Abstract
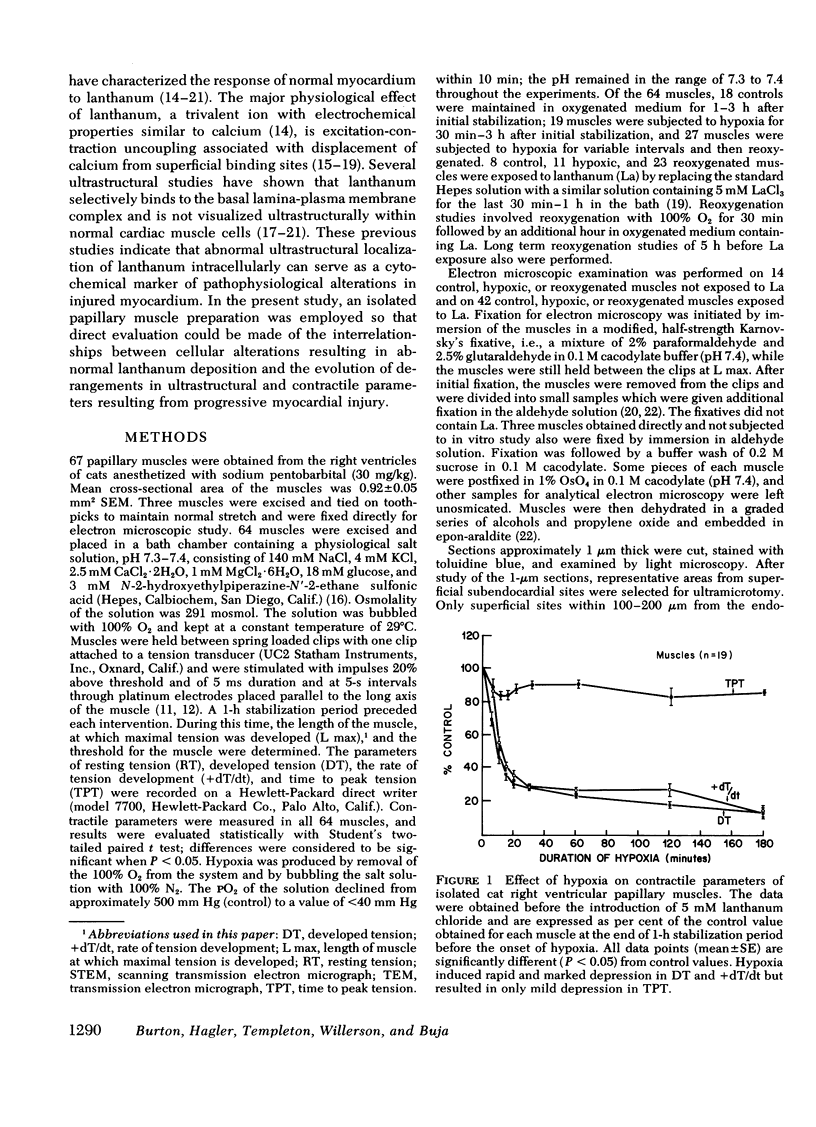
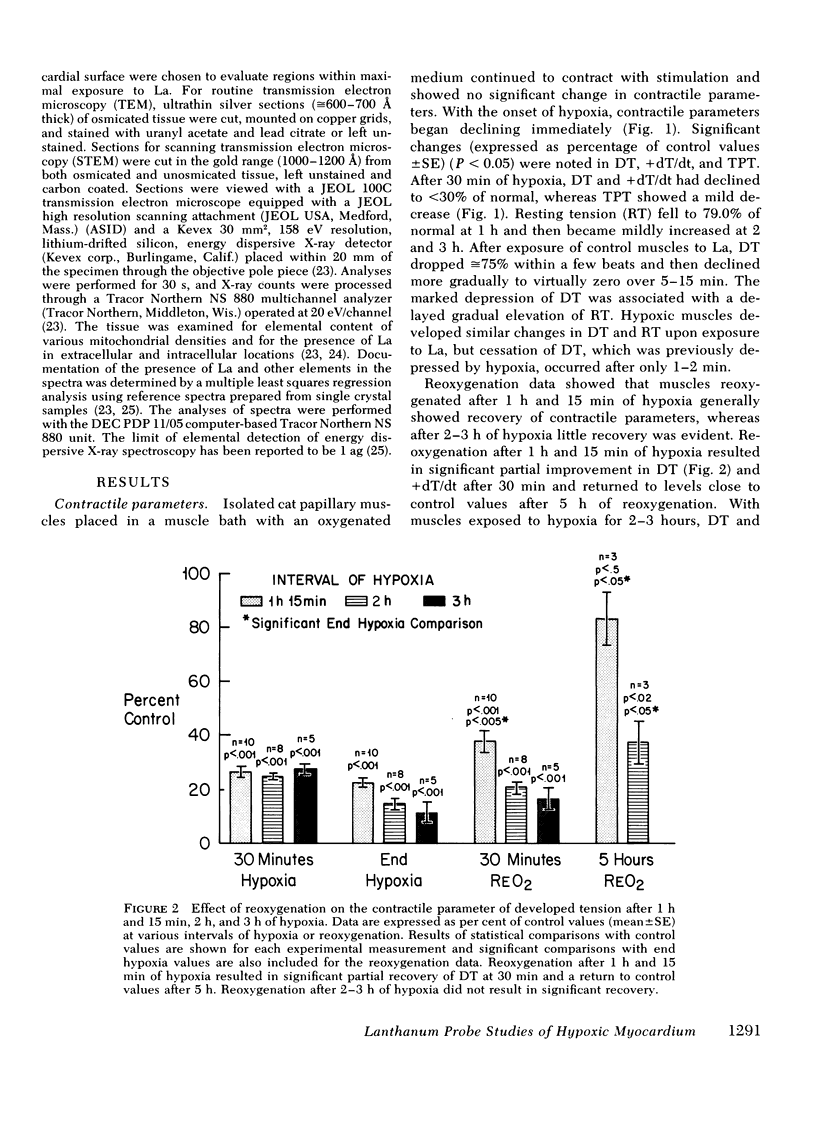
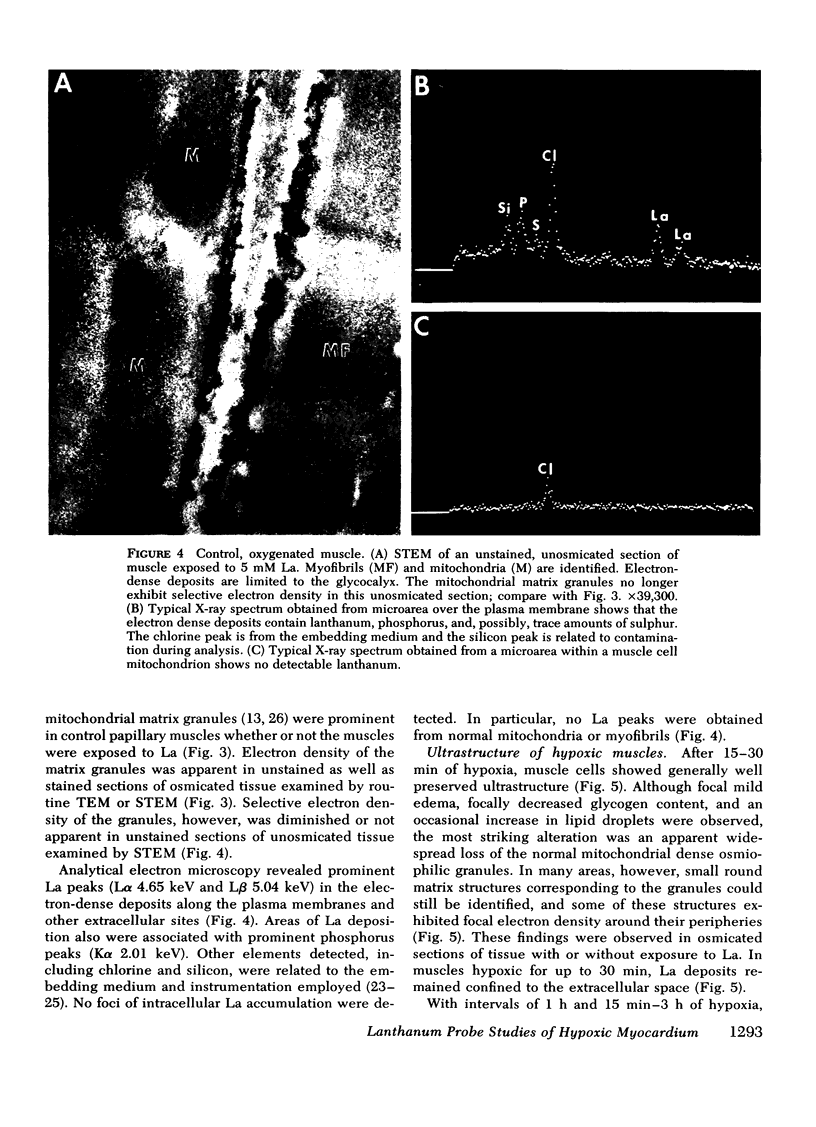
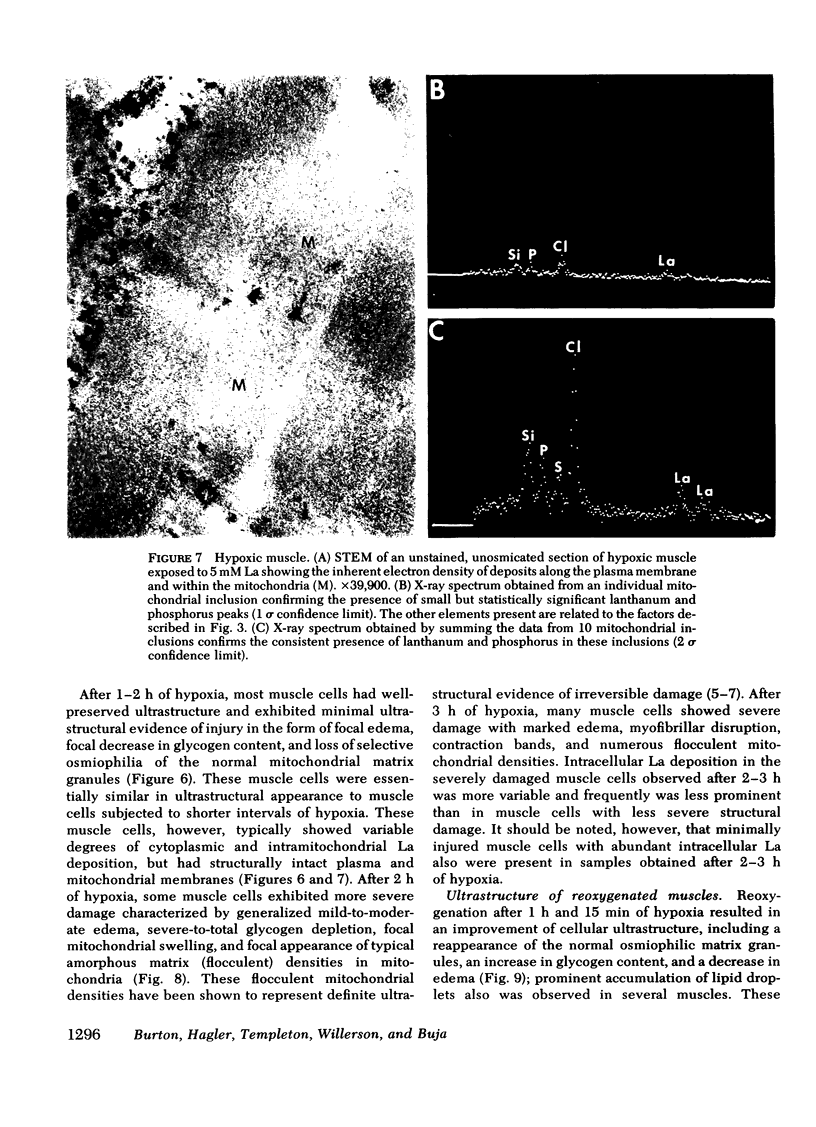
This study was undertaken to evaluate directly the relationship between evolution of irreversible myocardial injury induced by hypoxia in an isolated papillary muscle preparation and the development of pathophysiological alterations related to severely impaired membrane function. An ionic lanthanum probe technique was employed as a cytochemical marker to monitor the progression of cellular injury, and data from this cytologic technique were correlated with ultrastructure and measurements of contractile parameters in a total of 67 muscles subjected to control conditions or to graded intervals of hypoxia with or without reoxygenation. Marked depression of developed tension and rate of tension development occurred after 30 min of hypoxia. Contractile function showed significant recovery with reoxygenation after 1 h and 15 min of hypoxia but remained depressed when reoxygenation was provided after 2 or 3 h of hypoxia. Examination by transmission and analytical electron microscopy (energy dispersive X-ray microanalysis) revealed lanthanum deposition only in extracellular regions of control muscles and muscles subjected to 30 min of hypoxia. After hypoxic intervals of over 1 h, abnormal intracytoplasmic and intramitochondrial localization of lanthanum were detected. After 1 h and 15 min of hypoxia, abnormal intracellular lanthanum accumulation was associated with only minimal ultrastructural evidence of injury; muscle provided reoxygenation after 1 h and 15 min of hypoxia showed improved ultrastructure and did not exhibit intracellular lanthanum deposits upon exposure to lanthanum during the reoxygenation period. After 2 to 3 h of hypoxia, abnormal intracellular lanthanum accumulation was associated with ultrastructural evidence of severe muscle injury which persisted after reoxygenation. Thus, the data support the conclusion that cellular and membrane alterations responsible for abnormal intracellular lanthanum deposition precede the development of irreversible injury but evolve at a transitional stage in the progression from reversible to irreversible injury induced by hypoxia in isolated feline papillary muscles.