Abstract
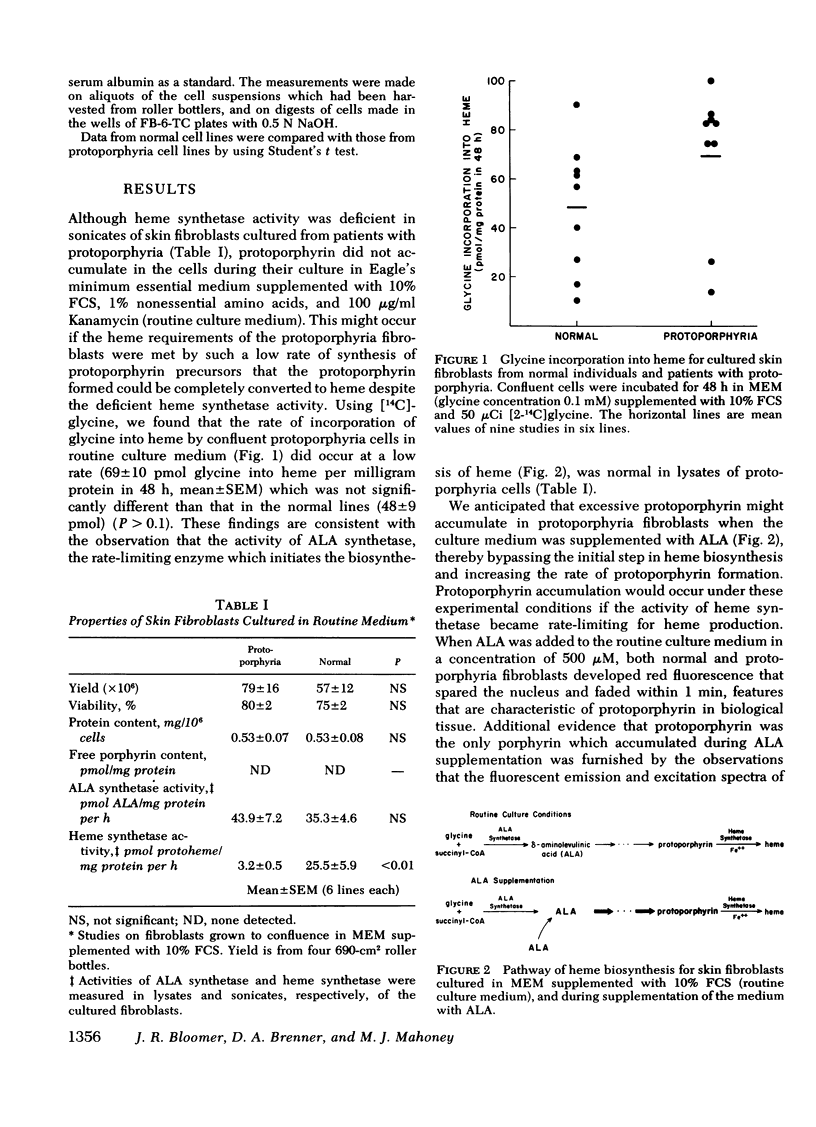
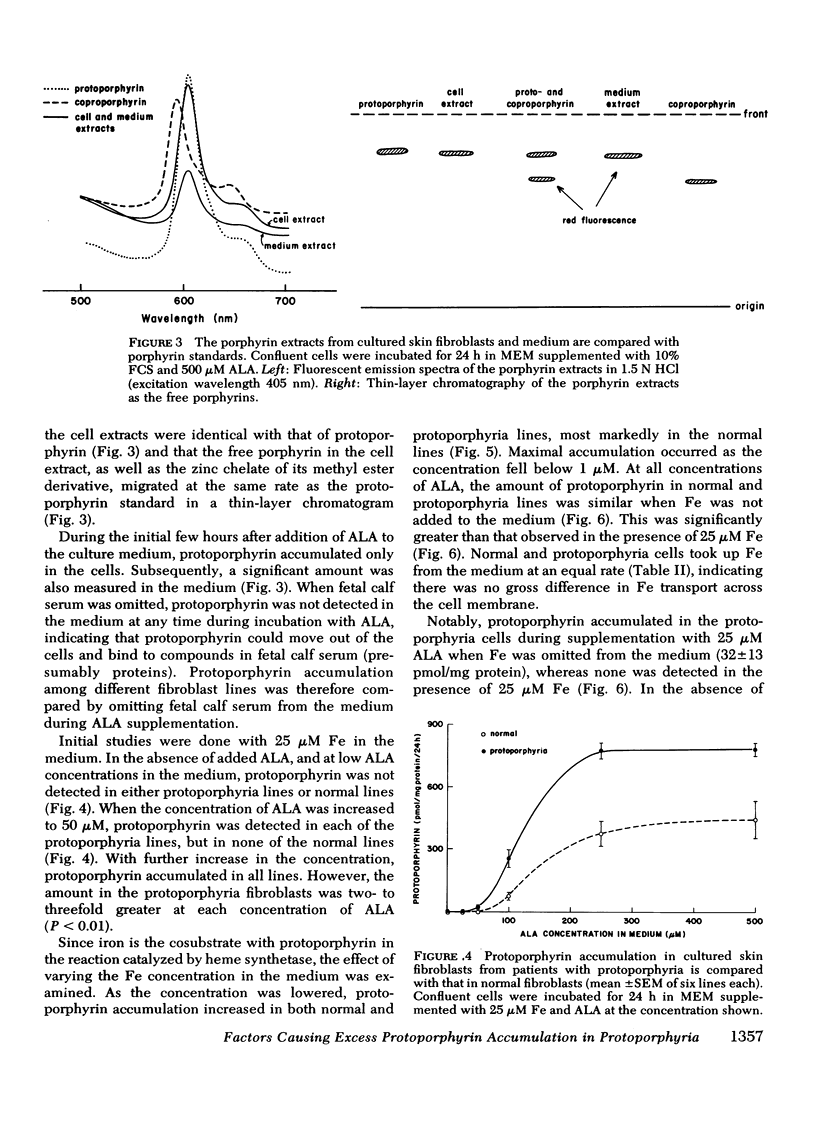
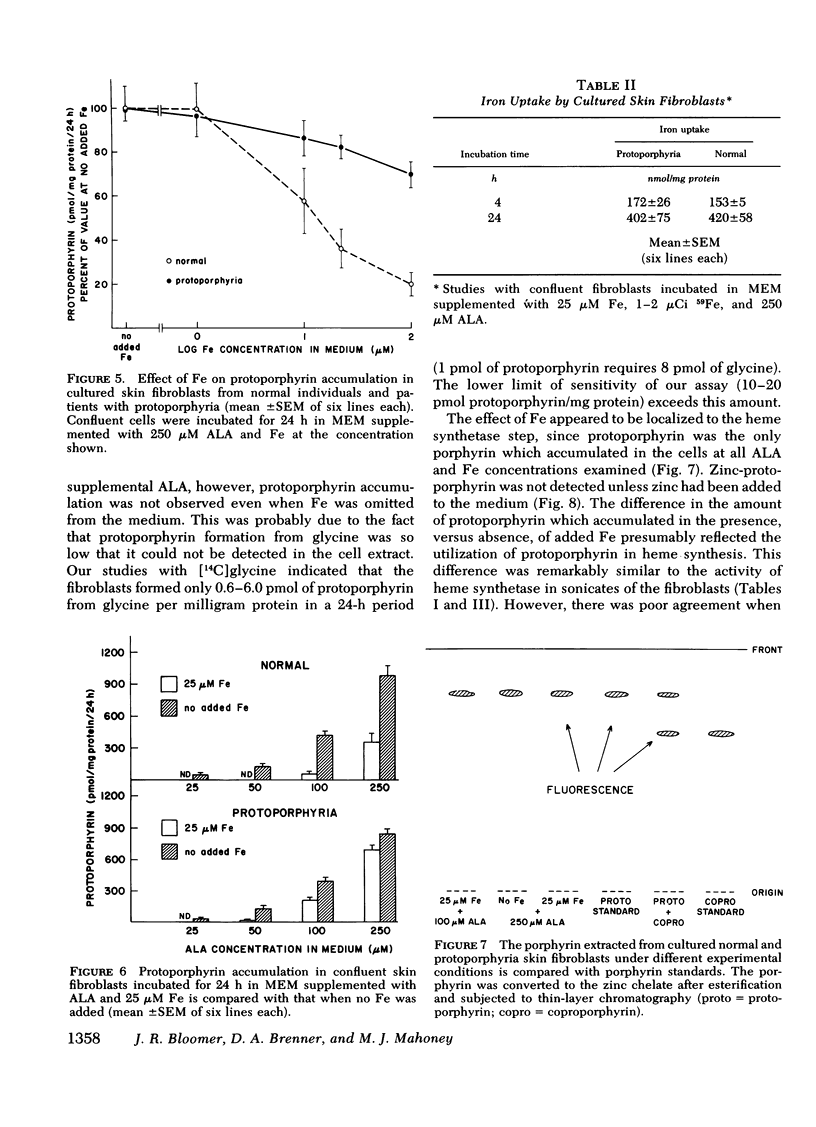
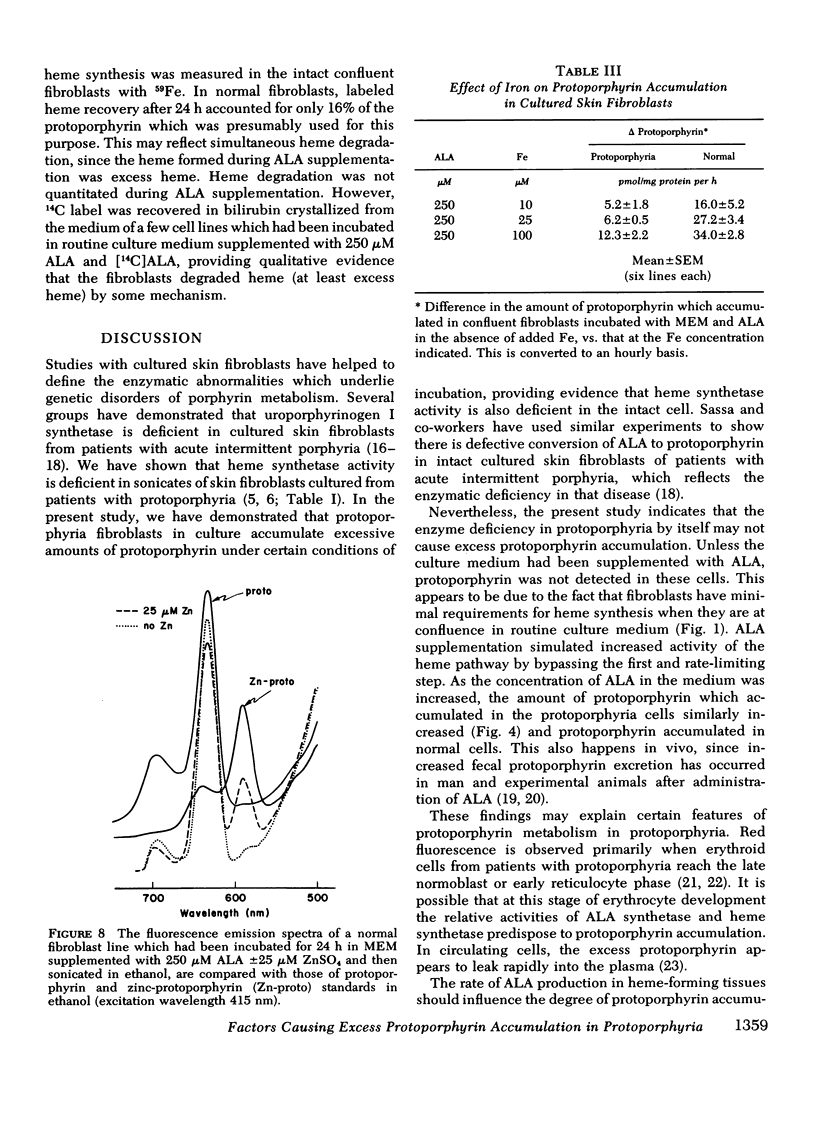
The activity of heme synthetase, which catalyzes the chelation of ferrous iron to protoporphyrin to form heme, is deficient in sonicates of skin fibroblasts cultured from patients with protoporphyria. During culture in Eagle's medium supplemented with fetal calf serum, these cells do not accumulate protoporphyrin, however. This may be due to a minimal requirement for heme synthesis, since glycine is incorporated into heme at a low rate which is similar to that in normal fibroblasts. In addition, the activity of delta-aminolevulinic acid (ALA) synthetase, the first and rate-limiting enzyme of heme biosynthesis which catalyzes the formation of ALA from glycine, is normal in lysates of the fibroblasts. Cultured fibroblasts were therefore incubated with ALA in order to bypass the rate-limiting step of heme biosynthesis. In the presence of 25 muM iron, protoporphyrin was detected in protoporphyria cell lines when the concentration of ALA in the medium reached 50 muM, but not in normal lines. As the concentration of ALA was increased above 50 muM, all lines accumulated protoporphyrin. However, the amount was 2-3 times more in cultured fibroblasts from patients with protoporphyria, reflecting their deficiency of heme synthetase activity. When iron was not added to the medium, protoporphyrin accumulated to a similar degree in normal and protoporphyria fibroblasts; this was significantly more than that in the presence of iron. These studies indicate that excessive protoporphyrin accumulation in protoporphyria, which is due principally to deficient heme synthetase activity, may be modified by the rate of ALA formation in heme-producing tissues, and by the availability of iron.