Abstract
The adipokine plasminogen activator inhibitor (PAI)-1 is increased in plasma of obese individuals and exhibits increased expression in the stomachs of individuals infected with Helicobacter. To investigate the relevance of gastric PAI-1, we used 1.1 kb of the H+/K+β subunit promoter to overexpress PAI-1 specifically in mouse gastric parietal cells (PAI-1-H/Kβ mice). We studied the physiological, biochemical, and behavioral characteristics of these and mice null for PAI-1 or a putative receptor, urokinase plasminogen activator receptor (uPAR). PAI-1-H/Kβ mice had increased plasma concentrations of PAI-1 and increased body mass, adiposity, and hyperphagia compared with wild-type mice. In the latter, food intake was inhibited by cholecystokinin (CCK)8s, but PAI-1-H/Kβ mice were insensitive to the satiating effects of CCK8s. PAI-1-H/Kβ mice also had significantly reduced expression of c-fos in the nucleus tractus solitarius in response to CCK8s and refeeding compared with wild-type mice. Exogenous PAI-1 reversed the effects of CCK8s on food intake and c-fos levels in the nucleus tractus solitarius of wild-type mice, but not uPAR-null mice. Infection of C57BL/6 mice with Helicobacter felis increased gastric abundance of PAI-1 and reduced the satiating effects of CCK8s, whereas the response to CCK8s was maintained in infected PAI-1–null mice. In cultured vagal afferent neurons, PAI-1 inhibited stimulation of neuropeptide Y type 2 receptor (Y2R) expression by CCK8s. Thus, gastric expression of PAI-1 is associated with hyperphagia, moderate obesity, and resistance to the satiating effects of CCK indicating a new role in suppressing signals from the upper gut that inhibit food intake.
Energy intake and expenditure are controlled by signals from various peripheral organs including the gut, endocrine pancreas, and adipose tissue (1). Gut-derived signals that inhibit energy intake include brain-gut peptides, eg, cholecystokinin (CCK); proinflammatory cytokines, eg, IL1β; and adipokines, eg, leptin (2). Recent work suggests that the adipokine plasminogen activator inhibitor (PAI)-1, which is elevated in plasma in obesity, is also produced in gut epithelial and subepithelial cells, raising the possibility of novel roles in gastrointestinal (GI) function and energy balance (3, 4).
PAI-1 is the principal extracellular inhibitor of urokinase plasminogen activator (uPA) and tissue plasminogen activator (tPA). It is an approximately 45-kDa molecule expressed in adipocytes and adipose stromal cells, liver, endothelial cells, platelets, macrophages, and monocytes. Because PAI-1 inhibits thrombolysis by inhibition of uPA and tPA and is elevated in obesity, it has been suggested to play a role in the increased risk of vascular occlusive disease in obesity (5, 6). In the stomach, PAI-1 is expressed in parietal and enterochromaffin-like cells, and expression is increased in Helicobacter pylori infection (3) and with elevated plasma gastrin concentrations (7). However, the role of PAI-1 in gastric function and a possible link to metabolic function is virtually unexplored.
Many GI signals influencing energy intake and expenditure do so by modulating vagal afferent signaling pathways (8, 9). There are multiple interactions including potentiation of vagal afferent responses to CCK by leptin (10–12) and inhibition by ghrelin (11, 13). Moreover, vagal afferent neurons are a target for cytokines released in infection and inflammation (14–16). In the present study, we hypothesized that gastric PAI-1 also influences gut-brain signaling. We now report that transgenic mice with overexpression of PAI-1 targeted to parietal cells exhibit hyperphagia and moderate lifelong obesity; we present evidence that PAI-1 suppresses vagal afferent signaling in response to CCK, indicating a previously unsuspected role in the stomach in reversing satiety signaling.
Materials and Methods
Animals
Wild-type C57BL/6 mice were obtained from Charles River (Wilmington, MA). Mice null for PAI-1 (PAI-1−/−) or for uPAR (uPAR−/−) on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were individually housed. Unless otherwise stated, experiments were conducted using male mice at 10 to 12 weeks. Adult male 200- to 300-g Wistar rats were purchased from Charles River and were group housed. All animals were maintained in an appropriately controlled environment with a 12-hour light, 12-hour dark cycle and were fed a commercial pellet diet with water ad libitum. Animals were killed by increasing CO2 concentration; blood was collected in trisodium citrate by cardiac puncture. All animal experiments were approved by the University of Liverpool Animal Welfare Committee and were conducted in compliance with United Kingdom Home Office requirements and the Animals (Scientific Procedures) Act 1986.