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apstein C. S., Bing O. H., Levine H. J. Cardiac muscle function during and after hypoxia: effects of glucose concentration, mannitol and isoproternol. J Mol Cell Cardiol. 1976 Aug;8(8):627–640. doi: 10.1016/0022-2828(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Bing O. H., Brooks W. W., Messer J. V. Prolongation of tension on reoxygenation following myocardial hypoxia: a possible role for mitochondria in muscle relaxation. J Mol Cell Cardiol. 1976 Mar;8(3):205–215. doi: 10.1016/0022-2828(76)90037-7. [DOI] [PubMed] [Google Scholar]
- Boutet M., Hüttner I., Rona G. Permeability alteration of sarcolemmal membrane in catecholamine-induced cardiac muscle cell injury. In vivo studies with fine structural diffusion tracer horse radish peroxidase. Lab Invest. 1976 May;34(5):482–488. [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Griffin E. E., Dingle J. T., Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977 May;59(5):911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahimi H. D., Cotran R. S. Permeability studies in heat-induced injury of skeletal muscle using lanthanum as fine structural tracer. Am J Pathol. 1971 Jan;62(1):143–157. [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A., Janke J., Döring H. J., Leder O. Myocardial fiber necrosis due to intracellular Ca overload-a new principle in cardiac pathophysiology. Recent Adv Stud Cardiac Struct Metab. 1974;4:563–580. [PubMed] [Google Scholar]
- Goldstein M. A., Thyrum P. T., Murphy D. L., Martin J. H., Schwartz A. Ultrastructural and contractile characteristics of isolated papillary muscle exposed to acute hypoxia. J Mol Cell Cardiol. 1977 Apr;9(4):285–295. doi: 10.1016/s0022-2828(77)80035-7. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Fox A. C., Hirsch J., Streuli F., Weissmann G. Colloidal lanthanum as a marker for impaired plasma membrane permeability in ischemic dog myocardium. Am J Pathol. 1975 May;79(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Kent S. P. Diffusion of plasma proteins into cells. A manifestation of cell injury in rabbit skeletal muscle exposed to lecithinase C. Arch Pathol. 1969 Oct;88(4):407–412. [PubMed] [Google Scholar]
