Abstract
Lysophosphatidic acids are structurally simple lipid phosphate esters with a now widely appreciated role as extracellular signaling molecules. LPA binds to selective cell surface receptors to promote cell growth, survival, motility and differentiation. Studies using LPA receptor knockout mice and experimental therapeutics targeting these receptors identify roles for LPA signaling in processes that include cardiovascular disease and function, angiogenesis, reproduction, cancer progression and neuropathic pain. These studies identify considerable functional redundancy between these receptors and raise the possibility that additional lysophosphatidic acid receptors remain to be identified. Lysophosphatidic acid is present in the blood and other biological fluids at physiologically relevant concentrations and can likely be rapidly generated and degraded in different locations, for example at sites of inflammation, vascular injury and thrombosis or in the tumor micro environment. Recent work identifies a secreted enzyme, autotaxin, as the key component of an extracellular pathway for generation of lysophosphatidic acid by lysophospholipase D catalyzed hydrolysis of lysophospholipid substrates. In contrast to the apparently redundant functions of LPA receptors, studies using autotaxin knock out and transgenic mice indicate that this enzyme is uniquely required for LPA signaling during early development and serves as the primary determinant of circulating LPA levels in adult animals. Accordingly, pharmacological inhibition of autotaxin may be a viable and potentially effective way to interfere with LPA signaling in the cardiovascular system and possibly other settings such as tumor metastasis for therapeutic benefit. In this review we provide an update on recent advances in defining roles for LPA signaling in major disease processes and discuss recent progress in understanding the regulation and function of autotaxin focusing on strategies for the identification and initial evaluation of small molecule autotaxin inhibitors.
Keywords: Lysophosphatidic acid, phospholipase, receptor, autotaxin, cancer, cardiovascular disease, molecular therapeutics
1. Introduction
The purpose of this review is to discuss the potential of the secreted plasma lysophospholipase D enzyme autotaxin (ATX/LysoPLD) as a target for drugs that inhibit the signaling functions of lysophosphatidic acid (1-acyl 2-hydroxyl glycerol 3-phosphate, LPA) which is the primary product of ATX catalyzed hydrolysis of circulating lysophospholipids. Accordingly we provide a brief overview of the enzymes receptors and pathways responsible for the synthesis, actions and inactivation of LPA. We highlight the physiologic and pathophysiologic functions of this mediator that ATX inhibitors might be expected to influence. Finally the review focuses on the structure, regulation and catalytic mechanism of ATX, recent advances in the design of high throughput-compatible ATX assays with optical readouts, and on the identification and preliminary characterization of small molecule ATX inhibitors.
2. General Principles of LPA Signaling
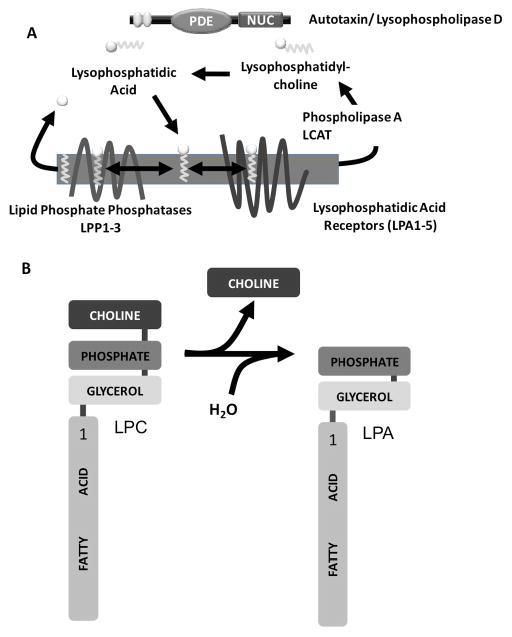
The biological activity of lysophosphatidic acid was first reported over 100 years ago. Work conducted in the 1970s and early 1980s established LPA as a modulator of vascular tone and platelet activation which ultimately led to the realization that many of these effects of LPA were receptor-mediated which culminated in the identification of a family of LPA-selective G-protein coupled receptors. Research in this field has accelerated rapidly in the intervening 15 years or so, driven in part by parallel advances in our understanding of the biology of the related lipid phosphate mediator sphingosine 1-phosphate (S1P). Several recent reviews discuss LPA receptors and LPA metabolism in considerable detail and we refer the reader to these for a more in depth treatment of this background information(1–5). Figure 1 presents a schematic overview of the enzymes, receptors and pathways involved in the synthesis, actions and inactivation of LPA.
Figure 1. A. Enzymes, receptors and pathways involved in the synthesis, actions and inactivation of LPA. B. Structural organization and lysophospholipase D activity of autotaxin.
The somatomedin B-like domains are in yellow, the PDE and nuclease domains are in blue and pink respectively. The hydrophobic signal sequence removed by proteolytic processing is in green.
2.1 LPA synthesis and inactivation
Definition of the enzymes and pathways involved in the generation and degradation of receptor-active “signaling” LPA is complicated by the well established intracellular role for LPA as a key intermediate in the de novo synthesis of phospholipids and triglycerides. As is the case with other established lipid signaling molecules including diaclglycerol, phosphoinositides and sphingolipids, metabolic and physical compartmentation of the relevant enzymes and substrates likely accounts for the ability of LPA to serve as both an intracellular metabolic intermediate and an extracellular signaling molecule. The predominant intracellular pathway for de novo synthesis of LPA is acylation of glycerol 3-phosphate. LPA can also be formed by phospholipase-catalyzed degradation of membrane phospholipids and here evidence for pathways involving hydrolysis of phosphatidic acid (PA) by a selective phospholipase A2 activity and lysophospholipase D (lysoPLD)-catalyzed hydrolysis of lysophospholipids have been presented(6). Finally a broad specificity acylglycerol kinase can form LPA by direct phosphorylation of monoglyceride(7). Although LPA may have actions at intracellular receptors(8) the predominant signaling actions of this lipid are mediated by cell surface receptors and therefore require delivery of LPA to the extracellular space or outer leaflet of the plasma membrane Mechanisms for “export” of intracellular generated LPA, for example involving membrane microparticles have been proposed but not yet convincingly demonstrated. Of particular interest here, isolated platelets can generate and release LPA suggesting a role in localized generation of this mediator(9). Experimental induction of thrombocytopenia did not significantly decrease bulk circulating LPA levels in rats (6), an anti-platelet drug that both blocks platelet activation and induces thrombocytopenia produced a marked reduction in circulating LPA levels in mice(10). Clearly this issue requires further investigation and it is possible that platelets could have an important function in localized production of LPA in the setting of hemostasis or in response to vascular injury. The most compelling discovery in this area is a series of recent reports that clearly establish the importance of a lysoPLD catalyzed extracellular pathway for generation of LPA in the blood and, based on the phenotype of mice lacking the enzyme responsible, vital production of LPA during early development(11–13). The enzyme responsible, ATX is the focus of this review and discussed in greater detail in Section 4.