PAI-1-H/Kβ mice
Mice with overexpression of PAI-1 targeted to the stomach were generated using the proximal 1.1 kb of the H+/K+ATPase β-subunit promoter, which is known to be selectively expressed in parietal cells (17), coupled to 1896 bp of mouse PAI-1 cDNA incorporating 45 bp of the 5′ noncoding region, the coding sequence, and 645 bp of the 3′ noncoding region in a pEGFP-1 vector backbone. The PAI-1 transgene was generated by digestion with HindIII and AflII, purified, and microinjected into pronuclei of embryos isolated from C57BL/CBA F1 hybrid mice. Two female founders (PAI-1-H/Kβ mice) were identified by Southern blotting to have 1 to 2 and 2 to 4 transgene copies at single integration sites and were backcrossed into C57BL/6 mice; the data reported here were obtained using mice derived from the latter founder (data for the former are presented in Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Body weight and food intake studies
In longitudinal studies, mice were weighed weekly for up to 50 weeks. Food intake was measured over varying periods. In some experiments, mice were fasted for 24 hours and treated with CCK8s (Tocris Biosciences, Bristol, UK), stabilized human PAI-1 (Calbiochem, Hertfordshire, UK) (18), vehicle, or combinations, prior to refeeding. In paired feeding experiments, 6-week-old male PAI-1-H/Kβ mice were either fed ad libitum or were fed daily for 6 weeks with identical quantities of food to those consumed by C57BL/6 mice. For studies of diet-induced obesity, 8-week-old male C57BL/6 and PAI-1-H/Kβ mice were fed either normal chow or a diet with 45% kilocalories as fat (Special Diet Services, Essex, UK) for 6 weeks.
Brainstem c-fos labeling
For experiments involving nucleus tractus solitarius (NTS) c-fos expression, mice were killed 90 minutes post treatment or refeeding. Animals were anesthetized with sodium pentobarbitone and perfused transcardially with 0.14M NaCl followed by 4% paraformaldehyde, brains were removed, and brainstems dissected, postfixed for 4 hours, and transferred to 30% (wt/vol) sucrose. Sections (40 μm) from approximately −7.76 to −7.32 mm from Bregma were processed using rabbit anti-c-fos antibody (Calbiochem), biotinylated antirabbit secondary antibody (DAKO, Cambridge, UK), and an ABC-Vector kit (Vector Laboratories, Peterborough, UK). Sections from the level of the area postrema were viewed using an Axioplan-2 microscope (Zeiss, Oberkochen, Germany). Neurons in the NTS with nuclear black/brown staining were counted as positive. On average, 4 to 6 sections per brainstem were quantified and the mean number of positive neurons determined.
Assays
Concentrations of PAI-1 (Molecular Innovations, Novi, MI), leptin (BioVendor, Oxford, UK), total ghrelin (Linco Research, St. Charles, MO), and insulin (Millipore, Watford, UK) were determined by ELISA of plasma according to manufacturers' instructions. Concentrations of plasma cholesterol and nonesterified fatty acids were determined using an autoanalyzer (Roche, West Sussex, UK). Blood glucose was measured from tail-tip blood using an Ascensia Contour meter (Bayer, Berkshire, UK). Total amidated gastrin was determined as previously described using [125I]G17 (PerkinElmer, Cambridge, UK) (19). Plasma CCK was determined as described previously (20) using [125I]CCK8 (PerkinElmer).
