Abstract
Tibetan wild barley (Hordeum vulgare L. ssp. spontaneum), originated and grown in harsh enviroment in Tibet, is well-known for its rich germpalsm with high tolerance to abiotic stresses. However, the genetic variation and genes involved in Al tolerance are not totally known for the wild barley. In this study, a genome-wide association analysis (GWAS) was performed by using four root parameters related with Al tolerance and 469 DArT markers on 7 chromosomes within or across 110 Tibetan wild accessions and 56 cultivated cultivars. Population structure and cluster analysis revealed that a wide genetic diversity was present in Tibetan wild barley. Linkage disequilibrium (LD) decayed more rapidly in Tibetan wild barley (9.30 cM) than cultivated barley (11.52 cM), indicating that GWAS may provide higher resolution in the Tibetan group. Two novel Tibetan group-specific loci, bpb-9458 and bpb-8524 were identified, which were associated with relative longest root growth (RLRG), located at 2H and 7H on barely genome, and could explain 12.9% and 9.7% of the phenotypic variation, respectively. Moreover, a common locus bpb-6949, localized 0.8 cM away from a candidate gene HvMATE, was detected in both wild and cultivated barleys, and showed significant association with total root growth (TRG). The present study highlights that Tibetan wild barley could provide elite germplasm novel genes for barley Al-tolerant improvement.
Introduction
Aluminium (Al3+) toxicity is considered as a major factor limiting crop production on acid soils [1], [2]. Al3+ rapidly inhibits plant root growth, thus preventing uptake of water and nutrients [2]. The initial and visual symptom of Al toxicity is the inhibition of root elongation and development of lateral roots.
There has been no commonly-recoginized standard criterion for evaluating plant Al tolerance. Many researches have used relative root growth (RRG) to estimate Al tolerance [3]–[7]. However, a weak correlation (R2 = 0.17) was found between rice Al tolerance based on RRG of the longest root and RRG of the total root system [8]. In addition, an absolute root elongation was also used for Al tolerance evaluation in short-term Al stress experiments [9]–[12]. It was reported that utilization of different root index in QTL or genome-wide association analysis could be helpful to identify novel loci [13]. To make the association analysis more efficient, accurate determination of root parameters is crucial and essential. Famoso et al [8] developed a novel and high-throughput Al tolerance phenotyping platform, which consists of a root imaging system and root computer program. So far, this technology has been successfully applied in many research projects [14]–[15].
The Al tolerance machanism of cereals, such as wheat, barley, rice, maize and sorghum, has been extensively studied [4], [16]–[21]. The most common mechanism is secretion of organic acids, which may chelate Al in the rizosphere, thus preventing Al being absorbed by roots [22]. However, it has been reported that Al resistance in maize cannot be fully explained by root organic acid release [23]. Thus, multiple mechanisms for Al tolerance may exist in plants. Degenhardt et al. [24] reported that a decrease of Al3+ activity is caused by root-mediated increase in rizosphere pH in an Arabidopsis mutant. Furthermore, a recent research reported that a T-DNA insertional mutant of XTH31, a gene controlling xyloglucan endohydrolase (XEH) and xyloglucan endotransglucosylase (XET) activities, gains the function of Al resistance by modulating cell wall xyloglucan content and Al binding capacity in Arabidopsis [25]. In short, the underlying mechanism of Al tolerance is complex and still not totally understood.
Altough barley is considered as one of the most sensitive species to Al toxicity among cereal crops, there is still a wide variation in Al tolerance among cultivars or genotypes [7]. So it is meaningful to investigate the underlying mechanism of barley Al tolerance. It was widely reported that the aluminum tolerance of barley was controlled by a dominant loci on chromosome 4H. Furukawa et al [4] and Wang et al [26] identified the same candidate gene HvMATE (HvAACT1) in cv. Murasakimochi and Dayton respectively, which is responsible for Al-induced citrate secretion and located on the long arm of chromosome 4H. However, Gruber et al [27], [28] reported that over-expression of HvALMT1, which encodes an anion channel protein to facilitate organic anion transport, confers enhanced Al tolerance. Moreover, multigenic control of barley aluminum tolerance was reported in a few QTL studies [29], [30]. Hence, barley Al tolerance is a complex trait, which may be quantitatively inherited. On the other hand, QTL analysis has the limitation that only the alleles segregated between the parents of DH or RIL population could be identified. Hence the genetic diversity of tolerant genotypes used in QTL analysis was limited. Compared with QTL mapping, GWA can always detect more alleles in one experiment. Because natural populations, especially Tibetan wild barley accessions used in the present study, possess wider genetic variation and may contain richer tolerant alleles in comparison with a DH population.
During the extended period of domestication, especially the modern breeding and intensified cultivation, genetic diversity of cultivated barley declined gradually and rare alleles disappeared. Barren genetic background became the bottleneck of breakthrough for breeding. The Tibet plateau is considered as one of the centers of cultivated barley domestication [31]–[33]. The Tibetan wild barley has been proved to be rich in genetic diversity of stress adaptation or tolerance [34]–[35]. Due to harsh environments in Tibet, special resistant mechanisms may be developed and some excellent alleles may be formed in Tibetan wild barley. In fact, there is clear evidence that Tibetan wild barley contains elite alleles of HKT1, HKT2 [36] and HvCBF4 [37], conferring enhanced salinity tolerance.
In the present study, four root parameters were precisely determined by using a root morphology analysis system, which is modified according to Famoso et al [8], consisting of a root scanning system WinRHIZO and root image analysis software RootReader2D. The relationships between these root parameters were analyzed. Subsequently, population structure was analyzed in whole accessions, and linkage disequilibrium (LD) decays of cultivated and Tibetan wild barleys were determined and compared. Finally, genome-wide association analysis (GWAS) was conducted by using the four root parameters and 469 DArT markers within or across the cultivated barley and Tibetan wild accessions.