As with the synthetic pathway, degradation of LPA could proceed by several pathways including phospholipase catalyzed deacylation or reacylation to form receptor-inactive free fatty acids or phosphatidic acid. The primary pathway for inactivation of LPA by intact cells appears to be dephosphorylation catalyzed by a class of integral membrane enzymes termed lipid phosphate phosphatases (LPPs)(14). Overexpression of these enzymes can decrease LPA responsiveness in some systems and chemical inhibitors of their activities have been shown to potentiate LPA signaling in other experimental settings(15). However, whole animal experiments support the idea that the functions of these enzymes are more complex and because in addition to LPA the LPPs can dephosphorylate other phospho- and sphigno-lipid phosphate substrates, likely unreated to their effects on LPA signaling. Transgenic overexpression of LPP1 results in a relatively benign phenotype with no measurable change in circulating LPA levels (16)while mice lacking LPP2 are viable with no obvious phenotype. Knock out of LPP3 results in early embryonic lethality characterized by defects in patterning and vasculogenesis that may involve alterations in wnt signaling(17). LPP1 knockout mice have not yet been reported and further work perhaps using animals with tissue specific inactivation of these LPP genes will be needed to tease out their function as regulators of LPA metabolism and signaling in vivo.
An important point to emphasize is that LPAs are a “family” of lysoglycerophospholipids that differ in both the nature of the linkage between the acyl chains and the glycerol phosphate backbone and in the length and degree of saturation of the acyl chains. LPA subclasses including molecules with -acyl-, alkenyl- and alkyl linkages and acyl chains exhibit distinctive pharmacological properties and in some cases, selectivities for their cognate G-protein coupled receptors. Although this issue requires further investigation the occurrence of different positional isomers of LPA with varied fatty acid chains length and degrees of saturation have been reported in various tissues, altherosclerotic lesions and the molecular species of LPA present in plasma or produced following platelet activation have also been shown to be heterogeneous(18, 19). Although neither ATX (see below) or the LPPs exhibit obvious preferences for different molecular species of their lysolipid substrates, some level of metabolic compartmentalization must be responsible for generating and maintaining this heterogeneity.
2.2 LPA receptors
LPA and S1P exert most if not all of their effects on target cells through lysolipid-specific G-protein coupled receptors. The founding members of this class of receptors were initially denoted “Edg” (endothelial cell differentiation gene) receptors but this has been superseded by a more logical nomenclature in which these established and more recently identified lysolipid receptors are denoted as members of either LPA or S1P selective classes. The LPA selective group of these receptors comprises LPA1/Edg-2/VZG-1, LPA2/Edg-4, and LPA3/Edg-7, and the sphingosine-1-phosphate receptor subfamily includes S1P1/Edg-1, S1P2/Edg-5/H218/AGR16), S1P3/Edg-3, S1P4/Edg-6, and S1P5/Edg-8/NRG-1. Recently two additional LPA receptors LPA4/p2y9/GPR23 LPA5/GPR92, have been described. Interestingly these receptors are more closely related to nucleotide-selective purinergic receptors than to the prototypic LPA1, LPA2 and LPA3 receptors(20).
Biological activities ascribed to LPA were originally observed over 100 years ago and work conducted in the 1970s showed that exogenous administration of LPA had dose and species-specific effects on blood pressure in mammals(21, 22). Studies conducted in the 1980s identified LPA as a potent mitogen for cells in culture (23)and an effective stimulus of human platelet activation(24). Although these platelet studies strongly suggested that the actions of LPA were consistent with a G-protein coupled receptor mediated process, as discussed in section 3.2, the identity of the receptor responsible still remains to be determined. The prototypic LPA receptor LPA1/Edg-2 was originally identified in a search for genes upregulated in migrating cells of the cerebral cortex during early development(25). Identification of this receptor was a pivotal discovery that provided a clear molecular mechanism for the pleiotropic effects of LPA that had often been dismissed by skeptics as non-specific and perhaps arising from effects on plasma membrane integrity. Although LPA1 deficient mice have are viable and have a benign phenotype characterized by craniofacial dysmorphism, semilethality due to defective suckling behavior, and a minor incidence of frontal hematoma(26) ongoing studies of these animals have defined roles for this LPA receptor in physiological and pathophysiological processes that include cerebral development, olfaction and inborn neonatal behavior, brain plasticity neuronal differentiation and astrocyte proliferation, myelination, pulmonary function and neuropathic pain(27–30). Structural similarities between LPA1 and the related S1P1 receptor facilitated molecular cloning of members of this family of lysolipid phosphate selective receptors leading to the identification of the LPA-selective receptors now known as LPA2 and LPA3. LPA2 receptor knockout mice have no obvious phenotype and the phenotype of mice lacking both LPA1 and LPA2 is no more severe than that of LPA1 deficient animals(30). Because LPA1 and LPA2 exhibit substantially overlapping expression patterns it is possible that the major functions of these receptors in normal development and physiology are largely redundant. However, more recent work provides evidence for a unique LPA1-independent role of LPA2 in fertility (31)and cancer progression (see below).
Roles for lysolipids in physiology of reproduction were suggested by the finding that LPA is present at relevant concentration in follicular and seminal fluid of healthy individuals, serum of ovarian cancer patients and prostate tissues(32). Evidence came once again from studies of mice lacking the third LPA selective receptor, LPA3(33, 34). These animals exhibit defects in fertility, embryo implanation and spacing. The defect in implantation appears to involve ablation of an LPA3-dependent pathway for upregulation of uterine cyclooxygenase-2. Changes in LPA3 receptor expression during early pregnancy raise the possibility that modulation of LPA3 may regulate hormone-dependent uterine receptivity. Although the related lysolipid S1P is clearly an important regulator of immune system function through effects on lymphocyte trafficking (35)a recent report identifies a role for LPA3 in immune surveillance(36). The fourth LPA-specific GPCR, LPA4/p2y9/GPR23, was identified in a functional screen of orphan G protein-coupled receptors(37). LPA4 receptor is phylogenetically distant from the LPA1-3 and S1P receptor families having only ~20% amino acid identity with these lysolipid receptors and perhaps surprisingly LPA4 is more closely related to nucleotide-selective receptors. Ongoing work identifies LPA4 as a mediator of the effects of LPA on neuronal morphology and neurite retraction(38). LPA4 is widely expressed with particular abundance in mouse embryonic brain tissues, skin and heart and in human ovary. A fifth LPA-responsive GPCR, LPA5/GPR92 receptor, was recently described by two groups(39). Like the related LPA4 receptor, LPA5 is more homologous to nucleotide-selective G-protein coupled receptors than to the LPA 1-3 and S1P receptor family. LPA5 is highly expressed in mouse intestine, dorsal root ganglia and embryonic stem cells human heart, placenta, brain, gut, spleen and lymphocytes. Although ligand binding to any LPA receptor has not been demonstrated convincingly the identification of several cultured cell lines that lack functional LPA receptors has allowed definition of the G-protein coupling specificity and downstream signaling pathways activated by these receptors. These types of approaches have revealed coupling of the LPA1, 2, 3 and 5 receptors to a similar spectrum of signaling pathways that include activation of Gi, Gq and G12/13 G-proteins and activation of downstream responses that typically include activation of phospholipase C, increases in cytosolic Ca2+, activation of Rho family GTPases and the ERK/MAP kinase cascade(20). Of particular interest are recent demonstrations that the LPA4 receptor is distinctively coupled to Gs (at least in heterologous expression systems) resulting in an activation of cyclic AMP accumulation in cells expressing the appropriately regulated adenylyl cyclase isoform(37). This finding raises the intriguing possibility that LPA4 may initiate a different spectrum of signaling processes than are activated by the other LPA receptors. Mouse knockouts of LPA4 and LPA5 have yet to be reported and obviously it will be of great interest to determine the roles of these receptors in normal physiology and disease processes.
