Abstract
Objective
To determine differences in TNF-α, IL-1β, IL-10, sICAM-1 concentrations, leg hypoxia and whole blood viscosity (WBV) at shear rates of 46 sec-1 and 230 sec-1 in persons with homozygous S sickle cell disease (SCD) with and without chronic leg ulceration and in AA genotype controls.
Design
& Methods: fifty-five age-matched participants were recruited into the study: 31 SS subjects without leg ulcers (SSn), 24 SS subjects with leg ulcers (SSu) and 18 AA controls. Haematological indices were measured using an AC.Tron Coulter Counter. Quantification of inflammatory, anti-inflammatory and adhesion molecules was performed by ELISA. Measurement of whole blood viscosity was done using a Wells Brookfield cone-plate viscometer. Quantification of microvascular tissue oxygenation was done by Visible Lightguide spectrophotometry.
Results
TNF-α and whole blood viscosity at 46 sec-1 and 230 sec-1 (1.75, 2.02 vs. 0.83, 1.26, p<0.05) were significantly greater in sickle cell disease subjects than in controls. There were no differences in plasma concentration of sICAM-1, IL-1β and IL-10 between SCD subjects and controls. IL-1β (median, IQR: 0.96, 1.7 vs. 0, 0.87; p<0.01) and sICAM-1 (226.5, 156.48 vs. 107.63, 121.5, p<0.005) were significantly greater in SSu group compared with SSn. However there were no differences in TNF-α (2, 3.98 vs. 0, 2.66) and IL-10 (13.34, 5.95 vs. 11.92, 2.99) concentrations between SSu and SSn. WBV in the SSu group at 46 sec-1 and at 230 Sec 1 were 1.9 (95%CI; 1.2, 3.1) and 2.3 (1.2, 4.4) times greater than in the SSn group. There were no differences in the degree of tissue hypoxia as determined by lightguide spectrophotometry.
Conclusion
Inflammatory, adhesion markers and WBV may be associated with leg ulceration in sickle cell disease by way of inflammation-mediated vasoocclusion/vasoconstriction. Impaired skin oxygenation does not appear to be associated with chronic ulcers in these subjects with sickle cell disease.
Introduction
Chronic leg ulceration is the most common cutaneous manifestation of homozygous sickle cell disease (SCD) [1], predominantly affecting the medial and lateral malleoli, and to a lesser extent, the anterior shin or dorsum of the foot [2]. A cumulative involvement of about 70% has been reported by the 30th year of life, establishing these lesions as a major source of morbidity among Jamaican SCD patients [3]. However, the most recent estimates among this group of patients have reported a prevalence of 29.5% and a cumulative incidence of 16.7% [4]. The tropical climate and low socio-economic status are likely contributors to the aetiology of chronic leg ulcers in the Jamaican population [4]. Other risk factors for ulceration include high white cell count, serum lactate dehydrogenase and venous incompetence [5,6].
The propensity of sickle red blood cells (RBC) for vasoocclusion and abnormal flow behaviours are central in propagating some of the diverse vascular symptoms associated with the condition, including systemic [7] and pulmonary [7–9] hypertension. Endothelial dysfunction is a feature of sickle cell disease [10,11] which likely influences whole blood viscosity and blood flow. A possible involvement of incompetent calf veins and elevated intravascular pressures in sickle ulceration, especially in the dependent position [5,6], suggest a role of abnormal flow behaviours in its development and/or maintenance. Moreover, a preponderance of abnormal adhesion properties [7–9] in SCD exacerbates this low flow state thereby influencing haemoglobin S polymerization, relative hyperviscosity, ischaemia and reperfusion tissue injury. The adhesion molecule sICAM-1 is constitutively expressed by endothelial cells and is up-regulated in response to inflammatory stimulus such as the cytokines TNF-α and IL-1β [12]. Shiu et al. demonstrated an increase in both membrane bound and soluble sICAM-1 expression upon perfusion of endothelial cells with sickle erythrocytes [13], where there was a greater concentration of inflammatory mediators suggesting a mechanistic link between vascular inflammation and adhesion. In addition, SCD is associated with increased propensity to infections, possibly a consequence of reticuloendothelial dysfunction. Infection-mediated endothelial activation by way of NFk-β nuclear translocation is important in the inflammatory response through the synthesis and secretion of pro-inflammatory cytokines [14,15], a correlate of clinical severity in SCD [16,17]. However, whether these inflammatory markers are associated with leg ulceration in sickle cell disease is unclear. Abnormal rheological properties of sickle cell disease characterized by an abnormal viscosity profile may be linked to a pro-adhesive state in the microcirculation. Reduced tissue perfusion has been reported in Jamaican ulcer patients [5] and could be related to viscosity changes.
The investigation of microvascular cutaneous blood flow has been used extensively in the assessment of vascular abnormalities in diseases such as diabetes [18,19] and sickle cell disease [6,20,21]. Laser Doppler fluxmetry and venous occlusion plethysmography are among the established noninvasive methods of quantifying microcirculatory blood flow. Visible lightguide spectrophotometry is another noninvasive measure which has been widely used in the assessment of amputation viability in critically ischaemic limbs [22–24] and lower limb cutaneous perfusion in diabetes [25] but remains unexplored in sickle cell disease. Spectrophotometry in the visible range has been developed for the determination of oxygenation in the inflamed skin [23]. Given the ubiquity of mediators of abnormal blood flow in SS disease, we decided to investigate the degree of tissue hypoxia in subjects with HbSS in order to ascertain whether tissue ischaemia may be implicated in sickle cell leg ulcers.
We hypothesize that cutaneous leg ulceration in HbSS is associated with an inflammatory aetiology marked by the up-regulation of pro-inflammatory cytokines and vascular adhesion molecules. In the present study the haematocrit-viscosity ratio (HVR), a measure of the effectiveness of erythrocytes in transporting oxygen [26–29], was used to assess blood flow conditions in the sickle cell disease subjects with ulcers and SCD controls. Additionally, cutaneous microvascular oxygenation was determined by visible lightguide spectrophotometry. We propose that abnormal rheology, inflammation and endothelial dysfunction have important roles in the pathogenesis of chronic leg ulceration in homozygous sickle cell disease.
Materials and Methods
A descriptive, cross-sectional study was conducted at the Sickle Cell Unit (SCU), Tropical Medicine Research Institute, University of the West Indies (UWI), Mona and the Department of Basic Medical Sciences (Physiology Section), UWI, Mona.
Ethics Statement
Ethical approval was granted by the University Hospital of the West Indies/Faculty of Medical Sciences/University of the West Indies (UHWI/FMS/UWI) Ethics Committee. The study was performed in accordance with the Declaration of Helsinki. Volunteers gave written, informed consent and completed an interviewer administered questionnaire.
