Abstract
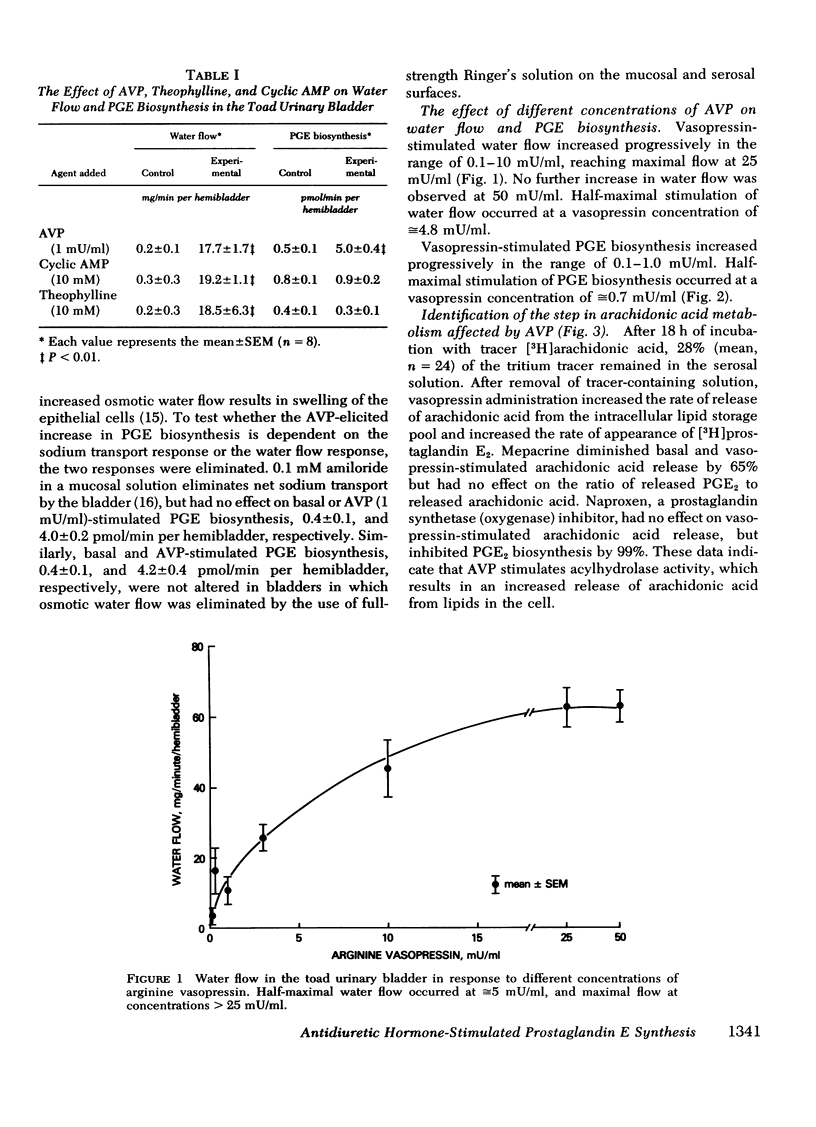
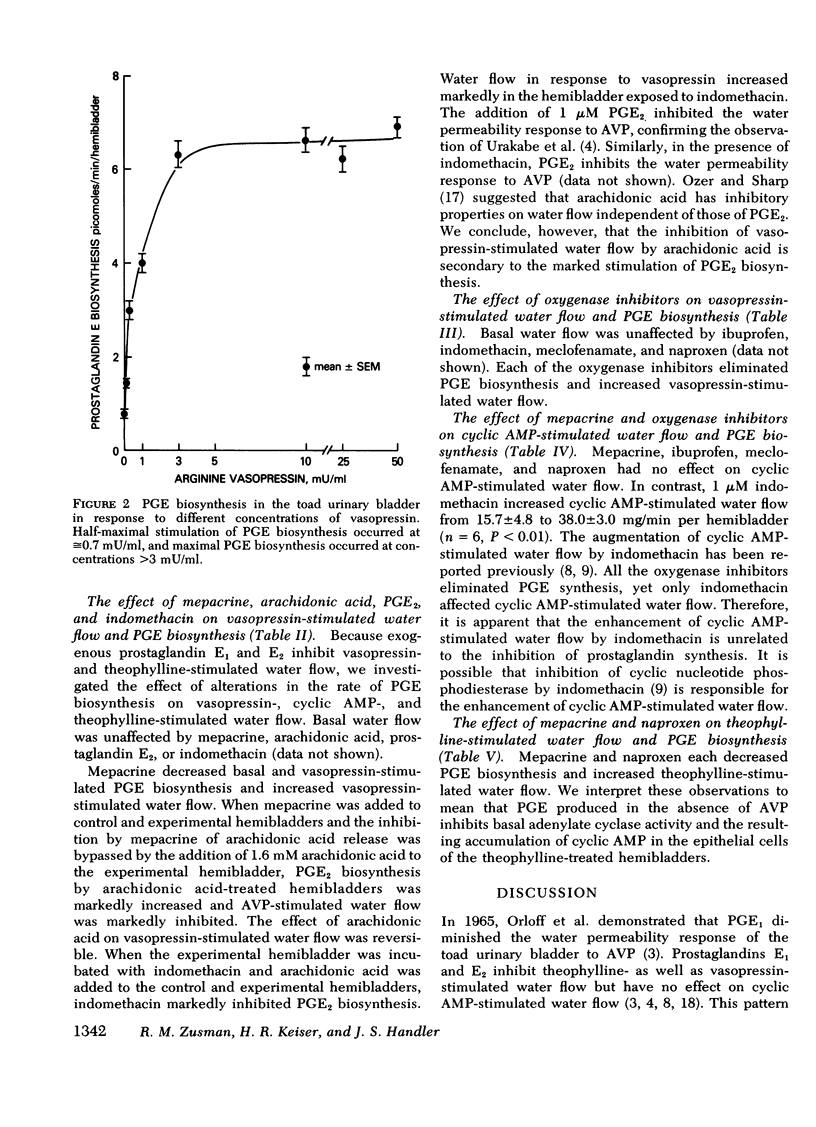
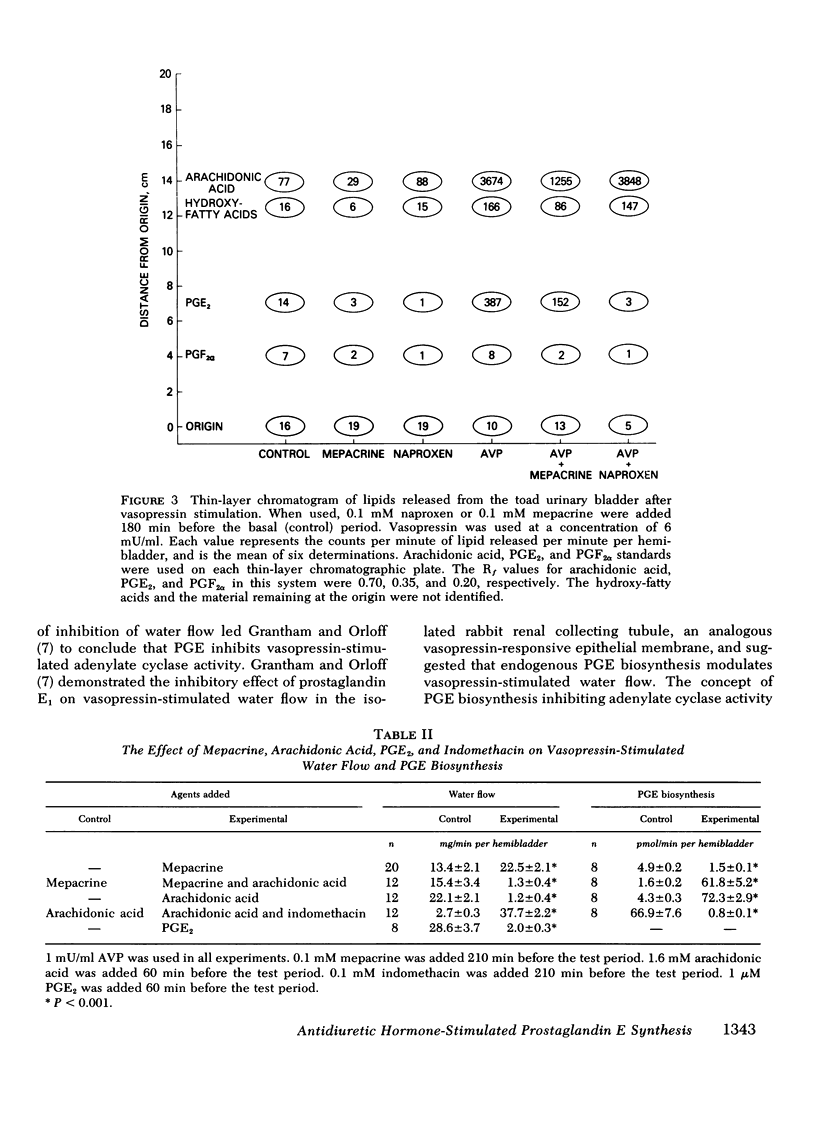
Prostaglandin E biosynthesis and its effect on water permeability were investigated in the toad urinary bladder. Arginine vasopressin (1 mU/ml) increased prostaglandin E (PGE) biosynthesis from 0.5+/-0.1 to 5.0+/-0.4 pmol/min per hemibladder (mean +/-SEM, n= 8, P less than 0.001). Maximal vasopressin-stimulated PGE biosynthesis, 6.4+/-0.2 pmol/min per hemibladder, occurred at vasopressin concentrations in excess of 3 mU/ml. Half-maximal stimulation of PGE biosynthesis occurred at a vasopressin concentration of approximately 0.7 mU/ml, whereas half-maximal stimulation of water flow occurred at a vasopressin concentration of approximately 5 mU/ml. Vasopressin-stimulated PGE biosynthesis did not depend on water flow along an osmotic gradient or upon sodium transport. Thin-layer chromatographic analysis of the lipids released from hemibladders labeled with tritium-arachidonic acid revealed that vasopressin stimulates the release of arachidonic acid from intracellular lipid stores without affecting the percentage of free arachidonic acid converted to PGE. Neither cyclic AMP nor theophylline stimulated PGE biosynthesis although they mimic arginine vasopressin (AVP) in stimulating water permeability. Biosynthesis of PGE was inhibited by mepacrine, a phospholipase inhibitor, and by agents that inhibit arachidonic acid oxygenase. The inhibition of PGE biosynthesis resulted in augmented vasopressin- and theophylline-stimulated water flow, but had no effect on cyclic AMP-stimulated water flow. We interpret these results to mean that endogenous PGE inhibits basal and vasopressin-stimulated adenylate cyclase activity. In contrast to the effects of AVP on permeability and transport, AVP stimulates PGE biosynthesis by a mechanism that does not depend on an increase in cellular cyclic