Abstract
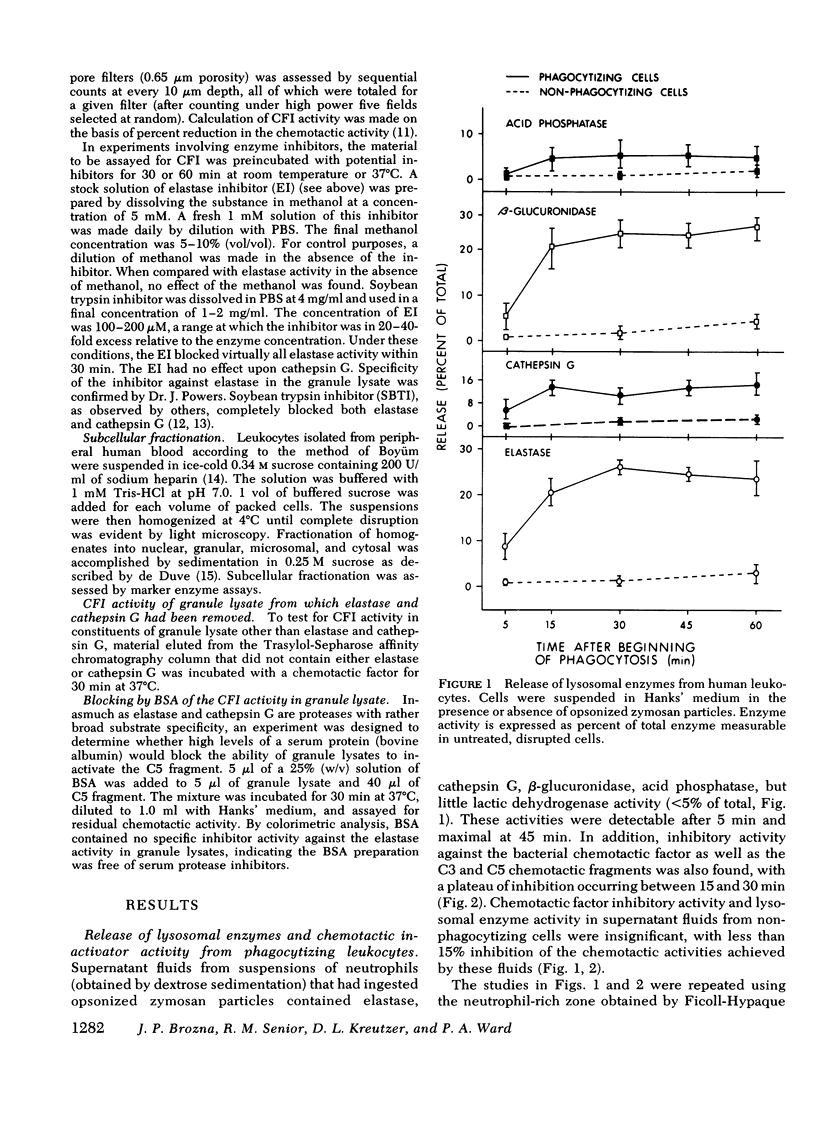
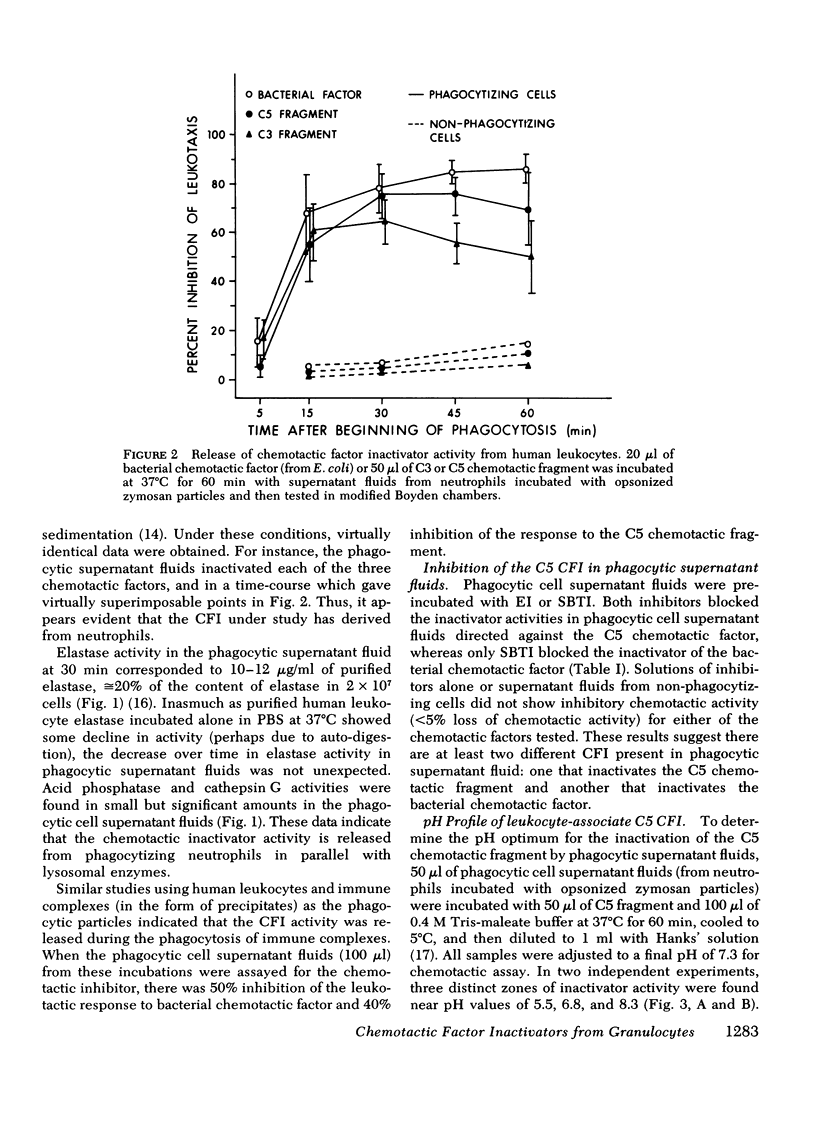
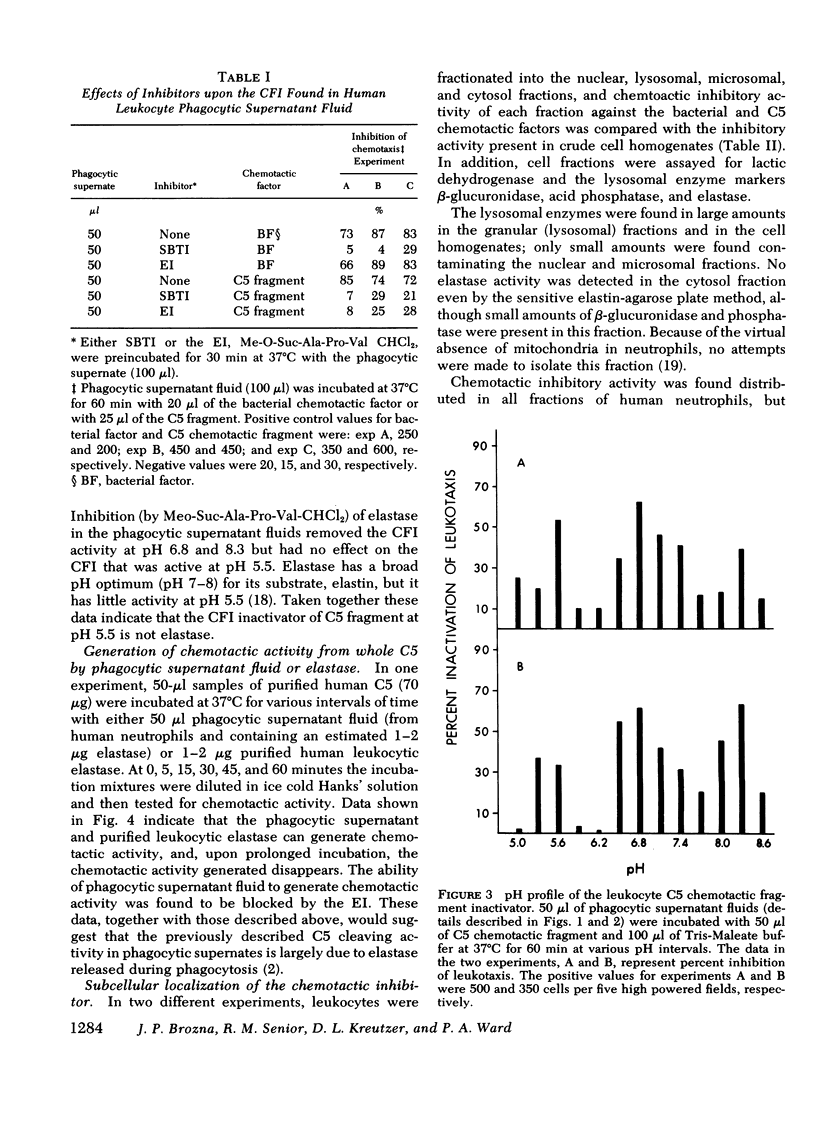
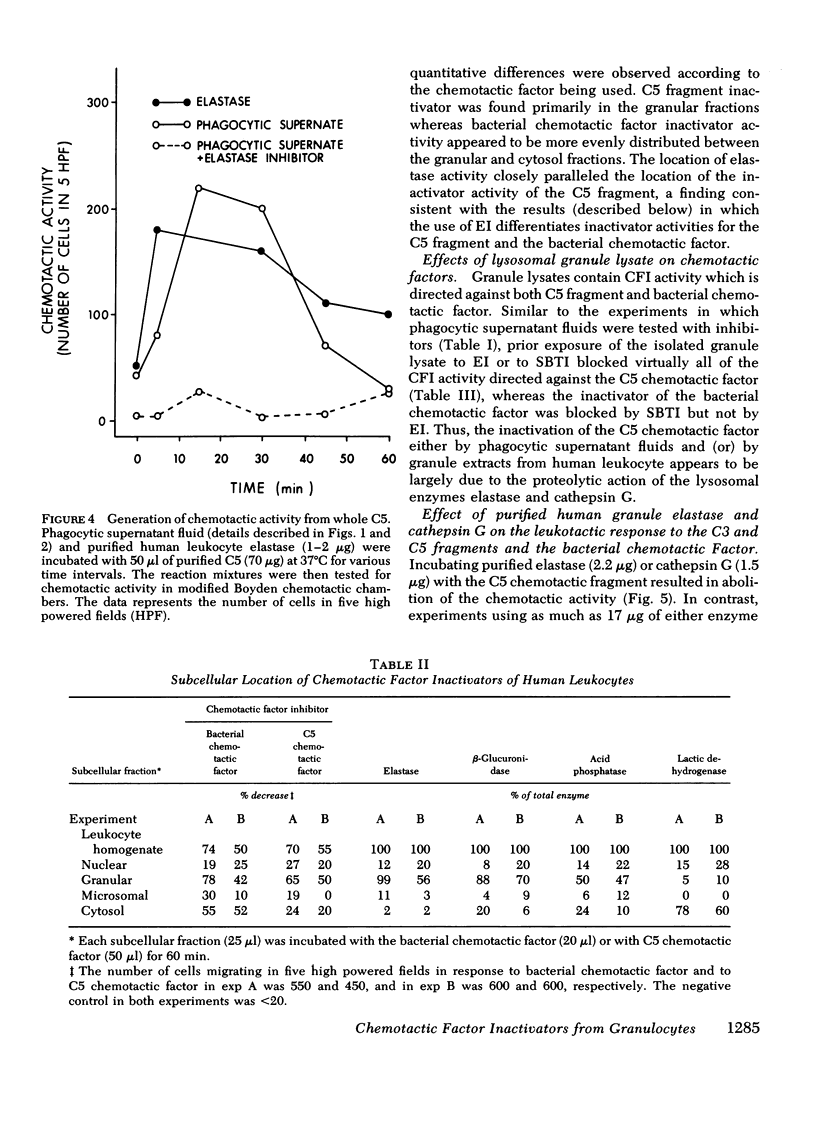
During phagocytosis, neutrophils release a variety of substances that include activators and inactivators of chemotactic factors. It is generally considered that these represent hydrolytic enzymes. Elastase and cathepsin G, major proteases released from lysosomal granules during phagocytosis, contain broad hydrolytic activity. This study examined granule elastase and cathepsin G for their role as inactivators of chemotactic factors. Elastase and cathepsin G were purified from human neutrophils by Trasylol-Sepharose and CM-cellulose chromatography. Small amounts (approximately equal to 3 microgram, 1 muM) of elastase and cathepsin G, comparable to quantities released by 10(6) neutrophils during phagocytosis, completely inactivated the C5 chemotactic factor generated in human serum. Larger concentrations were needed to inactivate the C3 chemotactic factor, and when the bacterial chemotactic factor from Escherichia coli was employed, five times more elastase