3. LPA signaling in health and disease
The impetus for development of therapeutics that target the LPA signaling system and in particular the ATX-dependent LPA synthetic pathway we focus on here comes in large part from an exponentially growing body of data implicating LPA in a range of disease processes which we briefly review below focusing on cancer initiation and progression, cardiovascular physiology and disease and nervous system function. As with the preceding section, this discussion is not complete non-exhaustive and we refer the reader to several more focussed disease oriented reviews(3, 40, 41).
3.1 Role of LPA in cancer initiation, progression and diagnosis
The mitogenic activity of LPA was among the first biological effects identified for this mediator and the actions of LPA as a growth factor-like pro-survival factor are consistently observed in many different cell types. In light of these effects, LPA has been considered to be and attractive mediator to promote the growth and survival of tumor cells. Although changes in both absolute levels and the pattern of LPA receptors have been associated with tumor progression as with other G-protein coupled receptors, somatic mutation of LPA receptors has not been reported in human cancers. On the other hand, mutational activation of downstream targets of LPA signaling, such as phosphoinositide 3-kinase are clearly associated with many cancers(40, 42). Perhaps of most direct relevance here is the finding that LPA may contribute to the tumor microenvironment because LPA is present at high concentration in cancer-associated malignant effusions and, in particular, in ascites fluid that accumulates in the abdominal cavity of ovarian cancer patients as a result of impaired lymphatic drainage(43). Ovarian cancer cells are highly responsive to LPA and studies using genetic and pharmacological approaches identify a role for LPA signaling in experimental models ovarian tumorigenesis and metastasis(44). Intriguing but presently unresolved and conflicting work suggests that increased plasma levels of LPA (or perhaps of particular molecular species of LPA) are a prospective marker for ovarian and certain other gynecological cancers(45). LPA1 has been shown to play a central role in motility of human pancreatic cancer cells and most strikingly in breast cancer derived-osteolytic bone metastasis suggesting that this receptor is a potential target for anti-metastatic therapy(10, 46, 47). LPA2 mediates mitogenic signals in human colon cancer cells(48) and over-expression of LPA2 has been observed in human invasive ductal carcinoma(49). LPA1/LPA2 are involved in the migration of human gastric cancer cells (29)and have been recently identified as a binomial receptor system able to calibrate LPA-dependent chemiotactic response in breast carcinoma cells. Interestingly, the expression ratio between LPA2 and LPA1 ratio increases significantly during malignant transformation of colorectal cancer cells(50). Evidence for a role of LPA signaling in angiogenesis is presented below and in this regard it is provocative that LPA has been shown to regulate VEGF expression in ovarian cancer patients through LPA2/LPA3 and the same receptors have been indicated as the mediators of LPA survival-inducing activity in B-cell lines and primary chronic lymphocytic leukemia cells, making LPA-targeting drugs an attractive candidate for therapy against B-cell-derived malignancies such as chronic lymphocytic leukemia. Other less, developed work implicates LPA as an important mediator in prostate thyroid and colon cancers and taken as a whole this body of work makes a strong case for further investigation into the potential of LPA-directed therapeutics as anti cancer agents. Work described in section 6 of this review discusses anti-metastatic effects of the first small molecule ATX inhibtors..
3.2 Cardiovascular function and disease
The first defined lysolipid receptor Edg1/S1P1 was originally identified as a gene upregulated in endothelial cells in an in vitro model of angiogenesis. The identification of S1P as high affinity ligand for this receptor provoked great interest in bioactive lysolipids as regulators of endothelial cell function and vasculature dynamics leading to the suggestion that platelet-derived LPA could play a role in regulating acute and sustained vascular responses that are initiated by platelet activation(51, 52). The mechanisms involved in promotion of vascular endothelial cell growth and migration by LPA appear complex. LPA-promoted endothelial cell mitogenesis is inhibited by the angiogenesisregulators thrombospondin-1 and 2. LPA increases endothelin-1 expression and mediates the production of matrix metalloprotease 2 which is a fundamental regulator of extra cellular matrix remodeling required for endothelial cell migration during angiogenesis(53). Increases in adhesion molecule expression in human endothelial cells are an important early response in angiogenesis, and white blood cell adhesion to these cells has been observed following stimulation by LPA and related lysolipids(54). However, other data indicate that LPA can inhibit motility and adhesion of vascular cells and the observed dependence of LPA responses on extracellular matrix composition and reports of cell-type specific responses indicates that signaling integration between LPA receptors, integrins and/or other indirect mediators may be required for a complete migratory response(55, 56). LPA treatment can promote matrix detachment of some preparations of human vascular endothelial cells raising the possibility that LPA signaling could impair vascular integrity in some settings. In this regard, several studies implicate LPA as a component of the oxidative stress response and LPA-promoted decreases in endothelial cell viability observed both in vitro and ex vivo studies of retina and brain explants microvasculature may involve effects on nitric oxide production(57).
As noted in the introduction, the finding that agonist-stimulated platelets produce LPA (58) and that LPA is itself an effective stimulus of platelet activation has provoked considerable interest in defining the role of LPA and other lysolipid mediators as regulators of platelet aggregation and signaling(59). Identification of the LPA receptor subtype(s) responsible for activation of human platelets has proved challenging, in particular because LPA does not activate mouse platelets which precludes studies of platelets from the various LPA receptor knockout mice as a way to address this question(22). Of particular interest here it is notable that human platelets respond much more effectively to alkyl-ether linked LPA and to highly unsaturated acyl-species carrying long fatty acyl groups (20:4) when compared to LPA species with 18:1(oleoyl-) or shorter fatty acids(60, 61). Although LPA can activate washed platelets directly in the presence of nucleotide scavengers, the possibility that LPA acts synergistically with other platelet activators merits further consideration. For example, some components of the effects of LPA on platelet activation in whole blood may be mediated by ADP-dependent activation of P2Y1/P2Y12 receptors(62) and synergy between LPA and other platelet activators/agonists may account for ability of LPA to promote platelet activation in whole blood. LPA stimulates homotypic interactions between platelets and monocytes homotypic which is a recognized early marker of acute myocardial infarction positioning LPA as potential mediator of this and other related cardiovascular complications. As noted in the original reports and more recently studied in greater detail ~20% of healthy individuals have platelets that are selectively unresponsive to LPA. Although this propensity does not correlate with age, gender or race in a group of patients presenting for diagnostic catheterization platelet LPA unresponsiveness was associated with the absence of atherothrombotic disease(63).
