Hynynen K., “Focal beam distortion and treatment planning in abdominal focused ultrasound surgery,” Med. Phys. 32, 1270–1280 (2005). 10.1118/1.1895525 [DOI] [PubMed] [Google Scholar]
- Liu H. L., Hsu C. L., Huang S. M., and Hsi Y. W., “Focal beam distortion and treatment planning for transrib focused ultrasound thermal therapy: A feasibility study using a two-dimensional ultrasound phased array,” Med. Phys. 37, 848–860 (2010). 10.1118/1.3298009 [DOI] [PubMed] [Google Scholar]
- Tanter M., Pernot M., Aubry J.-F., Montaldo G., Marquet F., and Fink M., “Compensating for bone interfaces and respiratory motion in high-intensity focused ultrasound,” Int. J. Hyperthermia 23, 141–151 (2007). 10.1080/02656730701209996 [DOI] [PubMed] [Google Scholar]
- Yao Y. and Ebbini E. S., “Refocusing dual-mode ultrasound arrays in the presence of strongly scattering obstacles,” IEEE Ultrasonics Symposium, 239–242 (2004). 10.1109/ULTSYM.2004.1417711 [DOI] [Google Scholar]
- Zhang L., Chen W. Z., Liu Y. J., Hu X., Zhou K., Chen L., Peng S., Zhu H., Zou H. L., Bai J., and Wang Z. B., “Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus,” Eur. J. Radiol. 73, 396–403 (2010). 10.1016/j.ejrad.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Fan X. and Hynynen K., “Ultrasound surgery using multiple sonications–treatment time considerations,” Ultrasound Med. Biol. 22, 471–482 (1996). 10.1016/0301-5629(96)00026-9 [DOI] [PubMed] [Google Scholar]
- Daum D. R. and Hynynen K., “Thermal dose optimization via temporal switching in ultrasound surgery,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 45, 208–215 (1998). 10.1109/58.646926 [DOI] [PubMed] [Google Scholar]
- Enholm J. K., Kohler M. O., Quesson B., Mougenot C., Moonen C. T., and Sokka S. D., “Improved volumetric MR-HIFU ablation by robust binary feedback control,” IEEE Trans. Biomed. Eng. 57, 103–113 (2010). 10.1109/TBME.2009.2034636 [DOI] [PubMed] [Google Scholar]
- Melodelima D., N’Djin W. A., Parmentier H., Rivoire M., and Chapelon J. Y., “Toric HIFU transducer for large thermal ablation,” Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 230–233 (2007). 10.1109/IEMBS.2007.4352265 [DOI] [PubMed] [Google Scholar]
- Zderic V., Foley J., Luo W., and Vaezy S., “Prevention of post-focal thermal damage by formation of bubbles at the focus during high intensity focused ultrasound therapy,” Med. Phys. 35, 4292–4299 (2008). 10.1118/1.2975149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. L. and ter Haar G. R., “Temperature rise recorded during lesion formation by high-intensity focused ultrasound,” Ultrasound Med. Biol. 23, 299–306 (1997). 10.1016/S0301-5629(96)00198-6 [DOI] [PubMed] [Google Scholar]
- Watkin N. A., ter Haar G. R., and Rivens I., “The intensity dependence of the site of maximal energy deposition in focused ultrasound surgery,” Ultrasound Med. Biol. 22, 483–491 (1996). 10.1016/0301-5629(95)02062-4 [DOI] [PubMed] [Google Scholar]
- Holt R. G. and Roy R. A., “Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material,” Ultrasound Med. Biol. 27, 1399–1412 (2001). 10.1016/S0301-5629(01)00438-0 [DOI] [PubMed] [Google Scholar]
- McDannold N. J., Vykhodtseva N. I., and Hynynen K., “Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits,” Radiology 241, 95–106 (2006). 10.1148/radiol.2411051170 [DOI] [PubMed] [Google Scholar]