AMP levels. The water permeability response of the toad urinary bladder to vasopressin is inhibited by PGE synthesized by the bladder in response to vasopressin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert W. C., Handler J. S. Effect of PGE1, indomethacin, and polyphloretin phosphate on toad bladder response to ADH. Am J Physiol. 1974 Jun;226(6):1382–1386. doi: 10.1152/ajplegacy.1974.226.6.1382. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Berl T., McDonald K. D., Schrier R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J Clin Invest. 1975 Aug;56(2):420–426. doi: 10.1172/JCI108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLEY P. J. The effects of neurohypophysial extracts on the water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol. 1958 Sep;17(3):201–209. doi: 10.1677/joe.0.0170201. [DOI] [PubMed] [Google Scholar]
- Bentley P. J. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol. 1968 Mar;195(2):317–330. doi: 10.1113/jphysiol.1968.sp008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A. G., Sharp G. W. Endogenous prostaglandins and osmotic water flow in the toad bladder. Am J Physiol. 1972 Dec;223(6):1392–1397. doi: 10.1152/ajplegacy.1972.223.6.1392. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszen F. H., Nugteren D. H. Histochemical localisation of prostaglandin synthetase. Histochemie. 1971;27(2):159–164. doi: 10.1007/BF00284957. [DOI] [PubMed] [Google Scholar]
- LEAF A., DEMPSEY E. Some effects of mammalian neurohypophyseal hormones on metabolism and active transport of sodium by the isolated toad bladder. J Biol Chem. 1960 Jul;235:2160–2163. [PubMed] [Google Scholar]
- Lipson L. C., Sharp G. W. Effect of prostaglandin E1 on sodium transport and osmotic water flow in the toad bladder. Am J Physiol. 1971 Apr;220(4):1046–1052. doi: 10.1152/ajplegacy.1971.220.4.1046. [DOI] [PubMed] [Google Scholar]