In addition to its thrombogenic activity, LPA is also implicated as a mediator of atherogenesis. Unsaturated but not saturated LPA species have been shown to elicit a potent dedifferentiation response in cultured rodent and human vascular smooth muscle cells raising the possibility that localized increases in LPA following plaque rupture or vascular injury could result in vascular cell activation and migration(52, 64, 65). LPA accumulates in human atherosclerotic lesions as a component of mildly oxidized low density lipoprotein and could therefore also be released during plaque rupture(62, 66). Exogenous application of unsaturated LPA induces vascular remodeling in rodent models and brief exposure of vessels to either unsaturated acyl forms or alkyl ether analogs of LPA has been shown to elicit the development of intimal hyperplasia in rat carotid artery(67). Thus, LPA accumulates in human atherosclerotic lesions and, in isolated or cultured cell systems, mediates activation of endothelial cells VSMC differentiation and platelet activation. Taken together these findings make LPA an attractive candidate mediator of vascular responses to injury including platelet deposition and thrombus formation following plaque rupture which in turn leads to the development of intimal hyperplasia. In comparison to studies of the role of LPA in cancer progression and nervous system development and function the role of LPA signaling in the cardiovasculature is relatively under studied but progress in this area is being rapidly facilitated by the ongoing availability of mouse models with targetted alterations in genes involved in LPA metabolism and responsiveness.
3.3 Central and Peripheral Nervous System
The effects of LPA and related lysolipids on the nervous system and its implications in diseases have been recently reviewed(68). Although historically a highly relevant area (and one that led to the initial discovery of the first defined LPA receptor) much more needs to be done to relate studies conducted with cells in culture or isolated tissue preparations to systemic effects on neuroanatomy, nervous system function and ultimately behavior. LPA promotes the growth, survival, differentiation and motility of a range of neuronal cells including schwann cells, oligodendrycytes, astrocytes neurons and microglia(41). Exposure to LPA produces dramatic LPA receptor-dependent effects on the folding and organization of the cerebral cortex and a more recent report identifies a role for LPA receptors as mediators of neuropathic pain(69). The continuing development of mouse models with alterations in LPA metabolism and signaling promises to continue to drive advances in this area.
4. Autotaxin
As discussed above, although LPA can potentially be formed by a number of alternate pathways the only mechanism for extracellular production of LPA presently established to have clear physiological importance is lysoPLD-catalyzed hydrolysis of lysophospholipid substrates. The enzyme responsible, ATX, was originally identified as an autocrine factor present in melanoma cell culture medium that stimulated tumor cell motility(70). Overexpression experiments subsequently identified potential roles for ATX in cancer progression, tumor cell invasion and metastasis including promotion of tumor angiogenesis. Structural characterization of ATX identified a relationship to a family of cell surface enzymes with ecto nucleotide pyrophosphatse/phosphodiesterase activities and mutagensis studies showed that these activities were critical for the signaling functions of the enzyme(71, 72). These observations were perplexing because the observed biological activities of ATX could not be obviously linked to alterations in nucleotide metabolism. Studies originating in the 1970s identified a lysoPLD activity in blood that could produce LPA by hydrolysis of both endogenous and exogenously provided lysophospholipid substrates(73). When purification of this activity was eventually accomplished sequencing revealed that it was identical to a secreted form of ATX(11, 74). Recombinantly expressed ATX exhibits an active lipid phosphodiesterase activity, and the enzyme functions as a D-type phospholipase with a strong preference for lysophospholipid substrates but little headgroup selectivity(75). The mitogenic and motility promoting effects of autotaxin were convincingly established to be dependent on the production of LPA and mediated by target cell LPA receptors(29).
Definitive evidence that ATX is a physiologically relevant regulator of LPA synthesis and signaling comes from studies in mice. ATX knockout mice are not viable and die in utero as a result of defects in vasculogenesis which is notably a more severe phenotype than that of any of the “single” LPA receptor knockout animals reported to date. Mice that are heterozygous for the wild type and null allele of ATX are viable but have circulating LPA levels that are 50% of wild type(12, 13, 76). We have characterized mice that transgenically overexpress ATX in the liver under control of the α-antitrypsin promoter and found that circulating levels of ATX and LPA are elevated in these animals (unpublished observations). Interestingly, although ATX has both nucleotide phosphatase and phosphodiesterase activities and could potentially catalyze the dephosphorylation of circulating nucleotide diphosphates this activity does not seem to be relevant in vivo because circulating levels of ATP and adenosine are unaltered in these autotaxin over expressing mice (unpublished results). The availability of mice with alterations in ATX activity and circulating LPA levels promises to be of great value for further elucidation of the role of LPA in normal physiology and disease and in particular for in vivo investigations of the growing number of small molecule ATX inhibitors discussed in sections 5 and 6 below.
4.1 Primary structure and Catalytic Mechanism
ATX belongs to a family of seven structurally related cell surface or secreted enzymes that exhibit phosphodiesterase activities against nucleotides or lipids but with varying substrate selectivities and biological functions(77). These enzymes are termed nucleotide phosphatases/pyrophosphatases (NPPs) and numbered in order of discovery. NPP1 generates pyrophosphates that are important for bone mineralization. The precise functions of NPPs 3, 4 and 5 are not known although NPP3 has been shown to regulate glial cell migration and differentiation in vivo through a mechanism that requires phosphodiesterase activity. In light of the preferential phospholipase activity of ATX it is interesting that NPP6 and NPP7 are choline headgroup-specific phospholipases C with activity against phosphatidylcholine and sphingomyelin(78). ATX and the related NPP1 and NPP3 enzymes share a common structure with two closely opposed N-terminal somatomedin B-like domains, a central phosphodiesterase domain containing sequence determinants that are critical for catalysis and a C-terminal domain with homology to nucleases (Figure 1). The function of the somatomedin B-like domain is presently unclear although other examples of this motif are known to interact with plasma proteins and integrin receptors. A very recent report indicates that ATX can bind to integrin receptors on primary and immortalised lymphocytes and suggests that this interaction may play a central role in the regulation of lymphocyte trafficking(79). The phosphodiesterase domain is responsible for hydrolysis of lipid and nucleotide substrates through a “ping-pong” mechanism involving a critical threonine residue that forms an enzyme phosphate intermediate(75, 80). The nuclease domain is important for activity but seems unlikely to play a direct role in catalysis and may therefore be important for folding or structural integrity of the enzyme. Clearly determination of the structure of ATX or of the relevant catalytic domain of the enzyme will be required to understand the basis for the unique preference of this enzyme for lysophospholipid substrates.
4.2 Post Translational Processing
Although all of the other members of the ENPP family are integral membrane cell surface proteins, the N-terminal hydrophobic sequence of ATX functions as a signal peptide and not a membrane anchor. ATX is therefore processed in the secretory pathway by sequential protease cleavage of the “pre-pro” enzyme to form a “pro-enzyme” that is subseqently converted to the mature form by furin-catalysed proteolysis(81). Because genetic manipulation of ATX expression in mice has established that circulating LPA levels parallel those of ATX, understanding how the balance between synthetic and degradative pathways determines extracellular levels of this enzyme is likely to provide valuable insights into how extracellular levels of LPA are controlled. Extracellular release of ATX involves the well defined secretory pathway although at present it is unclear if this is a constitutive or regulated process. Similarly, more work is needed to establish mechanisms responsible for clearance of ATX from the circulation. ATX is a glycoprotein with N-glycosylation on three distinct sites recently identified. Surprisingly glycosylation of one of these sites (N542) is critical for catalysis. Because the glycan modification of this residue is resistant to enzymatic degradation it may interact with the protein, perhaps stabilizing the catalytically competent structure(82). This set of studies clearly establish that proper proteolytic processing and glycosylation of ATX are critical for efficient expression of catalytic activity which raises some obvious concerns about the optimal way to generate the enzyme for biological or pharmacological studies. For example, although ATX can be made recombinantly in a variety of heterologous expression systems, specific activities approaching those exhibited by native ATX isolated from blood plasma have only been approached when the enzyme is expressed recombinantly in cultured mammalian cells and purified from cell-conditioned culture medium(83). Affinity tagging at the C-terminus is well tolerated and can be used to facilitate purification of the enzyme from these expression systems.