- Kopelman D., Inbar Y., Hanannel A., Freundlich D., Vitek S., Schmidt R., Sokolov A., Hatoum O. A., and Rabinovici J., “Magnetic resonance-guided focused ultrasound surgery using an enhanced sonication technique in a pig muscle model,” Eur. J. Radiol. 59, 190–197 (2006). 10.1016/j.ejrad.2006.04.018 [DOI] [PubMed] [Google Scholar]
- Stauffer P. R., Diederich C. J., and Seegenschmiedt M. H., “Interstitial heating technologies,” in Principles and Practices of Thermoradiotherapy and Thermochemotherapy, edited by Seegenschmiedt M. H., Fessenden P., and Vernon C. C. (Springer-Verlag, Berlin, 1995). [Google Scholar]
- Lafon C., Melodelima D., Salomir R., and Chapelon J. Y., “Interstitial devices for minimally invasive thermal ablation by high-intensity ultrasound,” Int. J. Hyperthermia 23, 153–163 (2007). 10.1080/02656730601173029 [DOI] [PubMed] [Google Scholar]
- Lafon C., Chapelon J. Y., Prat F., Gorry F., Margonari J., Theillere Y., and Cathignol D., “Design and preliminary results of an ultrasound applicator for interstitial thermal coagulation,” Ultrasound Med. Biol. 24, 113–122 (1998). 10.1016/S0301-5629(97)00203-2 [DOI] [PubMed] [Google Scholar]
- Lafon C., de L., Theillere Y., Prat F., Chapelon J. Y., and Cathignol D., “Optimizing the shape of ultrasound transducers for interstitial thermal ablation,” Med. Phys. 29, 290–297 (2002). 10.1118/1.1445416 [DOI] [PubMed] [Google Scholar]
- Mast T. D., Barthe P. G., Makin I. R., Slayton M. H., Karunakaran C. P., Burgess M. T., Alqadah A., and Rudich S. M., “Treatment of rabbit liver cancer in vivo using miniaturized image-ablate ultrasound arrays,” Ultrasound Med. Biol. 37, 1609–1621 (2011). 10.1016/j.ultrasmedbio.2011.05.850 [DOI] [PubMed] [Google Scholar]
- Makin I. R., Mast T. D., Faidi W., Runk M. M., Barthe P. G., and Slayton M. H., “Miniaturized ultrasound arrays for interstitial ablation and imaging,” Ultrasound Med. Biol. 31, 1539–1550 (2005). 10.1016/j.ultrasmedbio.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Owen N. R., Chapelon J. Y., Bouchoux G., Berriet R., Fleury G., and Lafon C., “Dual-mode transducers for ultrasound imaging and thermal therapy,” Ultrasonics 50, 216–220 (2010). 10.1016/j.ultras.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Melodelima D., Prat F., Fritsch J., Theillere Y., and Cathignol D., “Treatment of esophageal tumors using high intensity intraluminal ultrasound: First clinical results,” J. Trans. Med. 6, 28 (2008). 10.1186/1479-5876-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melodelima D., Salomir R., Chapelon J. Y., Theillere Y., Moonen C., and Cathignol D., “Intraluminal high intensity ultrasound treatment in the esophagus under fast MR temperature mapping: in vivo studies,” Magn. Reson. Med. 54, 975–982 (2005). 10.1002/mrm.20638 [DOI] [PubMed] [Google Scholar]
- Ross A. B., Diederich C. J., Nau W. H., Gill H., Bouley D. M., Daniel B., Rieke V., Butts K., and Sommer G., “Highly directional transurethral ultrasound applicators with rotational control for MRI guided prostatic thermal therapy,” Phys. Med. Biol. 49, 189–204 (2004). 10.1088/0031-9155/49/2/002 [DOI] [PubMed] [Google Scholar]
- Chopra R., Baker N., Choy V., Boyes A., Tang K., Bradwell D., and Bronskill M. J., “MRI-compatible transurethral ultrasound system for the treatment of localized prostate cancer using rotational control,” Med. Phys. 35, 1346–1357 (2008). 10.1118/1.2841937 [DOI] [PubMed] [Google Scholar]