Materials and Methods
Plant materials and growth conditions
A total of 110 Tibetan wild barley accessions and 56 cultivated cultivars were used for Al tolerance identification and association mapping. All wild barleys were kindly provided by professor Sun of Huazhong Agricultural University, China [36], [37], and 56 cultivated barleys are widely planted in the different areas of China, but no one was from Tibet. Barley seeds were soaked in deionized water for 6 h at 20°C in the dark and then germinated on moist filter paper in an incubator (20/14°C, day/night) for 2 days. Then, nine seedlings (about 2 cm root length) were selected for both control and treatment, scanned by WinRHIZO [38], a root-measuring system, and images were acquired for precise analysis of root length. After scanning, seedlings were placed on the net cups floating on a 0.2 mM CaCl2 solution, pH 4.3, in 50 ml centrifugal tubes as control. For Al stress treatment, seedlings were exposed to a 0.2 mM CaCl2 solution, pH 4.3, containing 5 μM AlCl3. The roots were imaged for both control and Al3+-treated plants after 6 days treatment.
Measurement and calculation of root parameters
The images were analyzed by RootReader2D (http://www.plantmineralnutrition.net/rootreader. htm), and longest root length (LR) and total root length (TR) were recorded. To evaluate Al3+ tolerance for each genotype, root growth parameters were calculated as follows:
Longest root growth (LRG) = LRT6d – LRT0d*, which represents absolute longest root elongation under Al stress;
* T and CK represent Al3+ treatment and control respectively, and 0d and 6d represent plants with initial and 6 days treatments, respectively.
The correlation analysis among these root parameters was conducted by SPSS (v19.0).
Population structure, cluster and linkage disequilibrium (LD) analysis
The samples of the whole-genome profiling were analyzed using the Barley PstI (BstNI) version 1.7 array with Diversity Arrays Technology (DArT P/L) in Australia (http://www.triticarte.com.au/content/barley_diversity_analysis.html). A total of 469 barley DArT markers (MAF>0.03) distributed over the whole genome were used to detect population structure using the STRUCTURE software v2.3.3 [39], [40], performing ten independent runs, setting the number of clusters (k) from 1 to 10, with 100,000 MCMC (Markov Chain Monte Carlo) iterations in an admixture model. The largest value of statistic index Δk, which was the change rate of LnP(D) (from STRUCTURE output), was used as an indicator of true number of clusters (k) [41]. We calculated genetic distance with NTSYSpc (version 2.10e) and developed a neighbor-joining tree according to Nei [42].
Linkage disequilibrium (LD) between every two linear DArT markers was estimated by TASSEL (v3.0) [43] for Tibetan wild and cultivated barleys individually. The r2 values, the squared allele frequency correlations, which is a measurement of the correlation between a pair of variables, and genetic distances were selected to display LD decay curve and fitted equation using Origin Pro (v8.0). LD decay distance in the whole genome was calculated as the genetic distance, when r2 = 0.1 using the fitted equation.
Genome-wide association analysis (GWAS)
Association analysis between 469 DArT markers and four root parameters was performed using three approaches in all accessions (166) using TASSEL (v3.0) [43], [44]. The first approach was Q method, in which Q matrix was included as a cofactor in the regression model to correct population structure. The second approach was k method, and a kinship, which represented the pair-wise relationship matrix, was estimated using software SPAGeDi [44], [45], and considered as a cofactor in the regression model. The third approach was Q+k method, considering both population structure and kinship as cofactors. According to the Q−Q plot from the output of TASSEL, both k and Q+k methods were appropriate for the present study. Then, we conducted GWAS using k method in Tibetan wild and cultivated barleys, respectively. The Benjamini-Hochberg false discovery rate (BH-FDR) [46] of q-value = 0.01 was applied in association significance test. Manhattan plots were displayed by R software v2.14.2.
Root index distribution analysis
According to the polymorphism (two type: 1 and 2) of detected markers and subpopulation groups (Wild barley, w; cultivated barley, c) and the root index of accessions were classified into four groups in a box plot. Five detected markers (bpb-6949, bpb-9458, bpb-8524, bpb-0631 and bpb-8021) were analyzed independently. ANOVA analysis was conducted among four groups (w1, w2, c1 and c2) using LSD test.
Results
Al tolerance evaluation based on root indexes and their relationships
A total of 110 Tibetan wild accessions and 56 cultivated barleys (Table 1) were evaluated for Al tolerance in response to 5 μM Al3+. A wide range of variations in LRG, TRG, RLRG and RTRG were observed (Fig. 1a–d). Under Al3+ stress, LRG and TRG were distributed around a mean of 1.40±0.28 (SD) and 6.68±1.70, ranging from 0.64–2.14 and 1.84–10.92, respectively. RLRG and RTRG were present with a mean of 0.373±0.094 and 0.397±0.107, ranging from 0.183–0.767 and 0.116–0.696, respectively. Weak correlations (less than 0.15) were found between root absolute elongation (LRG, TRG) and relative growth (RLRG, RTRG) (Table 1), suggesting that absolute and relative growth could be different in evaluation of barley Al tolerance.
Table 1. The correlations between root parameters.
| RTRG* | TRG | RLRG | LRG | |
| RTRG | 1.000 | |||
| TRG | 0.141 | 1.000 | ||
| RLRG | 0.776** | −0.005 | 1.000 | |
| LRG | 0.075 | 0.740** | 0.123 | 1.000 |
, RTRG, relative total root growth; TRG, total root growth; RLRG, relative longest root growth; LRG, longest root growth.
represent significant correlation (P<0.01).
Figure 1. Distribution of four root parameters.
Four root parameters were used for evaluation of Al tolerance in 166 accessions. a, RTRG; b, RLRG; c, TRG; d, LRG. The y axis represents frequency of root index.