Subjects
Fifty five subjects with homozygous sickle cell disease and 18 AA controls were identified and recruited at the SCU, UWI, Mona. Twenty four of the volunteers had an active ulcer at the time of the study and 31 had no history of ulceration. Twenty seven of the subjects with SCD, 11 with and 16 without active ulcers, participated in skin perfusion studies. Subjects were studied in the steady sate to exclude any possible effects caused by sickle painful crisis-related alterations in haemorheological and inflammatory markers. Steady state was defined as no sickle related event within the 4 weeks or blood transfusion within 3 months of the experimental study [6].
Sample collection
Venous blood (10 mL) was drawn from an antecubital vein into potassium EDTA-anticoagulated (1.5 mg/mL) vacutainer tubes. Five mL of whole blood were stored at room temperature (25°C) for viscometry and haematological analysis. The remaining 5 mL were centrifuged at 1000 g within 30 minutes of collection. Plasma aliquots were then stored into 1.5 mL Eppendorf tubes at -20°C for use in ELISA determinations.
Haematological analysis
Five ml of venous blood were drawn from an arm vein into EDTA anti-coagulated vacutainer tubes. Measurement of red blood cell, platelet counts and haemoglobin concentration were done using an AC.Tron Coulter Counter with Act.Diff Pak 4C controls (Coulter Electronics, Hialeah, FL, USA). The indices measured were haemoglobin concentration (Hb) (g/dL), red blood cell concentration (RBC) (x1012 cells/L), haematocrit (Hct) (%), platelet count (Plt) (x109/L), white cell count (WBC) (x109/L), mean corpuscular volume (MCV) (fL), mean corpuscular haemoglobin (MCH) (pg), mean corpuscular haemoglobin concentration (MCHC) (%) and red cell distribution width (RDW).
Cytokine assay
The concentrations of IL-1β, TNF-α, IL-10 and sICAM-1 in the circulation of all participants were determined by sandwich enzyme-linked immunosorbent assay (R&D Systems, 614 McKinley Place NE, Minneapolis, MN, USA).
Viscometry
Whole blood viscosity was measured with a Wells Brookfield cone-plate viscometer at a low and a high shear rate of 46 sec-1 and 230 sec-1 respectively. Measurements were performed within 15 minutes to an hour from time of sampling at native haematocrit at 37°C. Haemorheological determinations conformed to standard procedures for haematological and viscometric procedures [30].
Haematocrit-viscosity ratio (HVR)
The HVR was calculated as the ratio of the native haematocrit to whole blood viscosity [26,27,29,31].
Microvascular perfusion studies of the lower leg
Determination of microvascular tissue oxygenation (SO2) was performed on 27 subjects with HbSS, 16 with active ulcers at time of study and 11 subjects without ulcers using a Visible Lightguide Spectrophotometer (RM200 S02 Monitor, Whitland Research, Whitland, UK). The optical determination of skin oxygenation by lightguide spectrophotometry (O2C) has been described in previous studies [25,32]. The instrument functions by detecting the ratio of haemoglobin: oxyhaemoglobin in the microvasculature.
The Visible Lightguide Spectrophotometer was set up in a temperature controlled environment (24°C) free from the presence of fluorescent lighting. The machine was then calibrated using readings at the opposite ends of the colour spectrum (Black and White). Participants were then asked to sit on the examination bed with their feet positioned such that the shin was parallel to the floor. The probe was then lightly placed on the leg, 3cm medial to the shin and an initial So2 reading was obtained. The probe was then moved down the leg at 1 cm intervals, down to the ankle.
In determining the degree of hypoxia, critical ischaemia was defined when 15% or more of the measured values fell below an oxygen saturation threshold of 15% SO2.
Statistical analysis
Data were presented as median (inter-quartile range) or means (standard deviations). Differences in median values per group were determined by the Kruskal-Wallis equality-of-populations rank test: SCD vs. AA; SSn vs. SSu. Regression analysis was used to test for relationships between independent variables and leg ulceration. Data were analyzed using Stata Statistics Data Analysis v10.1 (Statacorp, College Station, Texas).
Results
There were no differences in the anthropometric variables (age, height, weight, body mass index) and LDH by genotype. As expected, subjects with sickle cell disease had significantly lower haematocrit, haemoglobin concentrations and RBC concentrations but significantly increased leucocyte counts, platelet concentrations and RDW (Table 1). However there was no difference in MCV value between AA and subjects with sickle cell disease.
Table 1. Anthropometric variables, haematological indices and lactate dehydrogenase concentrations in sickle cell disease patients and control group.
| Variables | AA (n = 18) | SS (n = 55) |
|---|---|---|
| Age (yrs) | 34.31±9.7 | 34.84±10.4 |
| Height (cm) | 168.1±8.3 | 163±21.2 |
| Weight (Kg) | 68.7±12.5 | 60.5±9.7 |
| Body mass index | 24.23±3.57 | 24.04±8.43 |
| LDH (IU/L) | (n = 12)378±172.02 | (n = 48)1216.62±403.8 |
| Hb (g/dL) | 13.1 (2.7); 10.4, 15.7 | 7.9 (2.2); 4.9, 10.6* |
| Hct (%) | 40.8 (8.2); 33, 49.8 | 23.7 (6.5); 15.5, 32.5* |
| RBC (x1012 cells/µL) | 4.7 (1.3); 3.6, 5.7 | 2.7 (0.8); 1.6, 4.2* |
| MCV (fL) | 88 (7.6); 63.3, 101 | 88 (8.2); 71.6, 103 |
| MCH (pg) | 28.9 (1.7); 18.6, 33.1 | 29.3 (3.4); 21.7, 34.8 |
| MCHC (%) | 32.2 (1.8); 29.5, 35.3 | 33.1 (22.4);21, 37.2* |
| Plt (x109/L) | 264.5(127); 171, 451 | 386 (151);185, 746* |
| WBC (x109/L) | 5.4 (1.6); 3.6, 9.6 | 11 (3.9);5.1, 37.8* |
| RDW | 12.9 (1.3); 11.1, 16.8 | 22.3 (4.5);14.5; 33.9* |
Anthropometric and LDH values are mean±SD; haematological values are median(inter-quartile range) minimum value, maximum value. * significant difference at p<0.05.
The mean age, weight and BMI of subjects with sickle disease and leg ulcers, SSu, were significantly greater than subjects with sickle cell disease without leg ulcers, SSn. However there was no difference in mean height between the SSn and SSu groups (Table 2). Haematocrit, MCV and RBC counts were significantly lower in the SSu group compared with SSn group. In contrast, there were no significant difference between SSu group and the SSn for other haematological variables and LDH (Table 2).