4.3 Isoforms, expression pattern and biological activities
Three distinct variants of autotaxin have been described. The predominant form which corresponds to the enzyme originally identified as plasma lysoPLD has 863 amino acids. Two other variants containing 52 and 24 residue insertions close to the phosphodiesterase domain have been reported but their significance remains to be established(78). ATX is very widely expressed with mRNA detected in essentially all tissues examined and high levels of expression notable in brain, ovary, lung, intestine and kidney. ATX expression is controlled by growth factors acting through diverse transcription factors. Although studies of ATX knockout mice identify a critical role for the enzyme in early development and in particular extra embryonic vasculogenesis less is known about the role of ATX in normal physiology. Given the participation of LPA receptors in the broad variety of processes discussed in section 3 one could reasonably expect these functions of ATX to be wide-ranging. Of particular interest s the link between ATX and cancer progression. ATX was originally identified as a tumor derived cytokine and upregulation of ATX expression has been associated with a variety of cancers and associated with pathways that drive tumor cell growth, motility and survival. In particular ATX stimulates tubulation of cultured human endothelial cells which, in light of the vasculogenesis defect observed in ATX deficient mice, suggests a possible involvement in tumor angiogenesis(84). As discussed further below, much of the impetus for development of small molecule ATX inhibitors comes from these studies implicating autotaxin as a viable target for cancer therapeutics.
5. Measurement of autotaxin activity
Although ATX can hydrolyze a variety of lysophospholipid substrates the primary physiological substrate is likely to be lysophosphatidylcholine (LPC). This lipid is very abundant in plasma, serum and some other biological fluids and can also be detected in conditioned media from a variety of cells. Under “optimal” assay conditions the Km of ATX for LPC is in the range of 100 μM which is close to the measured concentration of this lipid in blood plasma. Of the other “candidate” ATX lysolipid substrates, it is interesting that the enzyme can also hydrolyze sphingosylphosphorylcholine to generate the bioactive lipid mediator S1P. However, the observed Km for this substrate is significantly higher than that for lysophosphatidylcholine while plasma levels of sphingosylphosphorylcholine are more than 1000 times lower(85). Because mice that are heterozygous for the null autotaxin allele have normal circulating levels of S1P sphingosylphosphorylcholine is clearly not a physiologically relevant ATX substrate and of course extracellular production of S1P is now clearly established to involve a kinase pathway(86). The availability of a molecular structure for ATX will be required to advance our understanding the basis for selectivity of ATX for lyso versus diacylated lipid substrates. Like other members of the NPP family, ATX can also hydrolyze nucleotide triphosphates di-adenosine polyphosphates and artificial phosphodiester substrates such as paranitrophenyl thymidine phosphate (pNP-TMP) or bis paranitrophey phosphate (bis-PNP) with Km values close to 1mM and optimal activity observed at alkaline pH(77). Although nucleotides are unlikely to be relevant physiological substrates for ATX the use this class of substrates, particuarly pNP-TMP or bis-PNP) in colorimetric assays provides a simple and straightforward method to monitor enzyme activity in recombinant expression systems. Similarly, production of LPA from LPC can be measured directly using radiolabeled substrates and fluorescent derivates of LPC are also available and readily hydrolyzed by ATX allowing direct evaluation of enzyme activity after separation of substrate from unreacted product by thin layer chromatography. Of particular importance is the recent development of FRET-based and fluorogenic ATX substrates. The latter class are exemplified by compounds termed FS-2 and FS-3 which are LPC derivatives with a fluor and a quencher attached to the “headgroup” and acyl chains respectively(87). Hydrolysis of the phosphodiester bond results in fluorescence “dequenching” and an increase in fluorescence signal that can be monitored continuously. Another useful ATX substrate, CPF4, is a bis-PNP-based FRET sensor in which enzymatic hydrolysis results in a loss of the intramolecular FRET signal(88). Although the kinetic behavior of the enzyme with these substrates needs to be further investigated and issues of specificity for other phosphodiesterases may limit their use with anything other than purified enzyme preparations(89) these reagents are clearly of great value for the development of high throughput assays to screen for inhibitors of ATX activity.
6. Initial identification and characterization of autotaxin inhibitors
The work summarized about establishes ATX as a key determinant of circulating LPA levels and highlights the involvement of this enzyme, its lysolipid product and associated receptors in a broad range of pathophysiologies. Thus, this enzyme is in a pivotal position to be an attractive and intriguing therapeutic target. Moreover, because ATX knockout mice die in utero, small molecule inhibitors of autotaxin are very attractive tools for manipulation of ATX activity in mouse models of disease. With this goal in mind, several recent reports have begun to describe the identification, synthesis and characterization of the first ATX inhibitors which include provocative demonstrations of their potential value as anti-cancer therapeutics. The discovery that both LPA and S1P are potent “mixed” inhibitors of purified autotaxin has accelerated research in this area. Using the FRET sensor CPF4 as substrate, the Ki for inhibition of ATX by LPA is ~100 nM. Inhibition involves both a reduction in Vmax and an increase in Km and the high potency indicates that binding of LPA to the enzyme is ~1000-fold stronger than to lysophospholipid substrates(88). The biological importance of this mechanism remains to be established but clearly suggests that LPA and possibly also S1P could act as “feedback” inhibitors of ATX. While steady state plasma levels of LPA are low (>1μM) in comparison to the abundance of LPC possible roles for LPA binding to protein carriers and degradation by phospholipases and lipid phosphatases also need to be considered. However, feedback inhibition ATX could provide a relevant mechanism to limit localized accumulation of LPA in the blood and other tissues. The possibility that S1P could modulate the extracellular production of LPA also suggests another nexus for the already complex interactions between these signaling systems.