- Chopra R., Burtnyk M., N’Djin W. A., and Bronskill M., “MRI-controlled transurethral ultrasound therapy for localised prostate cancer,” Int. J. Hyperthermia 26, 804–821 (2010). 10.3109/02656736.2010.503670 [DOI] [PubMed] [Google Scholar]
- Ross A. B., Diederich C. J., Nau W. H., Rieke V., Butts R. K., Sommer G., Gill H., and Bouley D. M., “Curvilinear transurethral ultrasound applicator for selective prostate thermal therapy,” Med. Phys. 32, 1555–1565 (2005). 10.1118/1.1924314 [DOI] [PubMed] [Google Scholar]
- Kinsey A. M., Diederich C. J., Rieke V., Nau W. H., Pauly K. B., Bouley D., and Sommer G., “Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy: In vivo evaluation under MR guidance,” Med. Phys. 35, 2081–2093 (2008). 10.1118/1.2900131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly K. B., Diederich C. J., Rieke V., Bouley D., Chen J., Nau W. H., Ross A. B., Kinsey A. M., and Sommer G., “Magnetic resonance-guided high-intensity ultrasound ablation of the prostate,” Top. Magn. Reson. Imaging 17, 195–207 (2006). 10.1097/RMR.0b013e31803774dd [DOI] [PubMed] [Google Scholar]
- Chopra R., Luginbuhl C., Foster F. S., and Bronskill M. J., “Multifrequency ultrasound transducers for conformal interstitial thermal therapy,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 50, 881–889 (2003). 10.1109/TUFFC.2003.1214507 [DOI] [PubMed] [Google Scholar]
- Siddiqui K., Chopra R., Vedula S., Sugar L., Haider M., Boyes A., Musquera M., Bronskill M., and Klotz L., “MRI-guided transurethral ultrasound therapy of the prostate gland using real-time thermal mapping: initial studies,” Urology 76, 1506–1511 (2010). 10.1016/j.urology.2010.04.046 [DOI] [PubMed] [Google Scholar]
- Fosmire H., Hynynen K., Drach G. W., Stea B., Swift P., and Cassady J. R., “Feasibility and toxicity of transrectal ultrasound hyperthermia in the treatment of locally advanced adenocarcinoma of the prostate,” Int. J. Radiat. Oncol., Biol., Phys 26, 253–259 (1993). 10.1016/0360-3016(93)90205-A [DOI] [PubMed] [Google Scholar]
- Hurwitz M. D., Hansen J. L., Prokopios-Davos S., Manola J., Wang Q., Bornstein B. A., Hynynen K., and Kaplan I. D., “Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber Cancer Institute study 94-153,” Cancer 117, 510–516 (2011). 10.1002/cncr.25619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. B., Merrilees N. K., Dahleh M., and Hynynen K., “Control system for an MRI compatible intracavitary ultrasound array for thermal treatment of prostate disease,” Int. J. Hyperthermia 17, 271–282 (2001). 10.1080/02656730010025841 [DOI] [PubMed] [Google Scholar]
- Wootton J. H., Hsu I. C., and Diederich C. J., “Endocervical ultrasound applicator for integrated hyperthermia and HDR brachytherapy in the treatment of locally advanced cervical carcinoma,” Med. Phys. 38, 598 (2011). 10.1118/1.3512803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang T., Wootton J., Hsu I. C., and Diederich C., “Treatment delivery platform for conformal catheter-based ultrasound hyperthermia,” Proc. SPIE 7181, 718109 (2009). 10.1117/12.808339 [DOI] [Google Scholar]