Table 2. Anthropometric variables, haematological variables and lactate dehydrogenase concentrations in sickle cell disease patients with chronic leg ulcers and patients without ulcers.
| Variables | SSn (n= 31) | SSu (n= 24) |
|---|---|---|
| Age (yrs) | 31.6±10.1 | 38.7±9.7* |
| Height (cm) | 162±19.6; 111, 185.7 | 163.6±23.4; 111, 191.9 |
| Weight (Kg) | 58.1±10.5; 44, 98.9 | 63.6±7.7; 50, 82.7* |
| Body mass index | 23.29±9.17; 16.48, 51.75 | 25.0±7.44; 17.87, 44.23* |
| LDH (IU/L) | (n=29)1258.71±431.5; 545, 2129 | (n= 19) 1154.58±361.36;612, 1970 |
| Hb (g/dL) | 7.9 (2.1); 6.3, 10.6 | 7.6 (2.3); 4.9, 10.4 |
| Hct (%) | 24.7 (6.2); 18.9, 32 | 22.8 (6.6); 15.5, 32.5* |
| RBC (x1012 cells/µL) | 2.8 (0.7); 1.9, 4.2 | 2.5 (0.8); 1.6, 3.8* |
| MCV (fL) | 87 (6.4); 71.6, 103 | 91 (7.5); 80.8, 97.4* |
| MCH (pg) | 28.8(3); 21.7, 34.8 | 29.6 (3.2); 26.2, 34.8 |
| MCHC (%) | 33 (2.5); 21, 37 | 33.2 (2.5); 31.4, 37.2 |
| Plt (x109/L) | 409 (159); 185, 645 | 351.5(156); 246, 746 |
| WBC (x109/L) | 11 (4.6); 5.1, 37.8 | 11.2 (3.9); 7.9, 19.2 |
| RDW | 21.6 (4.6); 14.5, 33.9 | 23.1 (4.1); 17, 30.2 |
Anthropometric and LDH values are mean±SD; haematological values are median(inter-quartile range) minimum value, maximum value. * significant difference at p<0.05.
Plasma concentrations of the pro-inflammatory cytokines TNF-α and IL-1β, the adhesion molecule sICAM-1 and the anti-inflammatory cytokine IL-10 were measured in a total of 55 adult subjects with sickle cell disease and 17 haemoglobin AA controls. There were 32 males and 23 females with the SS genotype (median age 32; range 18-55). Of the SS subjects, 24 had an active ulcer (7 females & 17 males; median age 36.5; range 21-54) at the time of study (SSu) and 31 (16 females & 15 males; median age 32; range 18-55) were asymptomatic, HbSS subjects without ulcers (SSn).
Median TNF-α (p = 0.001) concentration was significantly increased in the sickle cell disease group. However, there were no differences in ICAM-1, IL-1β and IL-10 concentrations between patients with sickle cell disease and the control group (Table 3).
Table 3. Median inflammatory, anti-inflammatory and adhesion cytokine concentrations in sickle cell disease patients and AA controls.
| Variables | AA (n = 17) | SS (n = 55) |
|---|---|---|
| TNF-α (pg/mL) | 0(0); 0 7.2 | 0.63 (3.90); 0, 15.25* |
| IL-1β (pg/mL) | 0 (0.05); 0, 3.3 | 0 (0.91); 0, 6.33 |
| ICAM-1 (ng/mL) | 150.05 (89.91); 0, 249.01 | 93.81 (209.28); 0, 445.01 |
| IL-10 (ng/mL) | 6.36 (3.06); 0, 11.09 | 9.12 (12.76); 0, 33.56 |
Values are media(inter-quartile range) minimum, maximum value. * significant difference at p<0.05.
Median TNF-α (p = 0.001) concentration was significantly increased in the sickle cell disease group. Of the 24 subjects with active ulcers, TNF-α was detectable in 18 and IL-1β was detectable in 14 participants. The frequency of cytokine detection in sickle cell disease patients without ulcers was lower in comparison to the ulcer group, which showed 12 of 31 presenting with detectable concentrations of TNF-α and 10 with IL-1β.
Plasma concentrations of the proinflammatory cytokine IL1-β (pg/mL) (ssu vs. ssn median(IQR): 0.34 (1.28) vs. 0 (0.08); p = 0.0178), but not TNF-α (pg/mL) (1.99 (4.3) vs. 0.97 (3.38) was significantly greater in subjects with ulcers. Furthermore, comparing patients with leg ulcers with patients without ulcers, sICAM-1 (ng/mL) (141.8 (257.41) vs. 0.41 (107.3); p = 0.0152), but not IL-10 (ng/mL) (11.01 (15.95) vs. (2.98 (11.92) was significantly greater in the ulcer group (Figure 1).
Figure 1. Plasma concentration distributions of interleukin-1beta, tumor necrosis factor-alpha, inter-cellular adhesion molecule-1 and interleukin-10 in SCD patients with ulcers and without ulcers.
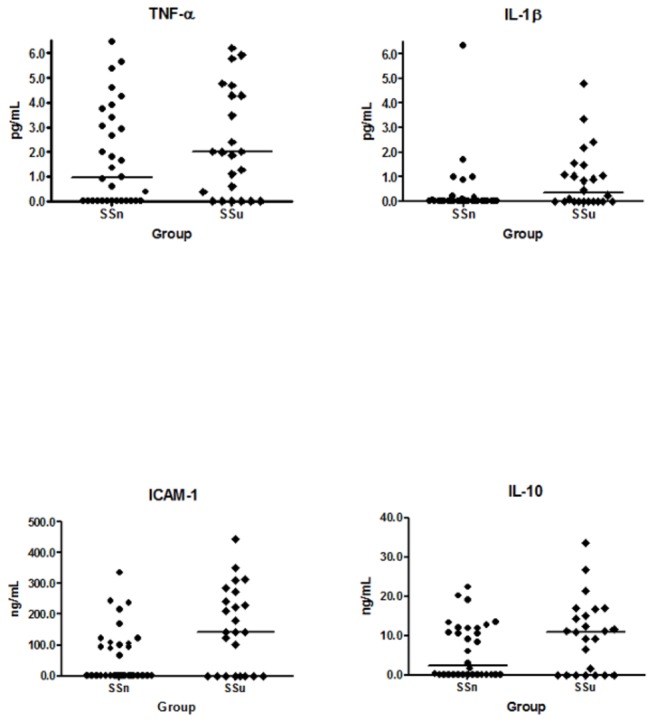
IL-1β concentration was greater in ulcer group ; TNF-α showed no difference between groups; ICAM-1 was greater in patients with ulcers; IL-10 was no different between groups.