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomer J. R., Bonkowsky H. L., Ebert P. S., Mahoney M. J. Inheritance in protoporphyria. Comparison of haem synthetase activity in skin fibroblasts with clinical features. Lancet. 1976 Jul 31;2(7979):226–228. doi: 10.1016/s0140-6736(76)91027-8. [DOI] [PubMed] [Google Scholar]
- Bloomer J. R., Phillips M. J., Davidson D. L., Klatskin G., Bloomer Hepatic disease in erythropoietic protoporphyria. Am J Med. 1975 Jun;58(6):869–882. doi: 10.1016/0002-9343(75)90644-0. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Weinbach E. C., Ebert P. S., Doherty J. M. Porphyrin synthesis and mitochondrial respiration in acute intermittent porphyria: studies using cultured human fibroblasts. J Lab Clin Med. 1975 Jan;85(1):93–102. [PubMed] [Google Scholar]
- Bottomley S. S., Tanaka M., Everett M. A. Diminished erythroid ferrochelatase activity in protoporphyria. J Lab Clin Med. 1975 Jul;86(1):126–131. [PubMed] [Google Scholar]
- Chang K. P., Chang C. S., Sassa S. Heme biosynthesis in bacterium-protozoon symbioses: enzymic defects in host hemoflagellates and complemental role of their intracellular symbiotes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2979–2983. doi: 10.1073/pnas.72.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K. G., Nicholson D. C. Erythrocyte protoporphyrin and iron uptake in erythropoietic protoporphyria. Clin Sci. 1971 Oct;41(4):363–370. doi: 10.1042/cs0410363. [DOI] [PubMed] [Google Scholar]