- Nau W. H., Diederich C. J., and Burdette E. C., “Evaluation of multielement catheter-cooled interstitial ultrasound applicators for high-temperature thermal therapy,” Med. Phys. (USA) 28, 1525–1534 (2001). 10.1118/1.1381550 [DOI] [PubMed] [Google Scholar]
- Kangasniemi M., Diederich C. J., Price R. E., Stafford R. J., Schomer D. F., Olsson L. E., Tyreus P. D., Nau W. H., and Hazle J. D., “Multiplanar MR temperature-sensitive imaging of cerebral thermal treatment using interstitial ultrasound applicators in a canine model,” J. Magn. Reson. Imaging 16, 522–531. (2002). 10.1002/jmri.10191 [DOI] [PubMed] [Google Scholar]
- Nau W. H., Diederich C. J., Ross A. B., Butts K., Rieke V., Bouley D. M., Gill H., Daniel B., and Sommer G., “MRI-guided interstitial ultrasound thermal therapy of the prostate: A feasibility study in the canine model,” Med. Phys. 32, 733–743 (2005). 10.1118/1.1861163 [DOI] [PubMed] [Google Scholar]
- Moros E. G., Roemer R. B., and Hynynen K., “Pre-focal plane high-temperature regions induced by scanning focused ultrasound beams,” Int. J. Hyperthermia 6, 351–366 (1990). 10.3109/02656739009141143 [DOI] [PubMed] [Google Scholar]
- Keall P. J., Mageras G. S., Balter J. M., Emery R. S., Forster K. M., Jiang S. B., Kapatoes J. M., Low D. A., Murphy M. J., Murray B. R., Ramsey C. R., Van Herk M. B., Vedam S. S., Wong J. W., and Yorke E., “The management of respiratory motion in radiation oncology report of AAPM Task Group 76,” Med. Phys. 33, 3874–3900 (2006). 10.1118/1.2349696 [DOI] [PubMed] [Google Scholar]
- Grissom W. A., Rieke V., Holbrook A. B., Medan Y., Lustig M., Santos J., McConnell M. V., and Pauly K. B., “Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs,” Med. Phys. 37, 5014–5026 (2010). 10.1118/1.3475943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict S. H., Yenice K. M., Followill D., Galvin J. M., Hinson W., Kavanagh B., Keall P., Lovelock M., Meeks S., Papiez L., Purdie T., Sadagopan R., Schell M. C., Salter B., Schlesinger D. J., Shiu A. S., Solberg T., Song D. Y., Stieber V., Timmerman R., Tome W. A., Verellen D., Wang L., and Yin F. F., “Stereotactic body radiation therapy: The report of AAPM Task Group 101,” Med. Phys. 37, 4078–4101 (2010). 10.1118/1.3438081 [DOI] [PubMed] [Google Scholar]
- InSightec L., ExAblate 2000 MR Guided Focused Ultrasound Surgery Operator's Manual, Rev 04/08 ed. (InSightec, Ltd., Tirat Carmel, 2008). [Google Scholar]
- Salomir R., Palussiere J., Vimeux F. C., de Zwart J. A., Quesson B., Gauchet M., Lelong P., Pergrale J., Grenier N., and Moonen C. T., “Local hyperthermia with MR-guided focused ultrasound: spiral trajectory of the focal point optimized for temperature uniformity in the target region,” J. Magn. Reson. Imaging 12, 571–583 (2000). [DOI] [PubMed] [Google Scholar]
- White P. J., Clement G. T., and Hynynen K., “Longitudinal and shear mode ultrasound propagation in human skull bone,” Ultrasound Med. Biol. 32, 1085–1096 (2006). 10.1016/j.ultrasmedbio.2006.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement G. T. and Hynynen K., “A non-invasive method for focusing ultrasound through the human skull,” Phys. Med. Biol. 47, 1219–1236 (2002). 10.1088/0031-9155/47/8/301 [DOI] [PubMed] [Google Scholar]
- McGough R. J., Kessler M. L., Ebbini E. S., and Cain C. A., “Treatment planning for hyperthermia with ultrasound phased arrays,” IEEE Trans. Ultrason. Ferroelectr. 43, 1074–1084 (1996). 10.1109/58.542051 [DOI] [Google Scholar]