Because of ongoing efforts to generate subtype selective agonists and antagonists of LPA and S1P selective G-protein coupled receptors several groups already have a substantial armamentarium of candidate LPA-mimetic potential ATX inhibitors available for testing. These studies have established that even fatty alcohol phosphates which are structurally simple LPA analogs can inhibit ATX(90). Cyclic phosphatidic acid (cyclicPA) is a naturally occurring analog of LPA in which the phosphate group substituted in the sn-3 hydroxyl group of the glycerol backbone forms a cyclic phosphodiester bond with the free sn-2 hydroxyl group. The biological mechanism responsible for generating this curious LPA analog is presently unclear. ATX can produce detectable quantities of cyclic PA or it would be reasonable to presume that actions of another phospholipase that favors nucleophilic attack by the sn-2 hydroxyl group over direct hydrolysis of the phosphodiester bond of a lysophospholipid substrate is involved in this process(91). Surprisingly the spectrum of biological responses to cyclicPA observed in cell culture systems and animal models can broadly be classified as “opposing” those of LPA(92). Although cyclic PA is a weak but measurable inhibitor of ATX stabilized cyclicPA analogs in which the sn-2 oxygen atom is substituted with a methylene group are highly effective ATX inhibitors with Ki values in the range of 100 nM(93). A particular challenge here is to identify LPA analogs that inhibit ATX without exhibiting significant agonist effects on LPA receptors and these “carba” cyclicPA analogs have very weak agonist activities at recombinant LPA receptors suggesting that they may be suitable for this purpose. These carba cyclic PA analogs inhibited tumor cell invasion when tested in an in vitro assay for ATX promoted increases in cell motility. Previous work demonstrated an anti-metastatic effect of exogenously administered cyclicPA on melanoma and intestinal tumors in mice and these carba cyclicPA analogs also produced significant inhibition of melanoma cell metastasis in this mouse model raising the possibility that inhibition of ATX accounts, at least in part, for the effects observed(93). Ongoing collaborations between chemists and pharmacologists continue to identify LPA mimetics with mixed effects on ATX and LPA receptors and one can reasonably expect that this will continue to be a fruitful area for investigation. In particular, the more recent identification of compounds with a dual action as both ATX inhibitors and LPA receptor subtype-selective antagonists may provide a novel synergistic approach for coincident inhibition of LPA production and signaling(94). Clearly much more needs to be done to establish the pharmacokinetics and mechanism of action of these experimental drugs as LPA inhibitors in vivo. In particular, clearly a demonstration that these compounds can alter circulating or localized levels of LPA when administered to animal models is presently lacking as is convincing evidence that effects observed in animal models actually involve actions at the intended ATX target. Nevertheless, this class of “lipid-like” ATX inhibitors clearly provide an exciting first step towards the eventual development of useful drugs targetting this enzyme.
The development of LPA and S1P analogs as ATX inhibitors necessitates careful evaluation of their effects on other receptor systems and enzymes involved in LPA signaling and metabolism making the identification of non-lipid ATX inhibitors which could reasonably be expected not to interact with these unintended targets an attractive goal. Although no potent non-lipid ATX inhibitors have yet been reported, a recent study evaluated effects of a subset of compounds previously identified as phosphodiesterase and kinase inhibitors on ATX activity using a colorimetric multiwell plate format assay with bispNPP as substrate which was augmented by secondary screening using LPC substrate. Two previously identified phosphodiesterase inhibitors calmidiaolium and vinpocetine and two kinase inhibitors damnacanthal and hypericin were identified as non-phospholipid autotaxin inhibitors albeit with relatively impotent with Ki values in the range of ~100 μM. One potentially insightful finding from this study was that certain of these compounds exerted selective inhibitory effects on the nucleotide and lipid-directed phosphodiesterase activities(83). Since both activities clearly involve the same active site compartive studies of these compounds and exploration of inhbitor structure activity relationships might provide insights into how the enzyme discriminates between nucleotide and lipid substrates.
7. Conclusions
In summary, ATX is now clearly established as a central regulator of LPA metabolism and signaling. Ongoing work identifying critical roles for LPA a broad range of physiological and disease processes focuses attention on ATX as an exciting target for pharmacological intervention. To date, work in this area had been largely driven by the identification of LPA and S1P as “product’inhibitors” of ATX and while this has led to the identification of useful “lead” compounds concerns about bioavailability and effects on LPA receptors and other enzymes and a failure to demonstrate effectiveness as inhibitors of LPA production in vivo tempers enthusiasm for this approach. At the same time, ATX activity can readily be measured colorimetric and fluorescence-based assays that are highly amenable to adaptation for high throughput. Although there are clues that recognition of nucleotide versus phospholipid substrates involves distinct determinants the basic catalytic machinery of the enzyme involved in hydrolysis of both classes of substrates is the same so one would predict that inhibitors identified using nucleotide-like substrates would also be effective inhibitors of the lipid phosphodiesterase activity. Although direct measurement of the hydrolysis of lipid substrates cannot by simply adapted for high throughput screening because of the requirement for physical separation of substrate and unreacted product its quite feasible to use radiolabeled or fluorescent lipid substrates in secondary screens for validation of “hits” obtained from the primary screen. Finally, effects of ATX on the growth, migration and survival of cultured cells have been well described and these assays can be used to evaluate effects of ATX inhibitors in a more biological context. While homozygous deletion of the ATX gene results in early embryonic lethality, mice that are heterozygous for the null autotaxin allele have reduced circulating levels of ATX and LPA, are viable and do not exhibit obvious phenotypes suggesting that inhibition of ATX activity would not produce dramatic effects on normal physiology. The availability of these animals (which one might predict would be more sensitive to effects of pharmacological inhibitors of ATX) promises to provide another useful experimental system to explore the effects of small molecule inhibitors of this interesting enzyme.
Table 1. Summary of identified autotaxin inhibitors.
Note that several of these papers describe the synthesis and characterization of a series of compounds with a shared basic structure. The Ki data noted in the table are from the most potent compounds described in these reports.
| Inhibitor | Ki | Assay/Substrate | Receptor Activity? | Reference |
|---|---|---|---|---|
| LPA | 110 nM | CPF4, LPC | Agonist | (88) |
| S1P | 40nM | Agonist | (88) | |
| Carba cyclic PA Analogs | 140 nM | BispNpp, LPC | Weak agonist | (93) |
| Sn-2 aminooxy LPA Analogs | ~1μM | BispNpp, LPC | (95) | |
| β-Keto and β-hydroxy phosphonate LPA Analogs | ~1μM | LPC, pNpTMp | No | (96) |
| Fatty alcohol phosphates | ~1μM | CPF4 | Subtype specific agonists/antagonists | (90) |
| L-Histidine | ~1mM | pNpTMp, LPC | No | (97) |
| calmidiaolium vinpocetine damnacanthal hypericin | ~100 μM | BispNpp, LPC | No | (83) |
Acknowledgments
Research in the authors laboratories is supported by grants from the National Institutes of Health, Department of Veterans Affairs and American Heart Association.