or cathepsin G was ineffective against this chemotactic factor. Supernatant fluid from human neutrophils that had ingested complement-coated zymosan particles contained elastase and cathepsin G and had inactivator activity for both the C5 chemotactic fragment and the bacterial factor. A specific inhibitor of elastase largely abolished the inactivator activity in the phagocytic supernates that was directed against C5 factor but did not affect the inactivator activity for the bacterial factor. Similar results occurred in studies of granule lysates. These data indicate heterogeneity in the chemotactic factor inactivator activity released by phagocytosing neutrophils. The predominant inactivator activity of the C5 chemotactic fragment is attributable to elastase and cathepsin G.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozna J. P., Ward P. A. Antileukotactic properties of tumor cells. J Clin Invest. 1975 Sep;56(3):616–623. doi: 10.1172/JCI108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- De Duve C. Tissue fractionation. Past and present. J Cell Biol. 1971 Jul;50(1):20d–55d. [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson U., Ohlsson K., Olsson I. Effects of granulocyte neutral proteases on complement components. Scand J Immunol. 1976;5(4):421–426. doi: 10.1111/j.1365-3083.1976.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Kang S. H., Fuchs M. S. An improvement in the Hummel chymotrypsin assay. Anal Biochem. 1973 Jul;54(1):262–265. doi: 10.1016/0003-2697(73)90270-4. [DOI] [PubMed] [Google Scholar]
- Levine E. A., Senior R. M., Butler J. V. The elastase activity of alveolar macrophages: measurements using synthetic substrates and elastin. Am Rev Respir Dis. 1976 Jan;113(1):25–30. doi: 10.1164/arrd.1976.113.1.25. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I., Spitznagel K. Localization of chymotrypsin-like cationic protein, collagenase and elastase in azurophil granules of human neutrophilic polymorphonuclear leukocytes. Hoppe Seylers Z Physiol Chem. 1977 Mar;358(3):361–366. doi: 10.1515/bchm2.1977.358.1.361. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Tegner H. Inhibition of elastase from granulocytes by the low molecular weight bronchial protease inhibitor. Scand J Clin Lab Invest. 1976 Sep;36(5):437–445. doi: 10.3109/00365517609054461. [DOI] [PubMed] [Google Scholar]
- SCHWIND J. L. The supravital method in the study of the cytology of blood and marrow cells. Blood. 1950 Jul;5(7):597–622. [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human cathepsin G. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):273–278. doi: 10.1042/bj1550273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human lysosomal elastase. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):265–271. doi: 10.1042/bj1550265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venge P., Olsson I. Cationic proteins of human granulocytes. VI. Effects on the complement system and mediation of chemotactic activity. J Immunol. 1975 Dec;115(6):1505–1508. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. I. A new method with immune complexes. J Immunol. 1973 Dec;111(6):1771–1776. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Kauffmann J. C., Chusid M. J., Herzig, Gallin J. I. Functional abnormalities of human neutrophils collected by continuous flow filtration leukopheresis. Blood. 1975 Dec;46(6):901–911. [PubMed] [Google Scholar]