- Cripps D. J., MacEachern W. N. Hepatic and erythropoietic protoporphyria. Delta-aminolevulinic acid synthetase, fluorescence, and microfluorospectrophotometric study. Arch Pathol. 1971 Jun;91(6):497–505. [PubMed] [Google Scholar]
- DeLeo V. A., Poh-Fitzpatrick M., Mathews-Roth M., Harber L. C. Erythropoietic protoporphyria. 10 years experience. Am J Med. 1976 Jan;60(1):8–22. doi: 10.1016/0002-9343(76)90528-3. [DOI] [PubMed] [Google Scholar]
- Doss M. Conversion of porphyrin methyl esters to zinc and copper chelates for spectrophotometric analysis. Anal Biochem. 1971 Jan;39(1):7–14. doi: 10.1016/0003-2697(71)90455-6. [DOI] [PubMed] [Google Scholar]
- Ebert P. S., Pearson G. R. Correlation of C-type virus (WF-1) production and heme synthesis in a rat fibroblastic cell line. Proc Soc Exp Biol Med. 1974 Jan;145(1):298–301. doi: 10.3181/00379727-145-37797. [DOI] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- HAEGER-ARONSEN B. ERYTHROPOIETIC PROTOPORPHYRIA. A NEW TYPE OF INBORN ERROR OF METABOLISM. Am J Med. 1963 Oct;35:450–454. doi: 10.1016/0002-9343(63)90144-x. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Steinmuller D. P., Lee G. R. The role of iron in the pathogenesis of porphyria cutanea tarda. II. Inhibition of uroporphyrinogen decarboxylase. J Clin Invest. 1975 Sep;56(3):661–667. doi: 10.1172/JCI108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamola A. A., Yamane T. Zinc protoporphyrin in the erythrocytes of patients with lead intoxication and iron deficiency anemia. Science. 1974 Dec 6;186(4167):936–938. doi: 10.1126/science.186.4167.936. [DOI] [PubMed] [Google Scholar]