Genetic division between Tibetan wild and cultivated barleys
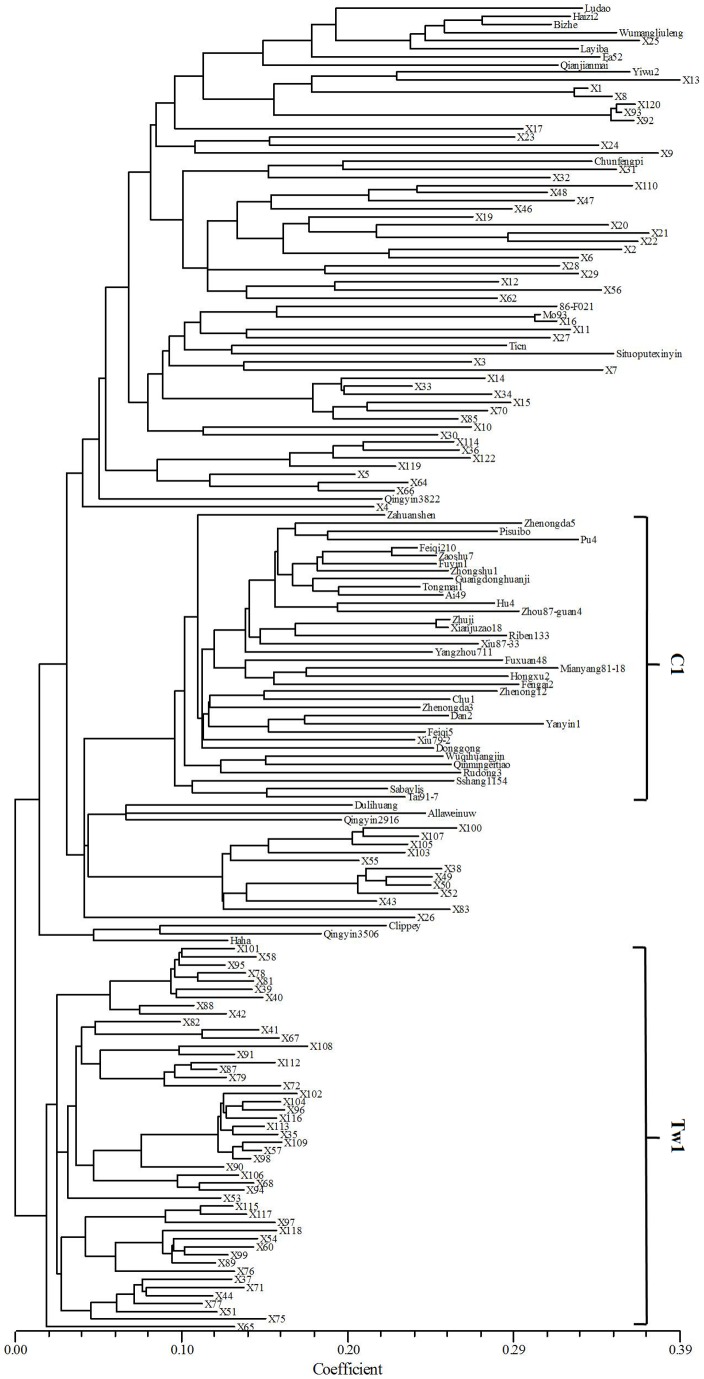
Population structure was analyzed using 469 DArT markers. The k2Q1 group, consisting of forty-eight Tibetan accessions with Q value >90%, could be distinctly detected when k was 2 or more (Fig. 2a, b). The result was consistent with the data from the cluster analysis (Fig. 3), in which Tw1 consisting of 48 Tibetan wild accessions showed significant genetic division from the remaining accessions. It is suggested that wide genetic diversity is present in Tibetan wild barley.
Figure 2. Population structure and Δk.
(a, b) Population structure of 166 barley accessions based on genetic diversity detected by 469 DArT markers with k = 2 (a) and k = 3 (b). X axis represents 1–166 accessions. Each accession ordered by membership coefficient (Q) is represented by a line partitioned in colored segments that represent the individual's estimated membership fractions. The number behind k means the total number of subpopulations, and the number behind Q represents which subpopulation the accession belongs to. (c) Δk. Estimation of the most probable number of clusters (k), based on 10 independent runs and k ranging from 1 to 10.
Figure 3. Phylogenetic tree (neighbor-joining) of 166 barley accessions based on 469 DArT markers.
Tw1 group consists of 48 Tibetan wild accessions, and C1 group includes 35 cultivated accessions.
To determine the number of subpopulations suitable for association analysis, Δk criterion was applied, and the most evident level of differentiation was observed with k = 3 (Fig. 2c). The k3Q1 consisted of 48 Tibetan accessions, and the same was for k2Q1. Interestingly, k3Q3 (Q>60%) consisted of 32 cultivated barleys (Fig. 2b), revealing the existence of considerable genetic diversity between Tibetan and cultivated barleys. Cluster analysis showed a similar result that 35 cultivated barleys belonged to C1 group (Fig. 3).
Linkage disequilibrium (LD) decay of genetic distance in the genome of Tibetan and cultivated barleys was 9.30 cM and 11.52 cM (r2 = 0.1) (Fig. 4), respectively. Thus 469 DArT markers used in the present study could cover the whole genomic region, and are sufficient for genome wide association analysis.
Figure 4. Decay of linkage disequilibrium (LD) of the whole genome of Tibetan wild barleys and cultivated barleys.
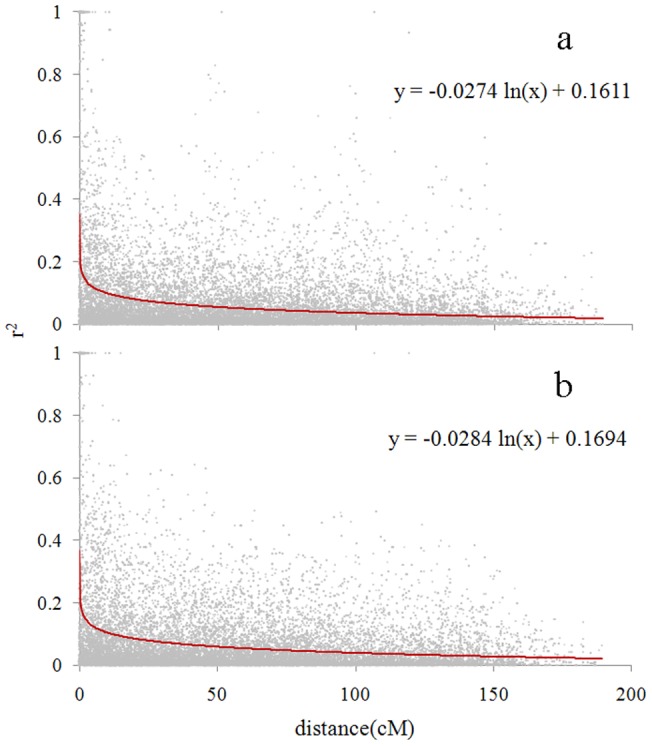
(a) LD decay in Tibetan wild barleys. (b) LD decay in cultivated barleys. The y axis r2 represents the squared allele frequency correlation coefficient between every two linear DArT markers. The decay of genetic distance is 9.30 cM (r2 = 0.1) in Tibetan wild barley and 11.52 cM in cultivated barley.