PCR analyses
RNA was extracted using Trizol (Invitrogen, Paisley, UK), treated with deoxyribonuclease (Ambion, Austin, TX), and reverse transcribed with avian myeloblastosis virus reverse transcriptase (Promega, Southampton, UK) and oligo-deoxythymidine primers (Fermentas, Baden-Wurttemberg, Germany). Three different assays were employed for PAI-1 mRNA: (1) wild-type PAI-1 (ie, excluding transgene), (2) total PAI-1 (ie, wild-type and transgene), and (3) transgenic PAI-1 (ie, excluding wild-type). Real-time PCR was performed on a 7500 real-time PCR system (Applied Biosystems, Warrington, UK) using either TaqMan primer/probe sets (wild-type PAI-1; ghrelin), or SYBR green (total PAI-1; transgenic PAI-1; GAPDH); endpoint RT-PCR was employed for uPAR mRNA. Quantifications were performed using a standard curve, and all values were standardized to GAPDH determined in the same sample. All primers and probes were purchased from Eurogentec (Seraing, Belgium) (see Supplemental Methods).
Helicobacter felis infection
Six-week-old male C57BL/6, PAI-1-H/Kβ, and PAI-1−/− mice were inoculated via oral gavage 3 times over a 2-week period with minimally passaged H. felis (ATCC 49179; 0.5 ml 1010 colony-forming units/ml) in TBS broth. Bacteria were routinely cultured in a microaerophilic atmosphere at 37°C on fresh chocolatized Columbia blood agar. Infection with H. felis was verified by an antral urease test (Prontodry; Medical Instruments Corporation, Solothrum, Switzerland) or by antral histology. Feeding experiments were performed after 6 months, and subsequently stomachs were fixed in paraformaldehyde and sections stained with hematoxylin and eosin; inflammatory cell infiltrates were scored as previously described (21).
Vagal afferent neurons
Nodose ganglia were dissected from 48-hour-fasted rats under aseptic conditions and were digested and cultured as previously described (22). Cultured neurons were used for immunohistochemistry or luciferase reporter assays. In some experiments, neurons were cultured in full medium for 48 hours in chamber slides, serum starved for 12 hours, and stimulated with 10nM CCK8s, 40nM PAI-1, or a combination of both for 2 hours.
Immunohistochemistry
The localization of urokinase plasminogen activator receptor (uPAR) was detected using a goat polyclonal antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and early growth response protein 1 (EGR-1) was localized using a rabbit polyclonal antibody (Santa Cruz). The appropriate donkey secondary antibodies conjugated to fluorescein isothiocyanate were used (Jackson ImmunoResearchWest Grove, PA). Samples were mounted in Vectashield containing 4′,6-diamidino-2-phenylindole (Vector Laboratories), visualized using an Axioplan Universal microscope and images processed using AxioVision version 3.0 imagining system (Zeiss). Neurons expressing EGR-1 in the nucleus or cytoplasm were quantified in a minimum of 20 fields per well.
Luciferase promoter-reporter assays
A construct consisting of 3034 bp of the wild-type human promoter sequence of Y2R (neuropeptide Y type 2 receptor) coupled to luciferase (Y2R-luc) has been previously described (23). Vagal afferent neurons cultured for 48 hours were transfected with Y2R-luc and a constitutively active Renilla-luciferase plasmid as an internal control (pRL-Sv40, 0.5 ng/well; Promega, Madison, WI) using CombiMag (OzBiosciences, Marseille, France) using Transfast reagent (Promega) according to the manufacturer's instructions. Cells were incubated in full medium for 48 hours, serum starved for 12 hours, and stimulated with 10nM CCK8s, 40nM PAI-1, or a combination of both for 6 hours. Luciferase activity was measured as previously described (22).
Statistics
Results are presented as means ± SEM; comparisons were made using either ANOVA or Student's t test as appropriate and were considered significant at P < .05.