- Hynynen K., “The role of nonlinear ultrasound propagation during hyperthermia treatments,” Med. Phys. 18, 1156–1163 (1991). 10.1118/1.596626 [DOI] [PubMed] [Google Scholar]
- Pulkkinen A. and Hynynen K., “Computational aspects in high intensity ultrasonic surgery planning,” Comput. Med. Imaging Graph. 34, 69–78 (2010). 10.1016/j.compmedimag.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Harpen M. D., “Basic nonlinear acoustics: An introduction for radiological physicists,” Med. Phys. 33, 3241–3247 (2006). 10.1118/1.2207128 [DOI] [PubMed] [Google Scholar]
- Kuroda K., Kokuryo D., Kumamoto E., Suzuki K., Matsuoka Y., and Keserci B., “Optimization of self-reference thermometry using complex field estimation,” Magn. Reson. Med. 56, 835–843 (2006). 10.1002/mrm.21016 [DOI] [PubMed] [Google Scholar]
- Rieke V., Vigen K. K., Sommer G., Daniel B. L., Pauly J. M., and Butts K., “Referenceless PRF shift thermometry,” Magn. Reson. Med. 51, 1223–1231 (2004). 10.1002/mrm.20090 [DOI] [PubMed] [Google Scholar]
- Rieke V., Kinsey A. M., Ross A. B., Nau W. H., Diederich C. J., Sommer G., and Pauly K. B., “Referenceless MR thermometry for monitoring thermal ablation in the prostate,” IEEE Trans. Med. Imaging 26, 813–821 (2007). 10.1109/TMI.2007.892647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Senneville B. D., Mougenot C., and Moonen C. T., “Real-time adaptive methods for treatment of mobile organs by MRI-controlled high-intensity focused ultrasound,” Magn. Reson. Med. 57, 319–330 (2007). 10.1002/mrm.21124 [DOI] [PubMed] [Google Scholar]
- Vigen K. K., Daniel B. L., Pauly J. M., and Butts K., “Triggered, navigated, multi-baseline method for proton resonance frequency temperature mapping with respiratory motion,” Magn. Reson. Med. 50, 1003–1010 (2003). 10.1002/mrm.10608 [DOI] [PubMed] [Google Scholar]
- Ries M., de Senneville B. D., Roujol S., Berber Y., Quesson B., and Moonen C., “Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues,” Magn. Reson. Med. 64, 1704–1712 (2010). 10.1002/mrm.22548 [DOI] [PubMed] [Google Scholar]
- Nehrke K., Bornert P., Groen J., Smink J., and Bock J. C., “On the performance and accuracy of 2D navigator pulses,” Magn. Reson. Imaging 17, 1173–1181 (1999). 10.1016/S0730-725X(99)00043-0 [DOI] [PubMed] [Google Scholar]
- Plewes D. B., Betty I., Urchuk S. N., and Soutar I., “Visualizing tissue compliance with MR imaging,” J. Magn. Reson. Imaging 5, 733–738 (1995). 10.1002/jmri.1880050620 [DOI] [PubMed] [Google Scholar]
- Walker W. F., Fernandez F. J., and Negron L. A., “A method of imaging viscoelastic parameters with acoustic radiation force,” Phys. Med. Biol. 45, 1437–1447 (2000). 10.1088/0031-9155/45/6/303 [DOI] [PubMed] [Google Scholar]
- McDannold N. and Maier S. E., “Magnetic resonance acoustic radiation force imaging,” Med. Phys. 35, 3748–3758 (2008). 10.1118/1.2956712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicke M., Engelbertz A., Habenstein B., Lewerenz M., Oehms O., Trautner P., Weber B., Wrede S., and Maier K., “New image contrast method in magnetic resonance imaging via ultrasound,” Hyperfine Interact. 181, 21–26 (2008). 10.1007/s10751-008-9628-6 [DOI] [Google Scholar]