- MAGNUS I. A., JARRETT A., PRANKERD T. A., RIMINGTON C. Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet. 1961 Aug 26;2(7200):448–451. doi: 10.1016/s0140-6736(61)92427-8. [DOI] [PubMed] [Google Scholar]
- McLaren G. D., Carpenter J. T., Jr, nino H. V. Erythrocyte protoporphyrin in the detection of iron deficiency. Clin Chem. 1975 Jul;21(8):1121–1127. [PubMed] [Google Scholar]
- Meyer U. A. Intermittent acute porphyria. Clinical and biochemical studies of disordered heme biosynthesis. Enzyme. 1973;16(1):334–342. [PubMed] [Google Scholar]
- Meyer U. A., Strand L. J., Doss M., Rees A. C., Marver H. S. Intermittent acute porphyria--demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972 Jun 15;286(24):1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Nicholson D. C., Cowger M. L., Kalivas J., Thompson R. P., Gray C. H. Isotopic studies of the erythropoietic and hepatic components of congenital porphyria and 'erythropoietic' protoporphyria. Clin Sci. 1973 Feb;44(2):135–150. doi: 10.1042/cs0440135. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli S., Lamola A. A., Poh-Fitzpatrick M. F., Seaman C., Harber L. C. Erythropoietic protoporphyria and lead intoxication: the molecular basis for difference in cutaneous photosensitivity. I. Different rates of disappearance of protoporphyrin from the erythrocytes, both in vivo and in vitro. J Clin Invest. 1975 Dec;56(6):1519–1527. doi: 10.1172/JCI108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker A. G., Sterling R. E. The "glucose effect" in erythropoietic protoporphyria. Arch Intern Med. 1968 May;121(5):446–448. [PubMed] [Google Scholar]