Identification of root parameters mediated-Al tolerance loci through GWAS
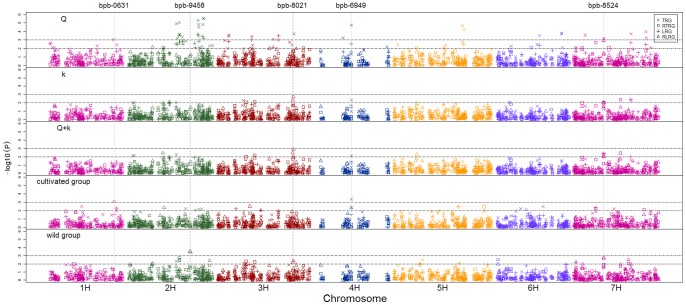
To identify Al tolerance loci, a genome wide association study was conducted using 469 DArT markers and four root growth parameters (LRG, TRG, RLRG and RTRG) (Fig. 5). When Q method was applied in association analysis across all accessions, 97 marker-trait associations, most of which belonged to TRG and LRG groups, were detected (p<0.01). However, most of these associations were identified as false positive results by Q−Q test (result by TASSEL), because the P values from TRG and LRG groups deviated from the expected value (Fig. 6a). P values from k model and Q+k model were close to expected values, indicating both models are suitable for association analysis (Fig. 6b, c). Eighteen and 16 associations were detected (p<0.01) using the k model and Q+k model, respectively, and 15 associations detected using both models.
Figure 5. GWA analysis of Al tolerance within and across 56 cultivated and 110 Tibetan wild subgroups.
Four root parameters were applied for evaluating Al tolerance: TRG (×), RTRG (□), LRG (+) and RLRG (□;). GWA analysis was firstly conducted using three different methods: Q, k and Q+k methods. Then, k method was selected to perform GWA analysis in cultivated and Tibetan wild subgroups individually. Significant associations were identified using criterion of −log10(P) >2 or 3.
Figure 6. Quantile–quantile (Q-Q) plots of estimated −log10(P).
Q−Q plots were displayed in four marker-trait association analysis using three models: a Q method, b k method, c Q+k method. The black line is the expected line under the null distribution. Red symbol represents the observed P values for LRG, yellow for TRG, blue for RLRG and green for RTRG.
Association analysis was also performed in subgroups. Twenty-seven and 24 associations were detected in the cultivated and wild groups, respectively (p<0.01). When the threshold of significant test was set at p<0.001, only three regions were detected. In the cultivated group, bpb-6949 on Chr.4H and bpb-0631 on Chr.1H explained 25.6% and 23.1% of the phenotypic variation, respectively. In the wild group, bpb-9458 and bpb-0590 on Chr.2H both explained 12.9% of the phenotypic variation (p<0.001). It was worth mentioning that three root parameters (LRG, RLRG and TRG) were associated with bpb-8524 and bpb-6707 on Chr.7H, suggesting these markers were powerfully correlated with Al tolerance, and explaining 9.7% and 9.5% of phenotypic variation. Bpb-8021 on Chr.3H was detected as the most significant marker across all accessions using both k and Q+k methods, but certified as false positive association by following distribution analysis.
Root index distribution of the detected five markers
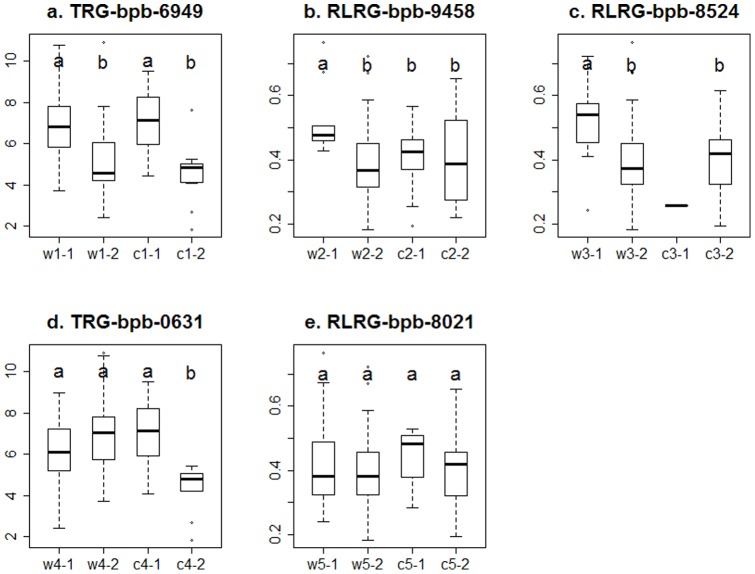
Based on marker polymorphisms, the distribution of root index were examined within cultivated and Tibetan barleys based on markers bpb-6949, bpb-9458, bpb-8524, bpb-0631 and bpb-8021 (Fig. 7a–e). W1-1 (95 accessions) showed higher TRG than w1-2 (15 accessions), which was similar to the result in the cultivated group (Fig. 7a). However, bpb-6949 was detected in all accessions and cultivated group by GWA, but not in the wild group. It may be explained that this Al3+-tolerant locus commonly existed in barley accessions, but its effect may be reduced or covered by other loci in the Tibetan group due to abundant genetic diversity.
Figure 7. Distribution of root index bases on marker polymorphism of five markers in Tibetan wild and cultivated subgroups.