The distribution of WBV was skewed and was normalized by Napier logarithmic transformation. WBV in the SSu group at 46 sec-1 and at 230 sec-1 was 1.9 (95%CI 1.2, 3.1) (p<0.04) and 2.3 (95%CI 1.2, 4.4) (p<0.007) times greater than the SSn group respectively (Figure 2).
Figure 2. Whole blood viscosity distribution at low and high shear rates in SCD patients with ulcers and those without.
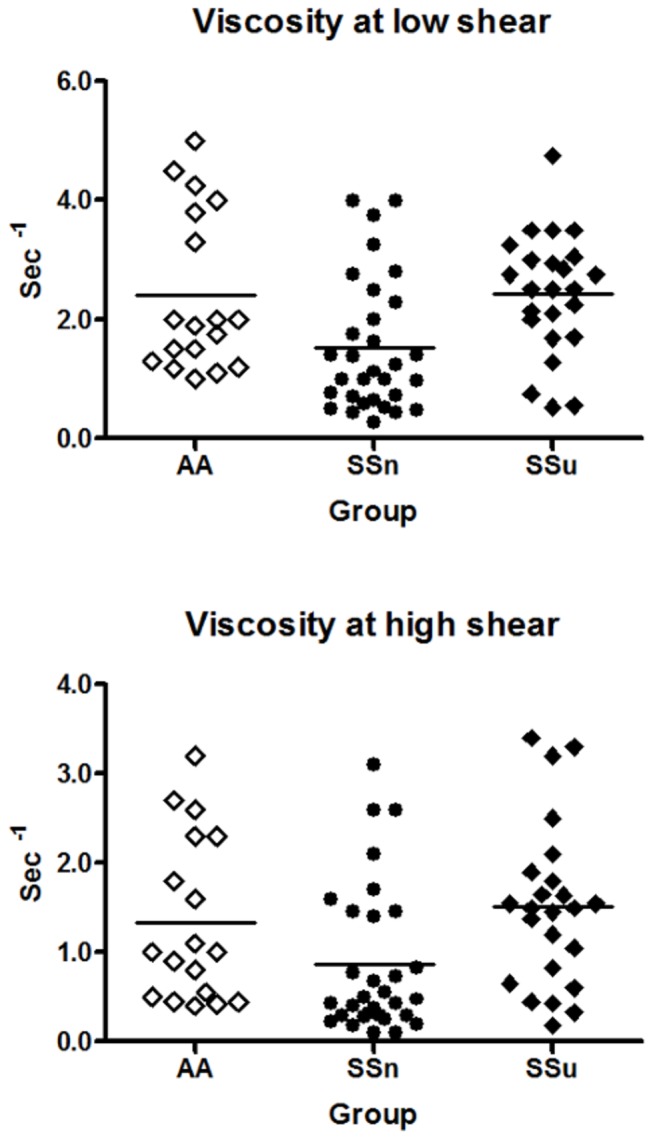
WBV was greater in the group with ulcers at both the low and high shear rates. Both groups had outliers above and below their respective WBV ranges.
The haematocrit–viscosity ratio was significantly lower in sickle cell disease subjects with ulcers in comparison to the non-ulcer group at 46 sec-1 (SSu vs. SSn: 8.73; 4.89, 45.47 vs. 22.85; 4.75, 101.78; p =0.011) and 230 sec-1 (SSu vs. SSn: 15.08; 7.4, 133.89 vs. 54.58; 7.96, 285; p =0.011), respectively. In SSu subjects the HVR was less than half that of the SSn subjects (Figure 3). There was a significant shear-dependent relationship between BMI and HVR. At low shear rate there was a -2.89 (95% CI; -0.003, 0.146; p = 0.015) change in the HVR with each unit increase in BMI. However, at high shear rate there was no significant association with the HVR and 1 unit change of BMI.
Figure 3. Erythrocyte transport effectiveness at low and high shear rates in SCD patients with ulcers and those without.
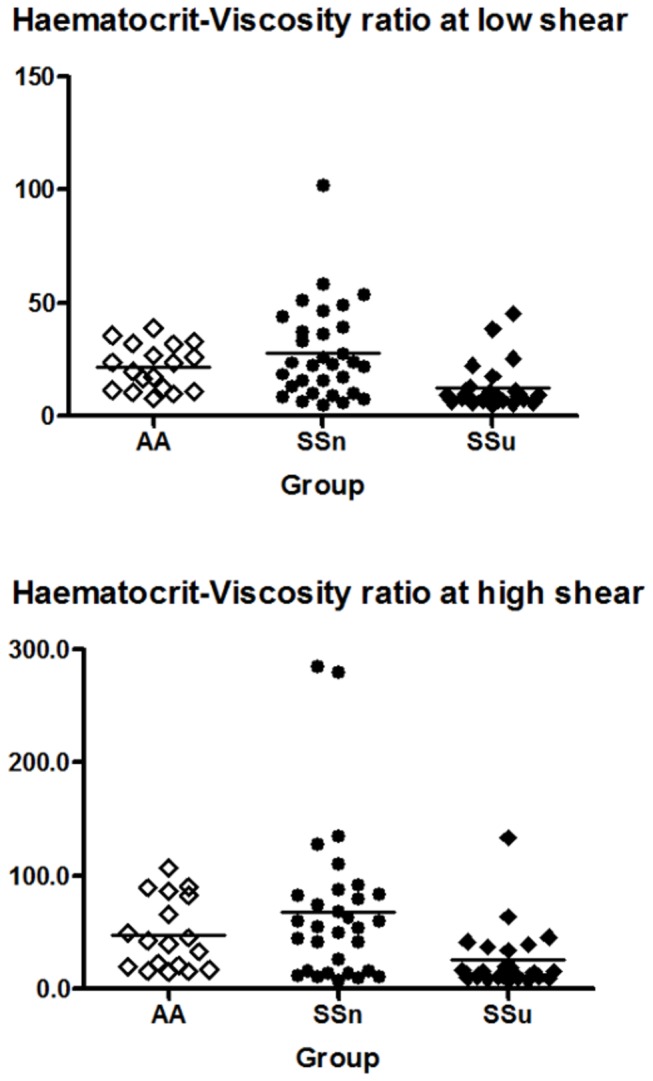
HVR was lower in the group with ulcers at both low and high shear rates of WBV.
There were no differences in cutaneous microvascular oxygen saturation as determined by lightguide spectrophotometry between SSn and SSu (Figure 4). Mean oxygen saturation was lower in subjects with ulcers than SS controls (mean +/- SD SO2: 45.02±12.97 versus 50.02±16.49). Both groups occupied similar SO2 ranges of 25-72.16 and 22-75.69 in cases and controls, respectively (Figure 4). However, none of the 11 subjects with active ulcers were classified as having hypoxia in the lower leg compared with 3 in the control group (Figure 5). Furthermore, SO2 were similar in the same subject from one leg to the next. There were no apparent relationships between the lightguide measurements and any of the investigated mediators of disease severity.