References
- 1.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–54. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 2.Chun J, Rosen H. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr Pharm Des. 2006;12:161–71. doi: 10.2174/138161206775193109. [DOI] [PubMed] [Google Scholar]
- 3.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Goetzl EJ, Tigyi G. Lysophospholipids and their G protein-coupled receptors in biology and diseases. J Cell Biochem. 2004;92:867–8. doi: 10.1002/jcb.20186. [DOI] [PubMed] [Google Scholar]
- 5.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–81. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 6.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–89. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol. 2005;169:801–11. doi: 10.1083/jcb.200407123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntyre TM, Pontsler AV, Silva AR, St HA, Xu Y, Hinshaw JC, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–6. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourcade O, Simon MF, Viode C, Rugani N, Leballe F, Ragab A, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–27. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 10.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–25. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 13.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem J. 2005;387:281–93. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem. 2004;92:900–12. doi: 10.1002/jcb.20126. [DOI] [PubMed] [Google Scholar]
- 16.Yue J, Yokoyama K, Balazs L, Baker DL, Smalley D, Pilquil C, et al. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell Signal. 2004;16:385–99. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Jr, Gang C, et al. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–37. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 18.Murph M, Tanaka T, Liu S, Mills GB. Of spiders and crabs: the emergence of lysophospholipids and their metabolic pathways as targets for therapy in cancer. Clin Cancer Res. 2006;12:6598–602. doi: 10.1158/1078-0432.CCR-06-1721. [DOI] [PubMed] [Google Scholar]
- 19.Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem. 2001;292:287–95. doi: 10.1006/abio.2001.5063. [DOI] [PubMed] [Google Scholar]
- 20.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–8. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 21.Tokumura A, Kume T, Fukuzawa K, Tsukatani H. Cardiovascular effects of lysophosphatidic acid and its structural analogs in rats. J Pharmacol Exp Ther. 1981;219:219–24. [PubMed] [Google Scholar]
- 22.Tokumura A, Fukuzawa K, Isobe J, Tsukatani H. Lysophosphatidic acid-induced aggregation of human and feline platelets: structure-activity relationship. Biochem Biophys Res Commun. 1981;99:391–8. doi: 10.1016/0006-291x(81)91758-7. [DOI] [PubMed] [Google Scholar]
- 23.Seckl MJ, Seufferlein T, Rozengurt E. Lysophosphatidic acid-depleted serum, hepatocyte growth factor and stem cell growth factor stimulate colony growth of small cell lung cancer cells through a calcium-independent pathway. Cancer Res. 1994;54:6143–7. [PubMed] [Google Scholar]
- 24.Gerrard JM, Robinson P. Lysophosphatidic acid can activate platelets without increasing 32P-labelling of phosphatidic acid. Biochim Biophys Acta. 1984;795:487–92. doi: 10.1016/0005-2760(84)90177-2. [DOI] [PubMed] [Google Scholar]
- 25.Hla T, Lee MJ, Ancellin N, Thangada S, Liu CH, Kluk M, et al. Sphingosine-1-phosphate signaling via the EDG-1 family of G-protein-coupled receptors. Ann N Y Acad Sci. 2000;905:16–24. doi: 10.1111/j.1749-6632.2000.tb06534.x. [DOI] [PubMed] [Google Scholar]
- 26.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–9. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 28.Simon MF, Daviaud D, Pradere JP, Gres S, Guigne C, Wabitsch M, et al. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem. 2005;280:14656–62. doi: 10.1074/jbc.M412585200. [DOI] [PubMed] [Google Scholar]
- 29.Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634–9. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 30.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–9. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budnik LT. Lysophosphatidic acid, LPA: a bad boy becomes good. Reprod Biol Endocrinol. 2003;1:37. doi: 10.1186/1477-7827-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H. Lysophosphatidic acid (LPA) receptors are activated differentially by biological fluids: possible role of LPA-binding proteins in activation of LPA receptors. FEBS Lett. 2002;523:187–92. doi: 10.1016/s0014-5793(02)02976-9. [DOI] [PubMed] [Google Scholar]
- 33.Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, et al. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod. 2007;77:954–9. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- 34.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 36.Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82:1193–200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 38.Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem. 2007;282:5814–24. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- 39.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 40.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–91. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 41.Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8:155–67. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- 42.Umezu-Goto M, Tanyi J, Lahad J, Liu S, Yu S, Lapushin R, et al. Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem. 2004;92:1115–40. doi: 10.1002/jcb.20113. [DOI] [PubMed] [Google Scholar]
- 43.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–64. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 44.Mills GB, Fang X, Lu Y, Hasegawa Y, Eder A, Tanyi J, et al. Specific keynote: molecular therapeutics in ovarian cancer. Gynecol Oncol. 2003;88:S88–S92. doi: 10.1006/gyno.2002.6692. [DOI] [PubMed] [Google Scholar]
- 45.Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, et al. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 2002;19(287):3081–2. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 46.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006;103:9643–8. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyruchaud O, Boucharaba A, Saulnier-Blache JS, Clezardin P. Lysophosphatidic acid: a new link between blood platelets and bone metastasis. Med Sci (Paris) 2005;21:353–5. doi: 10.1051/medsci/2005214353. [DOI] [PubMed] [Google Scholar]
- 48.Mori K, Kitayama J, Shida D, Yamashita H, Watanabe T, Nagawa H. Lysophosphatidic acid-induced effects in human colon carcinoma DLD1 cells are partially dependent on transactivation of epidermal growth factor receptor. J Surg Res. 2006;132:56–61. doi: 10.1016/j.jss.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 49.Kitayama J, Shida D, Sako A, Ishikawa M, Hama K, Aoki J, et al. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 2004;6:R640–6. doi: 10.1186/bcr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shida D, Kitayama J, Yamaguchi H, Hama K, Aoki J, Arai H, et al. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp Cell Res. 2004;301:168–78. doi: 10.1016/j.yexcr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Siess W, Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem. 2004;92:1086–94. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 52.Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim Biophys Acta. 2002;1582:204–15. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 53.Panetti TS, Hannah DF, Avraamides C, Gaughan JP, Marcinkiewicz C, Huttenlocher A, et al. Extracellular matrix molecules regulate endothelial cell migration stimulated by lysophosphatidic acid. J Thromb Haemost. 2004;2:1645–56. doi: 10.1111/j.1538-7836.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 54.Rizza C, Leitinger N, Yue J, Fischer DJ, Wang DA, Shih PT, et al. Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab Invest. 1999;79:1227–35. [PubMed] [Google Scholar]
- 55.Panetti TS, Magnusson MK, Peyruchaud O, Zhang Q, Cooke ME, Sakai T, et al. Modulation of cell interactions with extracellular matrix by lysophosphatidic acid and sphingosine 1-phosphate. Prostaglandins Other Lipid Mediat. 2001;64:93–106. doi: 10.1016/s0090-6980(01)00102-2. [DOI] [PubMed] [Google Scholar]