For each marker, four groups were included: w n-1, w n-2, c n-1 and c n-2, in which w represents Tibetan wild group, c represents cultivated group, 1 and 2 are DArT marker polumorphism, n = 1–5 represents five different DArT markers. The markers and root indexes include: (a) marker bpb-6949 associated with TRG, (b) bpb-9458with RLRG, (c) bpb-8524with RLRG, (d) bpb-0631with TRG, (e) bpb-8021with RLRG. For each marker, ANOVA analysis was conducted among four groups, and significant difference was marked by different letters.
W2-1 (10 accessions) and w3-1 (7 accessions) showed higher RLRG than that the other three groups, whereas no significant difference in RLRG was found between groups in the cultivated group (Fig. 7b, c). Moreover, only one accession was presented in haplotype c3-1 with low RLRG. These results confirmed that bpb-9458 and bpb-8524 associated with Al3+-tolerant genes were Tibetan group-specific.
C4-2 showed lower TRG than the other three groups, indicating bpb-0631 was cultivated group-specific, which was consistent with the results obtained by GWA analysis (Fig. 7d). Although bpb-8021 showed the most significance in GWA analysis across all accessions, no significant difference was found among groups within this marker, suggesting that it is a false positive association (Fig. 7e).
Discussion
GWAS results are affected by phenotyping methods
Many researchers usually consider relative longest root growth as an indicator for Al tolerance evaluation [3]–[7]. However, it was reported that the application of different phenotypic indexes directly impacted the significance of QTLs detected in rice Al tolerance [13], which was confirmed in the current study, that different loci were detected as associated with Al tolerance by using different root parameters in GWAS. Although there were significant corrections between LRG and TRG, and between RLRG and RTRG, most of loci detected are different.
Therefore, the question may be raised as to which index could be recognized as reasonable indicators for Al tolerance evaluation. In the present study, different subpopulations needed different root parameters to identify Al tolerance loci. When absolute root growth (LRG, TRG) indexes and relative root growth (RLRG, RTRG) indexes were used, 22 and 5 loci were detected respectively in the cultivated group (Table 2, Fig. 5). The different results were obtained in the Tibetan group, with 6 loci being detected using absolute root growth (LRG, TRG) indexes and 18 loci using relative root growth (RLRG, RTRG) indexes. The number of detected loci showed that absolute root growth (LRG, TRG) indexes are more suitable for the cultivated group, and relative root growth (RLRG, RTRG) indexes for the Tibetan group. However, the underlying reason is not known. Furthermore, the most significant locus bpb-6949 in the cultivated group was associated with TRG index, while bpb-9458 was detected using RLRG index in the Tibetan group. This result suggests that Al tolerance strategies differ among barley subpopulations and is mediated by developmental types of roots.
Table 2. Lists of DArT markers with a significant marker-trait association using different model (k and Q+k methods) and within subgroups.
| Trait | Marker | Chr. | Distance(cM) | Frequency(%) | −log10(p) | r2 | |
| k | LRG | bPb-9754 | 2H | 82.77 | 13.9 | 2.04 | 0.045 |
| LRG | bPb-6706 | 7H | 58.17 | 4.8 | 2.15 | 0.046 | |
| LRG | bPb-8524 | 7H | 58.02 | 4.8 | 2.10 | 0.044 | |
| LRG | bPb-9908 | 7H | 111.69 | 24.1 | 2.38 | 0.052 | |
| RLRG | bPb-1072 | 2H | 70.03 | 44.6 | 2.00 | 0.042 | |
| RLRG | bPb-3907 | 3H | 146.78 | 16.3 | 2.29 | 0.049 | |
| RLRG | bPb-8021 | 3H | 147.95 | 16.3 | 2.71 | 0.060 | |
| RLRG | bPb-8524 | 7H | 58.02 | 4.8 | 2.06 | 0.045 | |
| TRG | bPb-0858 | 2H | 95.68 | 39.2 | 2.03 | 0.043 | |
| TRG | bPb-4040 | 2H | 82.13 | 15.7 | 2.16 | 0.046 | |
| TRG | bPb-7024 | 2H | 12.92 | 5.4 | 2.01 | 0.041 | |
| TRG | bPb-4660 | 3H | 50.43 | 45.2 | 2.29 | 0.051 | |
| TRG | bPb-6949 | 4H | 72.21 | 16.3 | 2.33 | 0.050 | |
| TRG | bPb-9908 | 7H | 111.69 | 24.1 | 2.30 | 0.050 | |
| RTRG | bPb-3805 | 3H | 72.18 | 29.5 | 2.13 | 0.049 | |
| RTRG | bPb-6347 | 3H | 55.63 | 25.3 | 2.07 | 0.046 | |
| RTRG | bPb-8492 | 6H | 26.51 | 46.4 | 2.03 | 0.042 | |
| RTRG | bPb-2748 | 7H | 91.26 | 22.9 | 2.39 | 0.057 | |