Figure 4. Lightguide haemoglobin oxygen saturation observations in SCD subjects with active leg ulcers (cases) and controls.
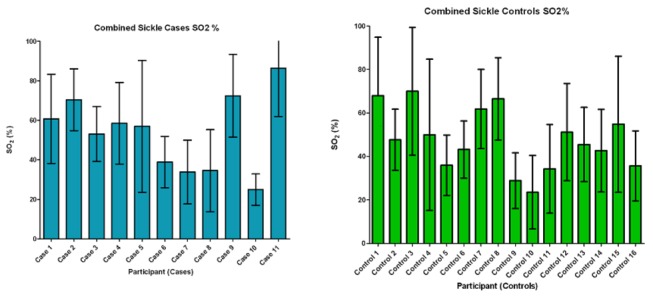
Pooled mean SO2 results taken at different sites along the length of the lower leg. Data are median with interquartile range.
Figure 5. Degree of tissue oxygen saturation distributions in SCD cases and controls.
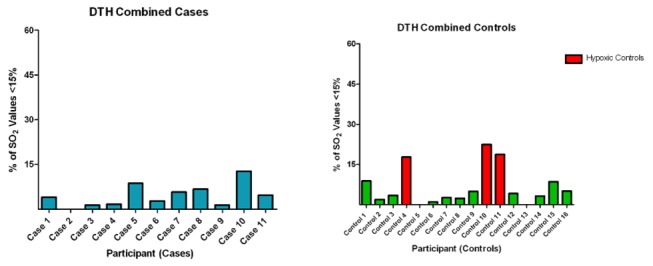
Figure shows percentage of observations with SO2 values below 15%.
Discussion
These data support the hypothesis that abnormal rheology, inflammation and endothelial dysfunction may be associated with chronic leg ulceration in sickle cell disease. Thus we found that sICAM-1 and IL-1β, markers of endothelial function and inflammation respectively, were significantly greater in SSu vs. SSn. Furthermore, consistent with previous reports suggesting an up-regulation of inflammatory pathways in sickle cell disease vs. controls, we found significantly greater concentrations of the inflammatory cytokine TNF-α [10,33–36], but not IL-1β or the adhesion molecule, ICAM-1 in the sickle cell disease group. There were no differences in the degree of tissue hypoxia between the sickle cell disease groups as measured by Visible Lightguide spectrophotometry.
Belcher et al. reported that and IL-1β serve as a marker of monocyte activation and that monocytosis is a common feature of sickle cell disease [10]. In addition, IL-1β is suggested to be involved in the activation of endothelial cells to an inflammatory phenotype. Endothelial adhesion enhances sickle cell polymerization by delaying the transit of red cells through micro-vessels, thereby promoting hypoxia and tissue infarction. These processes are amplified in the presence of the inflammatory cytokine IL-1β, which serve as a sort of triggering mechanism wherein the endothelium assumes an inflammatory state following a series of molecular conformational alterations. Shiu et al. demonstrated an increase in both membrane bound and soluble sICAM-1 expression upon perfusion of endothelial cells with sickle erythrocytes [13]. This marked an important finding since activation of the endothelium was brought about by red cells alone, not components of the plasma. Sickle cell disease subjects have been shown to be unusually susceptible to a variety of infections possibly as a consequence of reticuloendothelial dysfunction [33,37–40]. In light of such observations and the comparability of IL-10 concentration between SSn and SSu in the present study, it seems possible that there may be an imbalance between the expression of inflammatory and anti-inflammatory immune responses in sickle cell disease subjects with chronic leg ulcers.
Earlier in vitro studies have indicated that a molecular switch from a pro-inflammatory to anti-inflammatory phenotype is critical for the resolution of inflammation [25,41]. Inefficient inhibition of pro-inflammatory pathways could explain the chronic inflammatory phenotype of SCD and could be partly responsible for causing a predisposition for ulceration in a subset of SCD patients. An intriguing observation from the present findings was the discrepancy in inflammatory cytokine concentrations between the SCD patient groups, reported as significantly greater IL-1β but not TNF-α in SSu vs. SSn. Both TNF-α and IL-1β have a common activation pathway by way of nuclear factor kappa-beta (NFk-β) translocation [10]. However, since TNF-α and IL-1β have independent post-translational regulation mechanisms and endogenous inhibitors, these could account for differences in serum cytokine concentrations [42–45]. Therefore, treatment regimens targeted at reducing specific pro-inflammatory mediators involved in local inflammation in SCD may be useful in the management of sickle leg ulcers.
The involvement of the pro-inflammatory cytokines IL-1β and TNF-α in promoting endothelial adhesiveness, leukocyte activation [46] and the coagulation cascade [47] could render them potent mediators of episodic vasoocclusion in sickle cell disease and in promoting chronic leg ulcers. The properties of whole blood are such that changes in the whole blood viscosity have an inverse effect on the rate of blood flow. In vitro, this has important implications as normal blood flow can always be interrupted by any or a combination of factors which affect either shear rate or viscosity. In sickle cell blood, the abnormal rheological properties conferred by the polymerization dependent rigidification of the erythrocyte membrane are worsened by small increments in WBV. Cooperative vasoocclusion may result from clumping of rigid red cells and leucocytes and their adherence to the activated endothelium. The greater concentrations of sICAM-1, IL-1β, as wells as the raised WBV in SSu compared with SSn subjects may be indicative of inflammatory mediated vaso-occlusion in the pathogenesis of sickle cell leg ulcers. While it is conceivable how the up-regulation of adhesion factors may adversely affect WBV in vivo, it is unclear how these associations may be relevant in vitro since there is no endothelium for RBC adherence.
The present findings showed increased mean cell size in the ulcer group compared with HbSS controls. Meanwhile, RDW was similar between groups, suggestive of similar cell densities and therefore unlikely to be associated with the higher WBV in the ulcer patients. Acute phase increases in certain proteins with attendant decrease in haematocrit have been reported in diabetic foot ulcers [48] and arteriosclerosis obliterans [49]. Further investigations into the association between acute phase proteins and plasma viscosity among these ulcer patients may aid in explaining our WBV findings. It is possible that a significantly greater mean cell volume, although within normal limits, could be associated with greater WBV in patients with ulcers compared with those without ulcers. A lower HVR or otherwise termed ‘erythrocyte transport effectiveness’ [50,51] [31] describes the rheological potential of the blood and therefore serves an index for assessing the likelihood of oxygen reaching tissues in the microcirculation for a given haematocrit and WBV. However, oxygen delivery is a complex process and is ultimately determined by several other factors including pH, temperature and red cell 2,3-diphosphoglycerate concentration.