- 56.Avraamides C, Bromberg ME, Gaughan JP, Thomas SM, Tsygankov AY, Panetti TS. Hic-5 promotes endothelial cell migration to lysophosphatidic acid. Am J Physiol Heart Circ Physiol. 2007;293:H193–203. doi: 10.1152/ajpheart.00728.2006. [DOI] [PubMed] [Google Scholar]
- 57.Brault S, Gobeil F, Jr, Fortier A, Honore JC, Joyal JS, Sapieha PS, et al. Lysophosphatidic acid induces endothelial cell death by modulating the redox environment. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1174–83. doi: 10.1152/ajpregu.00619.2006. [DOI] [PubMed] [Google Scholar]
- 58.Mauco G, Chap H, Simon MF, Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie. 1978;60:653–61. doi: 10.1016/s0300-9084(78)80784-6. [DOI] [PubMed] [Google Scholar]
- 59.Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim Biophys Acta. 1989;1001:282–5. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 60.Tokumura A. Physiological and pathophysiological roles of lysophosphatidic acids produced by secretory lysophospholipase D in body fluids. Biochim Biophys Acta. 2002;1582:18–25. doi: 10.1016/s1388-1981(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 61.Gueguen G, Gaige B, Grevy JM, Rogalle P, Bellan J, Wilson M, et al. Structure-activity analysis of the effects of lysophosphatidic acid on platelet aggregation. Biochemistry. 1999;38:8440–50. doi: 10.1021/bi9816756. [DOI] [PubMed] [Google Scholar]
- 62.Haseruck N, Erl W, Pandey D, Tigyi G, Ohlmann P, Ravanat C, et al. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103:2585–92. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 63.Pamuklar Z, Lee JS, Cheng HY, Panchatcharam M, Steinhubl S, Morris AJ, et al. Individual heterogeneity in platelet response to lysophosphatidic acid: evidence for a novel inhibitory pathway. Arterioscler Thromb Vasc Biol. 2008;28:555–61. doi: 10.1161/ATVBAHA.107.151837. [DOI] [PubMed] [Google Scholar]
- 64.Xu YJ, Rathi SS, Chapman DC, Arneja AS, Dhalla NS. Mechanisms of lysophosphatidic acid-induced DNA synthesis in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2003;41:381–7. doi: 10.1097/00005344-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Gennero I, Xuereb JM, Simon MF, Girolami JP, Bascands JL, Chap H, et al. Effects of lysophosphatidic acid on proliferation and cytosolic Ca++ of human adult vascular smooth muscle cells in culture. Thromb Res. 1999;94:317–26. doi: 10.1016/s0049-3848(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 66.Essler M, Retzer M, Bauer M, Zangl KJ, Tigyi G, Siess W. Stimulation of platelets and endothelial cells by mildly oxidized LDL proceeds through activation of lysophosphatidic acid receptors and the Rho/Rho-kinase pathway. Inhibition by lovastatin. Ann N Y Acad Sci. 2000;905:282–6. doi: 10.1111/j.1749-6632.2000.tb06561.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–74. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chun J. Lysophospholipids in the nervous system. Prostaglandins Other Lipid Mediat. 2005;77:46–51. doi: 10.1016/j.prostaglandins.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 2008;152:296–8. doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 70.Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–9. [PubMed] [Google Scholar]
- 71.Lee HY, Clair T, Mulvaney PT, Woodhouse EC, Aznavoorian S, Liotta LA, et al. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J Biol Chem. 1996;271:24408–12. doi: 10.1074/jbc.271.40.24408. [DOI] [PubMed] [Google Scholar]
- 72.Clair T, Lee HY, Liotta LA, Stracke ML. Autotaxin is an exoenzyme possessing 5′-nucleotide phosphodiesterase/ATP pyrophosphatase and ATPase activities. J Biol Chem. 1997;272:996–1001. doi: 10.1074/jbc.272.2.996. [DOI] [PubMed] [Google Scholar]
- 73.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta. 1986;875:31–8. [PubMed] [Google Scholar]
- 74.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–42. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 75.Gijsbers R, Aoki J, Arai H, Bollen M. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS Lett. 2003;538:60–4. doi: 10.1016/s0014-5793(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 76.Ferry G, Giganti A, Coge F, Bertaux F, Thiam K, Boutin JA. Functional invalidation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 2007;581:3572–8. doi: 10.1016/j.febslet.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 77.Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 78.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–60. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–23. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cimpean A, Stefan C, Gijsbers R, Stalmans W, Bollen M. Substrate-specifying determinants of the nucleotide pyrophosphatases/phosphodiesterases NPP1 and NPP2. Biochem J. 2004;381:71–7. doi: 10.1042/BJ20040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jansen S, Stefan C, Creemers JW, Waelkens E, Van Eynde A, Stalmans W, et al. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J Cell Sci. 2005;118:3081–9. doi: 10.1242/jcs.02438. [DOI] [PubMed] [Google Scholar]
- 82.Jansen S, Callewaert N, Dewerte I, Andries M, Ceulemans H, Bollen M. An essential oligomannosidic glycan chain in the catalytic domain of autotaxin, a secreted lysophospholipase-D. J Biol Chem. 2007;282:11084–91. doi: 10.1074/jbc.M611503200. [DOI] [PubMed] [Google Scholar]
- 83.Moulharat N, Fould B, Giganti A, Boutin JA, Ferry G. Molecular pharmacology of adipocyte-secreted autotaxin. Chem Biol Interact. 2008;172:115–24. doi: 10.1016/j.cbi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, et al. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61:6938–44. [PubMed] [Google Scholar]
- 85.Clair T, Aoki J, Koh E, Bandle RW, Nam SW, Ptaszynska MM, et al. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003;63:5446–53. [PubMed] [Google Scholar]
- 86.Maceyka M, Sankala H, Hait NC, Le SH, Liu H, Toman R, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–29. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 87.Ferguson CG, Bigman CS, Richardson RD, van Meeteren LA, Moolenaar WH, Prestwich GD. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org Lett. 2006;8:2023–6. doi: 10.1021/ol060414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, et al. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–61. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 89.Morris AJ, Smyth SS. Measurement of autotaxin/lysophospholipase D activity. Methods Enzymol. 2007;434:89–104. doi: 10.1016/S0076-6879(07)34005-6. [DOI] [PubMed] [Google Scholar]
- 90.Durgam GG, Virag T, Walker MD, Tsukahara R, Yasuda S, Liliom K, et al. Synthesis, structure-activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPARgamma, and inhibitors of autotaxin. J Med Chem. 2005;48:4919–30. doi: 10.1021/jm049609r. [DOI] [PubMed] [Google Scholar]
- 91.Tsuda S, Okudaira S, Moriya-Ito K, Shimamoto C, Tanaka M, Aoki J, et al. Cyclic phosphatidic acid is produced by autotaxin in blood. J Biol Chem. 2006;281:26081–8. doi: 10.1074/jbc.M602925200. [DOI] [PubMed] [Google Scholar]
- 92.Murakami-Murofushi K, Uchiyama A, Fujiwara Y, Kobayashi T, Kobayashi S, Mukai M, et al. Biological functions of a novel lipid mediator, cyclic phosphatidic acid. Biochim Biophys Acta. 2002;1582:1–7. doi: 10.1016/s1388-1981(02)00131-2. [DOI] [PubMed] [Google Scholar]
- 93.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–93. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J, et al. alpha-Substituted Phosphonate Analogues of Lysophosphatidic Acid (LPA) Selectively Inhibit Production and Action of LPA. ChemMedChem. 2007;2:679–90. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gajewiak J, Tsukahara R, Fujiwara Y, Tigyi G, Prestwich GD. Synthesis, Pharmacology, and Cell Biology of sn-2-Aminooxy Analogues of Lysophosphatidic Acid. Org Lett. 2008;10:1111–4. doi: 10.1021/ol7030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui P, McCalmont WF, Tomsig JL, Lynch KR, Macdonald TL. alpha- and beta-substituted phosphonate analogs of LPA as autotaxin inhibitors. Bioorg Med Chem. 2008;16:2212–25. doi: 10.1016/j.bmc.2007.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clair T, Koh E, Ptaszynska M, Bandle RW, Liotta LA, Schiffmann E, et al. L-histidine inhibits production of lysophosphatidic acid by the tumor-associated cytokine, autotaxin. Lipids Health Dis. 2005;4:5. doi: 10.1186/1476-511X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]