- Konofagou E. E. and Hynynen K., “Localized harmonic motion imaging: Theory, simulations and experiments,” Ultrasound Med. Biol. 29, 1405–1413 (2003). 10.1016/S0301-5629(03)00953-0 [DOI] [PubMed] [Google Scholar]
- Curiel L. and Hynynen K., “Localized harmonic motion imaging for focused ultrasound surgery targeting,” Ultrasound Med. Biol. 37, 1230–1239 (2011). 10.1016/j.ultrasmedbio.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleke C. and Konofagou E. E., “Harmonic motion imaging for focused ultrasound (HMIFU): A fully integrated technique for sonication and monitoring of thermal ablation in tissues,” Phys. Med. Biol. 53, 1773–1793 (2008). 10.1088/0031-9155/53/6/018 [DOI] [PubMed] [Google Scholar]
- Du J., Hamilton G., Takahashi A., Bydder M., and Chung C. B., “Ultrashort echo time spectroscopic imaging (UTESI) of cortical bone,” Magn. Reson. Med. 58, 1001–1009 (2007). 10.1002/mrm.21397 [DOI] [PubMed] [Google Scholar]
- McNitt-Gray M. F., “AAPM/RSNA physics tutorial for residents: Topics in CT. Radiation dose in CT,” Radiographics 22, 1541–1553 (2002). 10.1148/rg.226025128 [DOI] [PubMed] [Google Scholar]
- Gatehouse P. D. and Bydder G. M., “Magnetic resonance imaging of short T2 components in tissue,” Clin. Radiol. 58, 1–19 (2003). 10.1053/crad.2003.1157 [DOI] [PubMed] [Google Scholar]
- Reichert I. L., Robson M. D., Gatehouse P. D., He T., Chappell K. E., Holmes J., Girgis S., and Bydder G. M., “Magnetic resonance imaging of cortical bone with ultrashort TE pulse sequences,” Magn. Reson. Imaging. 23, 611–618 (2005). 10.1016/j.mri.2005.02.017 [DOI] [PubMed] [Google Scholar]
- Robson M. D. and Bydder G. M., “Clinical ultrashort echo time imaging of bone and other connective tissues,” NMR Biomed. 19, 765–780 (2006). 10.1002/nbm.1100 [DOI] [PubMed] [Google Scholar]
- Du J., Carl M., Bydder M., Takahashi A., Chung C. B., and Bydder G. M., “Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone,” J. Magn. Reson. 207, 304–311 (2010). 10.1016/j.jmr.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Bae W. C., Chen P. C., Chung C. B., Masuda K., D’Lima D., and Du J., “Quantitative ultrashort echo time (UTE) MRI of human cortical bone: Correlation with porosity and biomechanical properties,” J. Bone Miner. Res. 27, 848–857 (2012). 10.1002/jbmr.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D., Cooley D., Perry T., Guo J., Richardson A., Moellmer J., Hadley R., Parker D., Skliar M., and Roemer R. B., “MR thermometry-based feedback control of efficacy and safety in minimum-time thermal therapies: Phantom and in-vivo evaluations,” Int. J. Hyperthermia 22, 29–42 (2006). 10.1080/02656730500412411 [DOI] [PubMed] [Google Scholar]
- Arora D., Minor M. A., Skliar M., and Roemer R. B., “Control of thermal therapies with moving power deposition field,” Phys. Med. Biol. 51, 1201–1219 (2006). 10.1088/0031-9155/51/5/011 [DOI] [PubMed] [Google Scholar]
- Dhiraj A., Daniel C., Trent P., Mikhail S., and Robert B. R., “Direct thermal dose control of constrained focused ultrasound treatments: phantom and in vivo evaluation,” Phys. Med. Biol. 50, 1919–1935 (2005). 10.1088/0031-9155/50/8/019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook A. B., Ghanouni P., Santos J. M., Medan Y., and Butts Pauly K., “In vivo MR acoustic radiation force imaging in the porcine liver,” Med. Phys. 38, 5081–5089 (2011). 10.1118/1.3622610 [DOI] [PMC free article] [PubMed] [Google Scholar]