- Rifkind A. B., Sassa S., Merkatz I. R., Winchester R., Harber L., Kappas A. Stimulators and inhibitors of hepatic porphyrin formation in human sera. J Clin Invest. 1974 Apr;53(4):1167–1177. doi: 10.1172/JCI107655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Solish G., Levere R. D., Kappas A. Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722–731. doi: 10.1084/jem.142.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick P., Marver H. S., Schmid R. Erythropoietic protoporphyria: evidence for multiple sites of excess protoporphyrin formation. J Clin Invest. 1971 Jan;50(1):203–207. doi: 10.1172/JCI106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Johnson J. A., Stephenson B. D., Anderson A. S., Edmondson P. R., Fusaro R. M. Erythropoietic defects in protoporphyria: a study of factors involved in labelling of porphyrins and bile pigments from ALA- 3 H and glycine- 14 C. J Lab Clin Med. 1971 Sep;78(3):411–434. [PubMed] [Google Scholar]
- Scott C. R., Labbe R. F., Nutter J. A rapid assay for urinary porphyrins by thin-layer chromatography. Clin Chem. 1967 Jun;13(6):493–500. [PubMed] [Google Scholar]
- Takaku F., Yano Y., Aoki Y., Nakao K., Wada O. -Aminolevulinic acid synthetase activity of human bone marrow erythroid cells in various hematological disorders. Tohoku J Exp Med. 1972 Jul;107(3):217–228. doi: 10.1620/tjem.107.217. [DOI] [PubMed] [Google Scholar]
- de Goeij A. F., Christianse K., van Steveninck J. Decreased haem synthetase activity in blood cells of patients with erythropoietic protoporphyria. Eur J Clin Invest. 1975 Sep 12;5(5):397–400. doi: 10.1111/j.1365-2362.1975.tb00470.x. [DOI] [PubMed] [Google Scholar]