The viscosity recordings in the present work were lower than reported for normal or sickle blood [28,31]. The lower values may be due to a systematic error in the cone-plate viscometer used in our study.
There are no reports in the literature regarding the involvement of body mass index (BMI) in sickle leg ulceration. However, we reported a significantly greater BMI in patients with ulcers than those without ulcers which was related a significantly greater mean weight in the ulcer group. Earlier works have suggested an association between ulceration and BMI in diabetics [52,53]. Sickle cell disease ulceration could share a common aetiology since both conditions show similarities in several vascular complications, notably retinopathy and leg ulceration [52,54,55]. However, reports among diabetics are conflicting with observations of both positive [52] and negative [53] associations between BMI and ulceration. It is also possible that the greater BMI in the present study is related to the higher mean age in the ulcer group. The age difference between the ulcer group and patients without ulcers is consistent with findings of the role of advancing age in ulceration [4,56]. However, it is unclear how this age discrepancy may influence haemorheological determinations across groups and between genotypes.
The Lightguide flow data indicated that microvascular oxygen saturation was not a precipitating factor in leg ulceration since there was no difference in the degree of tissue oxygenation in subjects with ulcers and those without. These data as determined by our definition for hypoxia appear conflicting in consideration of the lesser HVR in subjects with ulcers. However, whilst the HVR describes the efficiency of oxygen transport by RBC, it does not quantify local tissue perfusion in absolute terms. The mean SO2 values recorded along the length of the lower leg were lesser in subjects with ulcers for both the right and left leg measurements. It is likely that local hypoxia alone is not a strong indicator for the development and/or progression of leg ulcers in SCD. Mechanical injury to the endothelium by trapped rigid cells, increased number of leucocytes leading to chronic inflammation and vascular dysfunction could represent more important biomarkers in sickle cell leg ulceration. Studies have shown that the proposed ‘fibrin cuff’ in venous diseases do not cause a significant difference in the observed diffusion block to flowing blood between controls and subjects to implicate hypoxia in its aetiology [57]. Trapped leucocytes (by way of larger size and rigidity) in the lower leg could be a stimulus for ulceration by their damaging effects on connective tissue, cell membrane and the endothelium. Paradoxically, some authors believe that WBC in the interstitium may be targeted at fibroblasts where they promote increased cellular proliferation and fibrotic connective tissue growth and the characteristic thickened hyperpigmented skin associated with foot ulcers [57]. Furthermore, histological evidence has indicated the infiltration of the capillaries of the papillary plexus by inflammatory mediators such as monocytes, macrophages and fibrin.
Other reports have likened chronic leg ulcers to a sickle cell disease sub-phenotype characterized by chronic hyper-haemolysis and a significantly lowered haemoglobin and significantly increased lactate dehydrogenase levels [4,9,58]. These contrast the present findings where we observed no differences in these variables between the ulcer group and patients without ulcers. The reasons for these differences are not clear, especially regarding conflicts among findings within the Jamaican population [4]. However, these observations suggest the presence of leg ulcers in these patients may not always be associated with more severe haemolysis than patients without ulcers. Similarities observed here between SSn and SSu could also be due to the high variation in LDH values from one patient to the next as demonstrated by wide sample dispersion.
In conclusion, we have reported that sickle cell disease is associated with an up-regulation of inflammatory and adhesive plasmatic components. There does not appear to be a direct link between microvascular oxygen saturation and ulceration as determined by spectrophotometry. On the other hand, the HVR suggests that subjects with ulcers have a greater rheological deficit than those without, namely, lower haematocrit but a higher whole blood viscosity. While endothelial dysfunction and increased whole blood viscosity in ulcer patients could simply represent consequences of localized inflammation resulting from the ulcer scar and not a cause of ulceration, the complexity of SCD vasculopathy leaves much to be understood. Another possibility is that in predisposed patients, vaso-occlusion induced inflammation could lead to vascular damage, increased inflammation, endothelial activation and ischaemia-reperfusion injury. Prolonged inflammatory responses and reduced erythrocyte transport effectiveness could therefore be important in inciting a pro-inflammatory micro-environment. Therefore, an elevation in the concentrations of pro-inflammatory cytokines and adhesion molecules with concomitant increase in whole blood viscosity may play a role in the pathogenesis of leg ulceration in sickle cell disease.
Funding Statement
Funding was provided by the Office of Graduate Studies and Research, University of the West Indies, Mona. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Koshy M, Entsuah R, Koranda A, Kraus AP, Johnson R et al. (1989) Leg ulcers in patients with sickle cell disease. Blood 74: 1403-1408. PubMed: 2475188. [PubMed] [Google Scholar]
- 2. Serjeant RG, Serjeant EB (2001) Sickle cell disease. 56. Oxford University Press; & 59 p [Google Scholar]
- 3. Serjeant GR (1974) Leg ulceration in sickle cell anemia. Arch Intern Med 133: 690-694. doi:10.1001/archinte.1974.00320160184017. PubMed: 4818436. [PubMed] [Google Scholar]
- 4. Cumming V, King L, Fraser R, Serjeant G, Reid M (2008) Venous incompetence, poverty and lactate dehydrogenase in Jamaica are important predictors of leg ulceration in sickle cell anaemia. Br J Haematol 142: 119-125. doi:10.1111/j.1365-2141.2008.07115.x. PubMed: 18477043. [DOI] [PubMed] [Google Scholar]