- Lipson L., Hynie S., Sharp G. Effect of prostaglandin E-1 on osmotic water flow and sodium transport in the toad bladder. Ann N Y Acad Sci. 1971 Apr 30;180:260–277. doi: 10.1111/j.1749-6632.1971.tb53196.x. [DOI] [PubMed] [Google Scholar]
- Lum G. M., Aisenbrey G. A., Dunn M. J., Berl T., Schrier R. W., McDonald K. M. In vivo effect of indomethacin to potentiate the renal medullary cyclic AMP response to vasopressin. J Clin Invest. 1977 Jan;59(1):8–13. doi: 10.1172/JCI108624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S., BERGSTROM S. EFFECT OF PROSTAGLANDIN (PGE-1) ON THE PERMEABILITY RESPONSE OF TOAD BLADDER TO VASOPRESSIN, THEOPHYLLINE AND ADENOSINE 3',5'-MONOPHOSPHATE. Nature. 1965 Jan 23;205:397–398. doi: 10.1038/205397a0. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omachi R. S., Robbie D. E., Handler J. S., Orloff J. Effects of ADH and other agents on cyclic AMP accumulation in toad bladder epithelium. Am J Physiol. 1974 May;226(5):1152–1157. doi: 10.1152/ajplegacy.1974.226.5.1152. [DOI] [PubMed] [Google Scholar]
- Ozer A., Sharp G. W. Effect of prostaglandins and their inhibitors on osmotic water flow in the toad bladder. Am J Physiol. 1972 Mar;222(3):674–680. doi: 10.1152/ajplegacy.1972.222.3.674. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D., RASMUSSEN H. Structure of the toad's urinary bladder as related to its physiology. J Biophys Biochem Cytol. 1961 Aug;10:529–553. doi: 10.1083/jcb.10.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakabe S., Takamitsu Y., Shirai D., Yuasa S., Kimura G. Effect of different prostaglandins on the permeability of the toad urinary bladder. Comp Biochem Physiol C. 1975 Oct 1;52(1):1–4. doi: 10.1016/0306-4492(75)90002-7. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin E2 biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Mechanism of stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Biol Chem. 1977 Mar 25;252(6):2069–2071. [PubMed] [Google Scholar]