| Q+k | LRG | bPb-6706 | 7H | 58.17 | 4.8 | 2.42 | 0.053 |
| LRG | bPb-8524 | 7H | 58.02 | 4.8 | 2.36 | 0.052 | |
| RLRG | bPb-1072 | 2H | 70.03 | 44.6 | 2.45 | 0.055 | |
| RLRG | bPb-3907 | 3H | 146.78 | 16.3 | 2.45 | 0.054 | |
| RLRG | bPb-8021 | 3H | 147.95 | 16.3 | 2.88 | 0.066 | |
| RLRG | bPb-3792 | 5H | 45.58 | 22.3 | 2.27 | 0.049 | |
| RLRG | bPb-8524 | 7H | 58.02 | 4.8 | 2.07 | 0.045 | |
| TRG | bPb-0615 | 2H | 11.28 | 5.4 | 2.11 | 0.042 | |
| TRG | bPb-7024 | 2H | 12.92 | 5.4 | 2.15 | 0.043 | |
| TRG | bPb-0858 | 2H | 95.68 | 39.2 | 2.25 | 0.046 | |
| TRG | bPb-6949 | 4H | 72.21 | 16.3 | 2.11 | 0.042 | |
| TRG | bPb-6706 | 7H | 58.17 | 4.8 | 2.35 | 0.049 | |
| TRG | bPb-8524 | 7H | 58.02 | 4.8 | 2.25 | 0.046 | |
| RTRG | bPb-3805 | 3H | 72.18 | 29.5 | 2.19 | 0.051 | |
| RTRG | bPb-6347 | 3H | 55.63 | 25.3 | 2.01 | 0.045 | |
| RTRG | bPb-2748 | 7H | 91.26 | 22.9 | 2.42 | 0.058 | |
| cultivated group | LRG | bPb-1959 | 1H | 133.12 | 14.3 | 2.33 | 0.158 |
| LRG | bPb-2203 | 3H | 35.93 | 7.1 | 2.21 | 0.162 | |
| LRG | bPb-0353 | 3H | 84.38 | 7.1 | 2.04 | 0.133 | |
| LRG | bPb-8015 | 3H | 84.89 | 7.1 | 2.04 | 0.133 | |
| LRG | bPb-6949 | 4H | 72.21 | 21.4 | 2.31 | 0.157 | |
| LRG | bPb-4758 | 5H | 126.53 | 14.3 | 2.49 | 0.173 | |
| LRG | bPb-0071 | 5H | 126.77 | 14.3 | 2.45 | 0.171 | |
| LRG | bPb-9601 | 7H | 42.67 | 3.8 | 2.37 | 0.162 | |
| RLRG | bPb-8700 | 2H | 72.09 | 6.6 | 2.38 | 0.162 | |
| RLRG | bPb-1012 | 3H | 62.86 | 28.6 | 2.54 | 0.210 | |
| TRG | bPb-0631 | 1H | 128.45 | 17.9 | 3.11 | 0.231 | |
| TRG | bPb-5290 | 1H | 64.89 | 30.4 | 2.51 | 0.175 | |
| TRG | bPb-1959 | 1H | 133.12 | 14.3 | 2.00 | 0.130 | |
| TRG | bPb-6822 | 2H | 114.40 | 12.5 | 2.24 | 0.151 | |
| TRG | bPb-6087 | 2H | 145.55 | 37.5 | 2.18 | 0.147 | |
| TRG | bPb-1066 | 2H | 139.75 | 37.5 | 2.14 | 0.143 | |
| TRG | bPb-0326 | 2H | 139.91 | 3.6 | 2.14 | 0.144 | |
| TRG | bPb-3536 | 2H | 132.78 | 26.8 | 2.06 | 0.148 | |
| TRG | bPb-6949 | 4H | 72.21 | 21.4 | 3.36 | 0.256 | |
| TRG | bPb-7987 | 4H | 72.27 | 16.1 | 2.39 | 0.164 | |
| TRG | bPb-0432 | 6H | 91.99 | 32.1 | 2.49 | 0.176 | |
| TRG | bPb-0934 | 6H | 47.81 | 17.9 | 2.25 | 0.155 | |
| TRG | bPb-7179 | 6H | 58.56 | 16.1 | 2.12 | 0.140 | |
| TRG | bPb-1447 | 7H | 78.22 | 3.6 | 2.20 | 0.147 | |
| RTRG | bPb-5413 | 5H | 177.26 | 8.9 | 2.48 | 0.175 | |
| RTRG | bPb-9601 | 7H | 42.67 | 3.8 | 2.23 | 0.150 | |
| RTRG | bPb-1793 | 7H | 137.20 | 3.6 | 2.12 | 0.143 | |
| Tibetan group | LRG | bPb-4494 | 5H | 127.88 | 3.6 | 2.05 | 0.065 |
| LRG | bPb-7559 | 7H | 3.02 | 19.1 | 2.21 | 0.072 | |
| LRG | bPb-0844 | 7H | 5.01 | 19.1 | 2.21 | 0.072 | |
| RLRG | bPb-9458 | 2H | 122.28 | 9.1 | 3.55 | 0.129 | |
| RLRG | bPb-0590 | 2H | 123.70 | 9.1 | 3.43 | 0.129 | |
| RLRG | bPb-6048 | 2H | 161.12 | 14.5 | 2.41 | 0.080 | |
| RLRG | bPb-5629 | 2H | 49.03 | 10.9 | 2.03 | 0.065 | |
| RLRG | bPb-8021 | 3H | 147.95 | 20.9 | 2.36 | 0.078 | |
| RLRG | bPb-3907 | 3H | 146.78 | 20.0 | 2.19 | 0.071 | |
| RLRG | bPb-6710 | 5H | 51.63 | 23.6 | 2.02 | 0.066 | |
| RLRG | bPb-8524 | 7H | 58.02 | 6.4 | 2.78 | 0.097 | |
| RLRG | bPb-6706 | 7H | 58.17 | 6.4 | 2.70 | 0.095 | |
| RLRG | bPb-0202 | 7H | 106.63 | 17.3 | 2.53 | 0.087 | |
| TRG | bPb-0858 | 2H | 95.68 | 46.4 | 2.31 | 0.077 | |
| TRG | bPb-6260 | 5H | 56.76 | 42.7 | 2.48 | 0.084 | |
| TRG | bPb-9908 | 7H | 111.69 | 22.7 | 2.07 | 0.066 | |
| RTRG | bPb-9423 | 1H | 48.95 | 19.1 | 2.00 | 0.067 | |
| RTRG | bPb-7991 | 2H | 101.27 | 30.0 | 2.72 | 0.093 | |
| RTRG | bPb-1926 | 2H | 102.12 | 30.9 | 2.49 | 0.083 | |
| RTRG | bPb-6194 | 2H | 102.38 | 30.0 | 2.44 | 0.081 | |
| RTRG | bPb-6347 | 3H | 55.63 | 16.4 | 2.30 | 0.085 | |
| RTRG | bPb-3805 | 3H | 72.18 | 30.9 | 2.25 | 0.084 | |
| RTRG | bPb-3780 | 6H | 3.55 | 47.3 | 2.54 | 0.086 | |
| RTRG | bPb-0202 | 7H | 106.63 | 17.3 | 2.05 | 0.067 |
Tibetan wild barley provides elite germplasm for barley improvement in stress tolerance
Genetic differentiation is present between Tibetan wild and cultivated barleys. This is well supported by population structure partition (k = 2) and the genetic division of Tw1 group in cluster analysis in the current study (Fig. 2a and 3). Therefore, the Tibetan wild barley could provide elite germplasm for barley improvement in abiotic and biotic stress tolerance to fight against unpredictable climate changes in the future.