- 5. Mohan J, Marshall JM, Reid HL, Thomas PW, Hambleton I et al. (1998) Peripheral vascular response to mild indirect cooling in patients with homozygous sickle cell (SS) disease and the frequency of painful crisis. Clin Sci (Lond) 94: 111-120. PubMed: 9536918. [DOI] [PubMed] [Google Scholar]
- 6. Mohan JS, Marshall JM, Reid HL, Thomas PW, Serjeant GR (1997) Postural vasoconstriction and leg ulceration in homozygous sickle cell disease. Clin Sci (Lond) 92: 153-158. PubMed: 9059316. [DOI] [PubMed] [Google Scholar]
- 7. Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X et al. (2010) Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol 85: 36-40. PubMed: 20029950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashley-Koch AE, Elliott L, Kail ME, De Castro LM, Jonassaint J et al. (2008) Identification of genetic polymorphisms associated with risk for pulmonary hypertension in sickle cell disease. Blood 111: 5721-5726. doi:10.1182/blood-2007-02-074849. PubMed: 18187665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato GJ, Gladwin MT, Steinberg MH (2007) Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37-47. doi:10.1016/j.blre.2006.07.001. PubMed: 17084951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM (2000) Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood 96: 2451-2459. PubMed: 11001897. [PubMed] [Google Scholar]
- 11. de Montalembert M, Aggoun Y, Niakate A, Szezepanski I, Bonnet D (2007) Endothelial-dependent vasodilation is impaired in children with sickle cell disease. Haematologica 92: 1709-1710. doi:10.3324/haematol.11253. PubMed: 18055999. [DOI] [PubMed] [Google Scholar]
- 12. Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W et al. (1986) Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol 136: 1680-1687. PubMed: 3485132. [PubMed] [Google Scholar]
- 13. Shiu YT, Udden MM, McIntire LV (2000) Perfusion with sickle erythrocytes up-regulates ICAM-1 and VCAM-1 gene expression in cultured human endothelial cells. Blood 95: 3232-3241. PubMed: 10807794. [PubMed] [Google Scholar]
- 14. Swerlick RA, Lawley TJ (1993) Role of microvascular endothelial cells in inflammation. J Invest Dermatol 100: 111S-115S. doi:10.1038/jid.1993.33. PubMed: 8423379. [DOI] [PubMed] [Google Scholar]
- 15. Bijl M (2003) Endothelial activation, endothelial dysfunction and premature atherosclerosis in systemic autoimmune diseases. Neth J Med 61: 273-277. PubMed: 14692439. [PubMed] [Google Scholar]
- 16. Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH (1980) Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med 302: 992-995. doi:10.1056/NEJM198005013021803. PubMed: 7366623. [DOI] [PubMed] [Google Scholar]
- 17. Joneckis CC, Ackley RL, Orringer EP, Wayner EA, Parise LV (1993) Integrin alpha 4 beta 1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemia. Blood 82: 3548-3555. PubMed: 7505118. [PubMed] [Google Scholar]
- 18. Rajbhandari SM, Harris ND, Tesfaye S, Ward JD (1999) Early identification of diabetic foot ulcers that may require intervention using the micro lightguide spectrophotometer. Diabetes Care 22: 1292-1295. doi:10.2337/diacare.22.8.1292. PubMed: 10480773. [DOI] [PubMed] [Google Scholar]
- 19. Ratliff DA, Clyne CA, Chant AD, Webster JH (1984) Prediction of amputation wound healing: the role of transcutaneous pO2 assessment. Br J Surg 71: 219-222. doi:10.1002/bjs.1800710320. PubMed: 6697129. [DOI] [PubMed] [Google Scholar]
- 20. Mohan JS, Vigilance JE, Marshall JM, Hambleton IR, Reid HL et al. (2000) Abnormal venous function in patients with homozygous sickle cell (SS) disease and chronic leg ulcers. Clin Sci (Lond) 98: 667-672. doi:10.1042/CS20000004. PubMed: 10814603. [PubMed] [Google Scholar]
- 21. Gniadecka M, Gniadecki R, Serup J, Søndergaard J (1994) Microvascular reactions to postural changes in patients with sickle cell anaemia. Acta Derm Venereol 74: 191-193. PubMed: 7915459. [DOI] [PubMed] [Google Scholar]
- 22. Harrison DK, Hawthorn IE (2005) Amputation level viability in critical limb ischaemia: setting new standards. Adv Exp Med Biol 566: 325-331. doi:10.1007/0-387-26206-7_43. PubMed: 16594169. [DOI] [PubMed] [Google Scholar]
- 23. Harrison DK, McCollum PT, Newton DJ, Hickman P, Jain AS (1995) Amputation level assessment using lightguide spectrophotometry. Prosthet Orthot Int 19: 139-147. PubMed: 8927524. [DOI] [PubMed] [Google Scholar]
- 24. Harrison DK, Newton DJ, McCollum PT, Jain AS (1996) Lightguide spectrophotometry for the assessment of skin healing viability in critical limb ischaemia. Adv Exp Med Biol 388: 45-51. doi:10.1007/978-1-4613-0333-6_5. PubMed: 8798793. [DOI] [PubMed] [Google Scholar]
- 25. Quimby KR, Greenidge AR, Hennis AJ, Harrison DK, Landis RC (2010) Tumor necrosis factor receptor-associated periodic syndrome P46L and bilateral amputation in diabetes. Rheumatology (Oxf) 49: 2454-2455. doi:10.1093/rheumatology/keq227. PubMed: 20634234. [DOI] [PubMed] [Google Scholar]
- 26. Bowers AS, Pepple DJ, Reid HL (2008) Oxygen delivery index in subjects with normal haemoglobin (HbAA), sickle cell trait (HbAS) and homozygous sickle cell disease (HbSS). Clin Hemorheol Microcirc 40: 303-309. PubMed: 19126993. [PubMed] [Google Scholar]
- 27. Bowers AS, Pepple DJ, Reid HL (2009) Oxygen delivery index in homozygous sickle cell disease: steady and crisis states. Br J Biomed Sci 66: 148-149. PubMed: 19839226. [DOI] [PubMed] [Google Scholar]
- 28. Kameneva MV, Watach MJ, Borovetz HS (1999) Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc 21: 357-363. PubMed: 10711771. [PubMed] [Google Scholar]
- 29. Alexy T, Pais E, Armstrong JK, Meiselman HJ, Johnson CS et al. (2006) Rheologic behavior of sickle and normal red blood cell mixtures in sickle plasma: implications for transfusion therapy. Transfusion 46: 912-918. doi:10.1111/j.1537-2995.2006.00823.x. PubMed: 16734807. [DOI] [PubMed] [Google Scholar]
- 30. Baskurt OK, Boynard M, Cokelet GC, Connes P, Cooke BM et al. (2009) New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc 42: 75-97. PubMed: 19433882. [DOI] [PubMed] [Google Scholar]