- Kent S. P. Diffusion of plasma proteins into cells: a manifestation of cell injury in human myocardial ischemia. Am J Pathol. 1967 Apr;50(4):623–637. [PMC free article] [PubMed] [Google Scholar]
- Kent S. P. Intracellular plasma protein: a manifestation of cell injury in myocardial ischemia. Nature. 1966 Jun 18;210(5042):1279–1281. doi: 10.1038/2101279b0. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Jennings R. B. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974 Dec;54(6):1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LETTVIN J. Y., PICKARD W. F., MCCULLOCH W. S., PITTS W. A THEORY OF PASSIVE ION FLUX THROUGH AXON MEMBRANES. Nature. 1964 Jun 27;202:1338–1339. doi: 10.1038/2021338a0. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Frank J. S. Lanthanum in heart cell culture. Effect on calcium exchange correlated with its localization. J Cell Biol. 1972 Sep;54(3):441–455. doi: 10.1083/jcb.54.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A., Frank J. S., Nudd L. M., Seraydarian K. Sialic acid: effect of removal on calcium exchangeability of cultured heart cells. Science. 1976 Sep 10;193(4257):1013–1015. doi: 10.1126/science.948758. [DOI] [PubMed] [Google Scholar]
- Legato M. J., Langer G. A. The subcellular localization of calcium ion in mammalian myocardium. J Cell Biol. 1969 May;41(2):401–423. doi: 10.1083/jcb.41.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E. The interaction of La 3+ with mitochondria in relation to respiration-coupled Ca 2+ transport. Arch Biochem Biophys. 1971 Apr;143(2):506–515. doi: 10.1016/0003-9861(71)90235-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Benitez D., Alanis J. Selective deposition of lanthanum in mammalian cardiac cell membranes. Ultrastructural and electrophysiological evidence. J Cell Biol. 1973 Jul;58(1):1–10. doi: 10.1083/jcb.58.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerbaum S., Lang T. W., Corday E., Rubins S., Hirose S., Costantini C., Gold H., Dalmastro M. Progressive alterations of cardiac hemodynamic and regional metabolic function after acute coronary occlusion. Am J Cardiol. 1974 Jan;33(1):60–68. doi: 10.1016/0002-9149(74)90740-1. [DOI] [PubMed] [Google Scholar]