Structure analysis and Δk showed that three estimated populations were present in all 166 accessions (Fig. 2b, c). A subpopulation k3Q3 group consisting of 32 cultivated cultivars, was segregated from k2Q2 group. It may be suggested that cultivated barley gradually developed and engendered its own novel physiological or molecular traits or Al tolerant strategies during its expansion into specific environments. A good example is that an Al-resistant cultivar Murasakimochi acquires enhanced Al tolerance by a 1 kb insertion in the upstream region of HvAACT1 [47]. This insertion happened during the expansion of barley cultivation onto acid soil area and was identified to be originated from Eastern Asian region [47]. However, there is no such insertion in all wild barley accessions used in the present study (Data not shown).
LD decay analysis showed that DArT markers used in this study could cover four times as the size of the barley genome, indicating the density of DArT markers sufficient for GWAS. LD decayed more rapidly in Tibetan barley (9.30 cM) than cultivated barley (11.52 cM), thus the results of GWAS in Tibetan wild group can provide higher resolution of fine mapping and gene discovery than those in cultivated barley.
In short, it may be concluded that Tibetan wild barley is appropriate and efficient for genome-wide association by which we may detect rare elite alleles contributing to elevated Al tolerance in barley.
Novel Al-tolerant loci are detected by GWAS
GWA mapping by using 110 Tibetan wild and 56 cultivated barleys genotyped with 469 DArT markers identified four significant regions, all of which are subpopulation-specific.
In the cultivated group, bpb-6949 was localized at 0.8 cM away from a major QTL in Chr.4H and a candidate gene HvMATE, which is considered as a major gene conferring barley Al tolerance [26], [48]. This marker was also detected (p<0.01) when all barley genotypes were examined, suggesting that it is a common locus related to Al tolerance in barley.
However, the genetic diversity of tolerant genotypes used in a previous QTL study was limited [49]–[53]. Only the alleles segregating between parents of DH population could be identified by the QTL analysis. HvMATE (HvAACT1) was identified by using Dayton and Murasakimochi [4], [26]. The two genotypes, as well as WB229 and FM404 were used as tolerant parents in the previous QTL studies. All are cultivated barleys. In the present study, GWAS was also performed in the Tibetan group and novel loci were identified on Chr.2H and Chr.7H. This result strongly suggests that different Al tolerant mechanism exists in Tibetan barley. The genotypes selected from haplotype analysis and the detected loci could be used in marker-assistant breeding. However, these possible Al-tolerant genes are still unknown, and fine mapping is required for gene discovery in future research.
Acknowledgments
We thank professor Dongfa Sun of Huazhong Agriculture University for his kind help in providing Tibetan wild barley, and Ms. Mei Li and Ms. Jingqun Yuan, the technicians of 985-Institute of Agrobiology and Environmental Sciences of Zhejiang University, for providing convenience in using the experimental equipments.
Funding Statement
This research was supported by National Natural Science Foundation of China (No. 30971719), 863 projects of Ministry of Sciences and Technology, China (2011AA101105), and Zhejiang Provincial Natural Science Foundation of China (No. Z3110054). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Foy CD (1988) Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant Anal 19: 959–987. [Google Scholar]
- 2. Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260. [Google Scholar]
- 3. Caniato FF, Guimarães CT, Hamblin M, Billot C, Rami JF, et al. (2011) The relationship between population structure and aluminum tolerance in cultivated sorghum. PLoS One 6(6): e20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, et al. (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 5. Wu P, Liao CY, Hu B, Yi KK, Jin WZ, et al. (2000) QTLs and epistatis for aluminum tolerance in rice (Oryza sativa L.) at different seedling stages. Theor Appl Genet 100: 1295–1303. [Google Scholar]
- 6. Xue Y, Jiang L, Su N, Wang JK, Deng P, et al. (2007) The genetic basic and fine-mapping of a stable quantitative-trait loci for aluminum tolerance in rice. Planta 227: 255–262. [DOI] [PubMed] [Google Scholar]
- 7. Zhao ZQ, Ma JF, Sato K, Takeda K (2003) Differential Al resistance and citrate secretion in barley. Planta 217: 794–800. [DOI] [PubMed] [Google Scholar]
- 8. Famoso AN, Clark RT, Shaff JE, Craft E, McCouch SR, et al. (2010) Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol 153(4): 1678–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arroyave C, Barceló J, Poschenrieder C, Tolrà R (2011) Aluminium-induced changes in root epidermal cell patterning, a distinctive feature of hyperresistance to Al in Brachiaria decumbens. J Inorg Biochem 105: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 10. Jones DL, Kochian LV (1995) Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell 7: 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garzón T, Gunsé B, Moreno AR, Tomos AD, Barceló J, et al. (2011) Aluminium-induced alteration of ion homeostasis in root tip vacuoles of two maize varieties differing in Al tolerance. Plant Sci 180: 709–715. [DOI] [PubMed] [Google Scholar]
- 12. Silva S, Santos C, Matos M, Pinto-Carnide O (2012) Al toxicity mechanism in tolerant and sensitive rye genotypes. Environ Exp Bot 75: 89–97. [Google Scholar]
- 13. Famoso AN, Zhao K, Clark RT, Tung CW, Wright MH, et al. (2011) Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet 7(8): e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Negishi T, Oshima K, Hattori M, Kanai M, Mano S, et al. (2012) Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoS One. 7(8): e43189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang JL, Zhu XF, Zheng C, Zhang YJ, Zheng SJ (2011) Genotypic differences in Al resistance and the role of cell-wall pectin in Al exclusion from the root apex in Fagopyrum tataricum. Ann Bot 107(3): 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17 (6): 341–348. [DOI] [PubMed] [Google Scholar]