- 31. Tripette J, Alexy T, Hardy-Dessources MD, Mougenel D, Beltan E et al. (2009) Red blood cell aggregation, aggregate strength and oxygen transport potential of blood are abnormal in both homozygous sickle cell anemia and sickle-hemoglobin C disease. Haematologica 94: 1060-1065. doi:10.3324/haematol.2008.005371. PubMed: 19644138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh DB, Stansby G, Harrison DK (2008) Assessment of oxygenation and perfusion in the tongue and oral mucosa by visible spectrophotometry and laser Doppler flowmetry in healthy subjects. Adv Exp Med Biol 614: 227-233. doi:10.1007/978-0-387-74911-2_26. PubMed: 18290333. [DOI] [PubMed] [Google Scholar]
- 33. Francis RB Jr., Haywood LJ (1992) Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J Natl Med Assoc 84: 611-615. PubMed: 1629925. [PMC free article] [PubMed] [Google Scholar]
- 34. Croizat H (1994) Circulating cytokines in sickle cell patients during steady state. Br J Haematol 87: 592-597. doi:10.1111/j.1365-2141.1994.tb08318.x. PubMed: 7527647. [DOI] [PubMed] [Google Scholar]
- 35. Kuvibidila S, Gardner R, Ode D, Yu L, Lane G et al. (1997) Tumor necrosis factor alpha in children with sickle cell disease in stable condition. J Natl Med Assoc 89: 609-615. PubMed: 9302858. [PMC free article] [PubMed] [Google Scholar]
- 36. Malavé I, Perdomo Y, Escalona E, Rodriguez E, Anchustegui M et al. (1993) Levels of tumor necrosis factor alpha/cachectin (TNF alpha) in sera from patients with sickle cell disease. Acta Haematol 90: 172-176. doi:10.1159/000204452. PubMed: 8140855. [DOI] [PubMed] [Google Scholar]
- 37. Thomas AN, Pattison C, Serjeant GR (1982) Causes of death in sickle-cell disease in Jamaica. Br Med J (Clin Res Ed) 285: 633-635. doi:10.1136/bmj.285.6342.633. PubMed: 6819042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powars D, Chan LS, Schroeder WA (1990) The variable expression of sickle cell disease is genetically determined. Semin Hematol 27: 360-376. PubMed: 2255920. [PubMed] [Google Scholar]
- 39. Thomson AP, Dick M (1988) Endotoxinaemia in sickle cell disease. Clin Lab Haematol 10: 397-401. PubMed: 3074892. [DOI] [PubMed] [Google Scholar]
- 40. Musa BO, Onyemelukwe GC, Hambolu JO, Mamman AI, Isa AH (2010) Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clin Vaccine Immunol 17: 602-608. doi:10.1128/CVI.00145-09. PubMed: 20130127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM et al. (2004) Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 94: 119-126. doi:10.1161/01.RES.0000109414.78907.F9. PubMed: 14656926. [DOI] [PubMed] [Google Scholar]
- 42. Herzyk DJ, Allen JN, Marsh CB, Wewers MD (1992) Macrophage and monocyte IL-1 beta regulation differs at multiple sites. Messenger RNA expression, translation, and post-translational processing. J Immunol 149: 3052-3058. PubMed: 1401931. [PubMed] [Google Scholar]
- 43. Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J et al. (2001) Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol 21: 6461-6469. doi:10.1128/MCB.21.9.6461-6469.2001. PubMed: 11533235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS (1996) Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: evidence for TNF-alpha posttranslational regulation. Am J Respir Crit Care Med 154: 1843-1850. doi:10.1164/ajrccm.154.6.8970379. PubMed: 8970379. [DOI] [PubMed] [Google Scholar]
- 45. Volpe F, Clatworthy J, Kaptein A, Maschera B, Griffin AM et al. (1997) The IL1 receptor accessory protein is responsible for the recruitment of the interleukin-1 receptor associated kinase to the IL1/IL1 receptor I complex. FEBS Lett 419: 41-44. doi:10.1016/S0014-5793(97)01426-9. PubMed: 9426216. [DOI] [PubMed] [Google Scholar]
- 46. Movat HZ (1987) Tumor necrosis factor and interleukin-1: role in acute inflammation and microvascular injury. J Lab Clin Med 110: 668-681. PubMed: 3316455. [PubMed] [Google Scholar]
- 47. Bauer KA, ten Cate H, Barzegar S, Spriggs DR, Sherman ML et al. (1989) Tumor necrosis factor infusions have a procoagulant effect on the hemostatic mechanism of humans. Blood 74: 165-172. PubMed: 2752108. [PubMed] [Google Scholar]
- 48. Upchurch GR Jr., Keagy BA, Johnson G Jr. (1997) An acute phase reaction in diabetic patients with foot ulcers. Cardiovasc Surg 5: 32-36. doi:10.1016/S0967-2109(97)89848-1. PubMed: 9158120. [DOI] [PubMed] [Google Scholar]
- 49. Tsushima N, Matsuo H, Hayashi T, Homma S (1996) The clinical features and treatment of arteriosclerosis obliterans with diabetes. Diabetes 45 Suppl 3: S101-S104. doi:10.2337/diabetes.45.1.101. PubMed: 8674871. [DOI] [PubMed] [Google Scholar]
- 50. Bogar L, Juricskay I, Kesmarky G, Kenyeres P, Toth K (2005) Erythrocyte transport efficacy of human blood: a rheological point of view. Eur J Clin Invest 35: 687-690. doi:10.1111/j.1365-2362.2005.01562.x. PubMed: 16269018. [DOI] [PubMed] [Google Scholar]
- 51. Bogar L, Juricskay I, Kesmarky G, Feher G, Kenyeres P et al. (2006) Gender differences in hemorheological parameters of coronary artery disease patients. Clin Hemorheol Microcirc 35: 99-103. PubMed: 16899912. [PubMed] [Google Scholar]
- 52. Sohn MW, Budiman-Mak E, Lee TA, Oh E, Stuck RM (2011) Significant J-shaped association between body mass index (BMI) and diabetic foot ulcers. Diabetes/Metab Res Rev 27: 402-409. doi:10.1002/dmrr.1193. PubMed: 21360633. [DOI] [PubMed] [Google Scholar]
- 53. Abouaesha F, van Schie CH, Griffths GD, Young RJ, Boulton AJ (2001) Plantar tissue thickness is related to peak plantar pressure in the high-risk diabetic foot. Diabetes Care 24: 1270-1274. doi:10.2337/diacare.24.7.1270. PubMed: 11423514. [DOI] [PubMed] [Google Scholar]
- 54. Asleh R, Levy AP (2005) In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag 1: 19-28. doi:10.2147/vhrm.1.1.19.58930. PubMed: 17319095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morel C (2001) [Retinal involvement in hemoglobinopathy]. J Fr Ophtalmol 24: 987-992. PubMed: 11912846. [PubMed] [Google Scholar]
- 56. Alleyne SI, Wint E, Serjeant GR (1977) Social effects of leg ulceration in sickle cell anemia. South Med J 70: 213-214. doi:10.1097/00007611-197702000-00033. PubMed: 841405. [DOI] [PubMed] [Google Scholar]
- 57. Smith PC (2006) The causes of skin damage and leg ulceration in chronic venous disease. Int J Low Extrem Wounds 5: 160-168. doi:10.1177/1534734606292429. PubMed: 16928672. [DOI] [PubMed] [Google Scholar]
- 58. Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt et al. (2006) Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 107: 2279-2285. doi:10.1182/blood-2005-06-2373. PubMed: 16291595. [DOI] [PMC free article] [PubMed] [Google Scholar]