- Mela L. Inhibition and activation of calcium transport in mitochondria. Effect of lanthanides and local anesthetic drugs. Biochemistry. 1969 Jun;8(6):2481–2486. doi: 10.1021/bi00834a034. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Harris J. P. Inhibition by Lanthanum of the Na+ + K+ activated, ouabain-sensitive adenosinetriphosphatase enzyme. J Mol Cell Cardiol. 1976 Oct;8(10):811–822. doi: 10.1016/0022-2828(76)90087-0. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Stone J., Carson V., Chipperfield D. Effect of ischaemia on cardiac contractility and calcium exchangeability. J Mol Cell Cardiol. 1971 Jun;2(2):125–143. doi: 10.1016/0022-2828(71)90066-6. [DOI] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I., Greenawalt J. W., Carafoli E. On the nature of the dense matrix granules of normal mitochondria. J Cell Biol. 1969 Feb;40(2):565–568. doi: 10.1083/jcb.40.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan T. J., Harman M. A., Lehan P. H., Burke W. M., Oldewurtel H. A. Ventricular arrhythmias and K+ transfer during myocardial ischemia and intervention with procaine amide, insulin, or glucose solution. J Clin Invest. 1967 Oct;46(10):1657–1668. doi: 10.1172/JCI105657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Lowe J. E., Jennings R. B. Effect of the calcium antagonist verapamil on necrosis following temporary coronary artery occlusion in dogs. Circulation. 1977 Apr;55(4):581–587. doi: 10.1161/01.cir.55.4.581. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. S. Liver parenchymal cell injury. 3. The nature of calcium--associated electron-opaque masses in rat liver mitochondria following poisoning with carbon tetrachloride. J Cell Biol. 1965 Jun;25(3 Suppl):53–75. doi: 10.1083/jcb.25.3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn W. G., Langer G. A. Specific uncoupling of excitation and contraction in mammalian cardiac tissue by lanthanum. J Gen Physiol. 