- 17. Larsen P, Geisler M, Jones C, Williams K, Cancel J (2005) ALS3 encodes a phloem localized ABC transporter like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41: 353–363. [DOI] [PubMed] [Google Scholar]
- 18. Magalhaes J, Garvin D, Wang Y, Sorrells M, Klein P (2004) Comparative mapping of a major aluminum tolerance gene in sorghum and other species in the Poaceae. Genetics 167: 1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maron LG, Kirst M, Mao C, Milner MJ, Menossi M, et al. (2008) Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol179: 116–128. [DOI] [PubMed] [Google Scholar]
- 20. Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, et al. (2004) A gene encoding an aluminum-activated malate transporter segregates with aluminum tolerance in wheat. Plant J 37: 645–653. [DOI] [PubMed] [Google Scholar]
- 21. Xia J, Yamaji N, Kasai T, Ma J (2010) Plasma membrane-localized transporter for aluminum in rice. P Nati Acad Sci USA 107: 18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6: 273–278. [DOI] [PubMed] [Google Scholar]
- 23. Piñeros MA, Shaff JE, Manslank HS, Alves VM, Kochian LV (2005) Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol 137: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminuminduced increase in rhizosphere pH. Plant Physiol 117: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu XF, Shi YZ, Lei GJ, Fry SC, Zhang BC, et al. (2012) XTH31, Encoding an in Vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 24(11): 4731–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang JP, Raman H, Zhou MX, Ryan PR, Delhaize E, et al. (2007) High-resolution mapping of the Alp locus and identification of a candidate gene HvMATE controlling aluminium tolerance in barley (Hordeum vulgare L.). Theor Appl Genet 115: 265–276. [DOI] [PubMed] [Google Scholar]
- 27. Gruber BD, Ryan PR, Richardson AE, Tyerman SD, Ramesh S, et al. (2010) HvALMT1 from barley is involved in the transport of organic anions. J Exp Bot 61(5): 1455–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruber BD, Delhaize E, Richardson AE, Roessner U, James RA, et al.. (2011) Characterisation of HvALMT1 function in transgenic barley plants. Funct Plant Biol 38(2) 163–175. [DOI] [PubMed]
- 30.Raman H, Wang JP, Read B, Zhou MX, Vengatanagappa S, et al.. (2005) Molecular mapping of resistance to aluminium toxicity in barley. In: Proceedings of Plant and Animal Genome XIII Conference, January 15–19, San Diego, p. 154.
- 31. Dai F, Nevo E, Wu D, Comadran J, Zhou M, et al. (2012) Tibet is one of the centers of domestication of cultivated barley. Proc Natl Acad Sci USA 109(42): 16969–16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu TW (1975) On the origin and phylogeny of cultivated barley with preference to the discovery of Ganze wild two-rowed barley Hordeum spontaneum c. Koch Acta Genet Sin 2: 129–137. [Google Scholar]
- 33. Xu TW (1982) Origin and evolution of cultivated barley in China. Acta Genet Sin 9: 440–446. [Google Scholar]
- 34. Nevo E (2006) Genome evolution of wild cereal diversity and prospects for crop improvement. Plant Genet Resour 4: 36–46. [Google Scholar]
- 35.Zohary D, Hopf M, Weiss E (2012) Domestication of pants in the old world: The origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin (Oxford Univ Press, Oxford).
- 36. Qiu L, Wu D, Ali S, Cai S, Dai F, et al. (2011) Evaluation of salinity tolerance and analysis of allelic function of HvHKT1 and HvHKT2 in Tibetan wild barley. Theor Appl Genet 122(4): 695–703. [DOI] [PubMed] [Google Scholar]
- 37. Wu DZ, Qiu L, Xu LL, Ye LZ, Chen MX, et al. (2011) Genetic Variation of HvCBF Genes and Their Association with Salinity Tolerance in Tibetan Annual Wild Barley. PLoS One 6(7): e22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arsenault JL, Pouleur S, Messier C, Guay R (1995) WinRHIZOTM, a root-measuring system with a unique overlap correction method. Hort Sci 30: 906. [Google Scholar]
- 39. Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164: 1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- 42. Nei M (1972) Genetic distance between populations. Am Nat 106: 283–292. [Google Scholar]
- 43. Bradbury PJ, Zhang ZW, Kroon DE, Casstevens RM, Ramdoss Y, et al. (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19): 2633–2635. [DOI] [PubMed] [Google Scholar]
- 44. Yu JM, Pressoir G, Briggs WH, Bi IV, Yamasaki M, et al. (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38: 203–208. [DOI] [PubMed] [Google Scholar]
- 45. Hardy OJ, Vekemans X (2002) Spagedi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2: 618–620. [Google Scholar]
- 46. Benjamini Hochberg (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 57: 289–300. [Google Scholar]
- 47. Fujii M, Yokosho K, Yamaji N, Saisho D, Yamane M, et al. (2012) Acquisition of aluminium tolerance by modification of a single gene in barley. Nat Commun 3: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma JF, Nagao S, Sato K, Ito H, Furukawa J, et al. (2004) Molecular mapping of a gene responsible for Al-activated secretion of citrate in barley. J Exp Bot 55(401): 1335–1341. [DOI] [PubMed] [Google Scholar]
- 49. Echart CL, Barbosa-Neto JF, Garvin DF, Cavalli-Molina S (2002) Aluminum tolerance in barley: Methods for screening and genetic analysis. Euphytica 126: 309–313. [Google Scholar]
- 50. Minella E, Sorrells ME (1997) Inheritance and chromosome location of Alp, a gene controlling aluminum tolerance in ‘Dayton’ barley. Plant Breed 116: 465–469. [Google Scholar]
- 51. Raman H, Moroni JS, Sato K, Read BJ, Scott BJ (2002) Identification of AFLP and microsatellite markers linked with an aluminium tolerance gene in barley (Hordeum vulgare L.). Theor Appl Genet 105: 458–464. [DOI] [PubMed] [Google Scholar]
- 52.Reid DA (1970) Genetic control of reaction to aluminum in winter barley. In: Proceedings of the 2nd International Barley Genetics Symposium, Pullman, pp.409–413.
- 53. Tang Y, Sorrells ME, Kochian LV, Garvin DF (2000) Identification of RFLP markers linked to the barley aluminum tolerance gene Alp . Crop Sci 40: 778–782. [Google Scholar]