1970 Aug;56(2):191–217. doi: 10.1085/jgp.56.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea S. M. Lanthanum staining of the surface coat of cells. Its enhancement by the use of fixatives containing Alcian blue or cetylpyridinium chloride. J Cell Biol. 1971 Dec;51(3):611–620. doi: 10.1083/jcb.51.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Devine C. E., Peters P. D., Hall T. A. Electron microscopy and electron probe analysis of mitochondrial cation accumulation in smooth muscle. J Cell Biol. 1974 Jun;61(3):723–742. doi: 10.1083/jcb.61.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybers H. D., Helmer P. R., Murphy Q. R. Effects of hypoxia on myocardial potassium balance. Am J Physiol. 1971 Jun;220(6):2047–2050. doi: 10.1152/ajplegacy.1971.220.6.2047. [DOI] [PubMed] [Google Scholar]
- Taubert K., Templeton G., Willerson J. T., Shapiro W. Effects of digitalis and calcium on papillary muscles in normal and hypoxic states. Am J Physiol. 1976 Jul;231(1):66–72. doi: 10.1152/ajplegacy.1976.231.1.66. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Collan Y., Kahng M. W., Mergner W. J. Recent studies on the pathophysiology of ischemic cell injury. Beitr Pathol. 1976 Sep;158(4):363–388. doi: 10.1016/s0005-8165(76)80135-7. [DOI] [PubMed] [Google Scholar]
- Tyberg J. V., Yeatman L. A., Parmley W. W., Urschel C. W., Sonnenblick E. H. Effects of hypoxia on mechanics of cardiac contraction. Am J Physiol. 1970 Jun;218(6):1780–1788. doi: 10.1152/ajplegacy.1970.218.6.1780. [DOI] [PubMed] [Google Scholar]
- Willerson J. T., Crie J. S., Adcock R. C., Templeton G. H., Wildenthal K. Influence of calcium on the inotropic actions of hyperosmotic agents, norepinephrine, paired electrical stimulation, and treppe. J Clin Invest. 1974 Oct;54(4):957–964. doi: 10.1172/JCI107836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson J. T., Scales F., Mukherjee A., Platt M., Templeton G. H., Fink G. S., Buja L. M. Abnormal myocardial fluid retention as an early manifestation of ischemic injury. Am J Pathol. 1977 Apr;87(1):159–188. [PMC free article] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]