Results
PAI-1-H/Kβ mice are obese
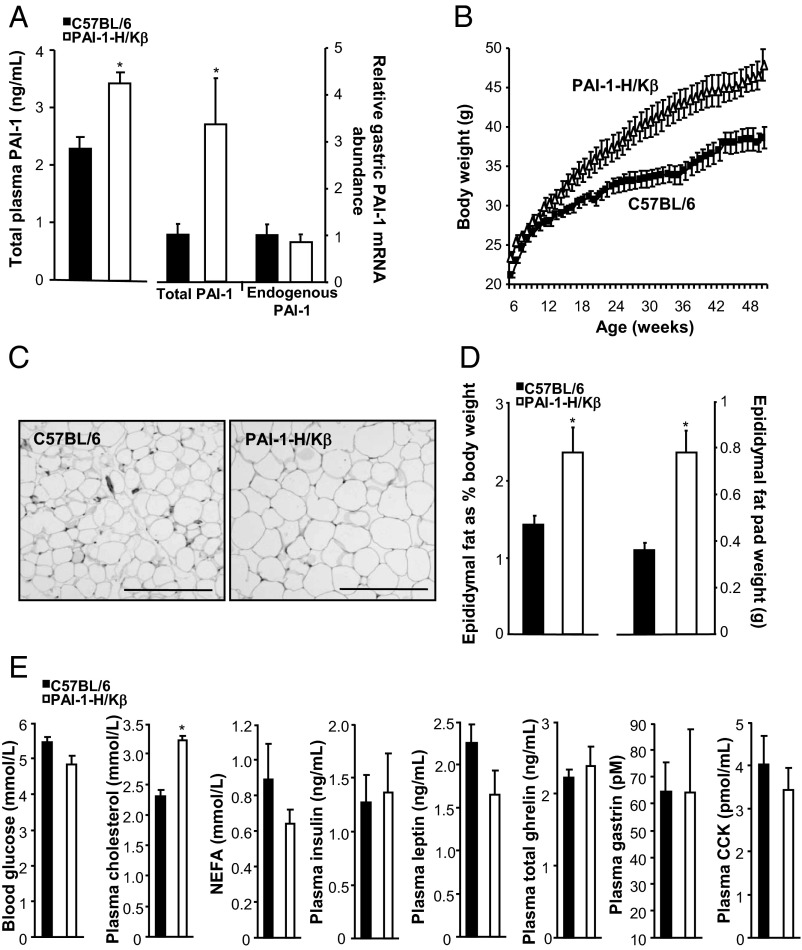
Transgenic mice with targeted overexpression of PAI-1 to parietal cells exhibited modest increases in plasma PAI-1 concentrations and approximately 3-fold higher total gastric PAI-1 mRNA abundance (Figure 1A). An assay specific for wild-type PAI-1 mRNA indicated no difference in abundance between transgenic and C57BL/6 mice, whereas an assay specific for the transgene verified expression of transgenic mRNA in PAI-1-H/Kβ but not C57BL/6 mouse stomach. Over the period of 12 to 50 weeks, the body weights of PAI-1-H/Kβ mice were significantly higher compared with C57BL/6 mice (Figure 1B and Supplemental Figure 1). Increased body weight was exhibited by both males and females in one line and by males of a second line (Supplemental Figure 1A). Transgenic mice exhibited adipocyte hypertrophy (Figure 1C) and increased epididymal fat pad mass expressed both on an absolute basis and relative to body weight (Figure 1D). Plasma concentrations of nonesterified fatty acids, glucose, insulin, leptin, gastrin, and CCK were similar in PAI-1H/Kβ and C57BL/6 mice fed ad libitum, but there was a modest increase in cholesterol in PAI-1H/Kβ mice (Figure 1E).
Figure 1.
PAI-1-H/Kβ mice are obese. A, Increased plasma PAI-1 and total gastric PAI-1 mRNA abundance, but not wild-type PAI-1 mRNA, in PAI-1-H/Kβ mice. B, Increased body weight from 8 to 50 weeks in PAI-1-H/Kβ mice. C, Hypertrophy of epididymal adipocytes in PAI-1-H/Kβ mice: C57BL/6, 485 ± 22 vs PAI-1-H/Kβ, 287 ± 28 cells per square millimeter. D, Increased epididymal fat pat weights in PAI-1-H/Kβ on an absolute basis (right) and as proportion of body weight (left). E, Blood glucose, plasma cholesterol, nonesterified fatty acids (NEFA), insulin, leptin, total ghrelin, gastrin, and CCK in ad libitum-fed C57BL/6 and PAI-1-H/Kβ mice; plasma CCK was assayed in a separate set of samples. In C–E, mice were 10 to 12 weeks old. *, P < .05; n = 6–13.
PAI-1-H/Kβ mice are hyperphagic and resistant to the satiety effects of CCK
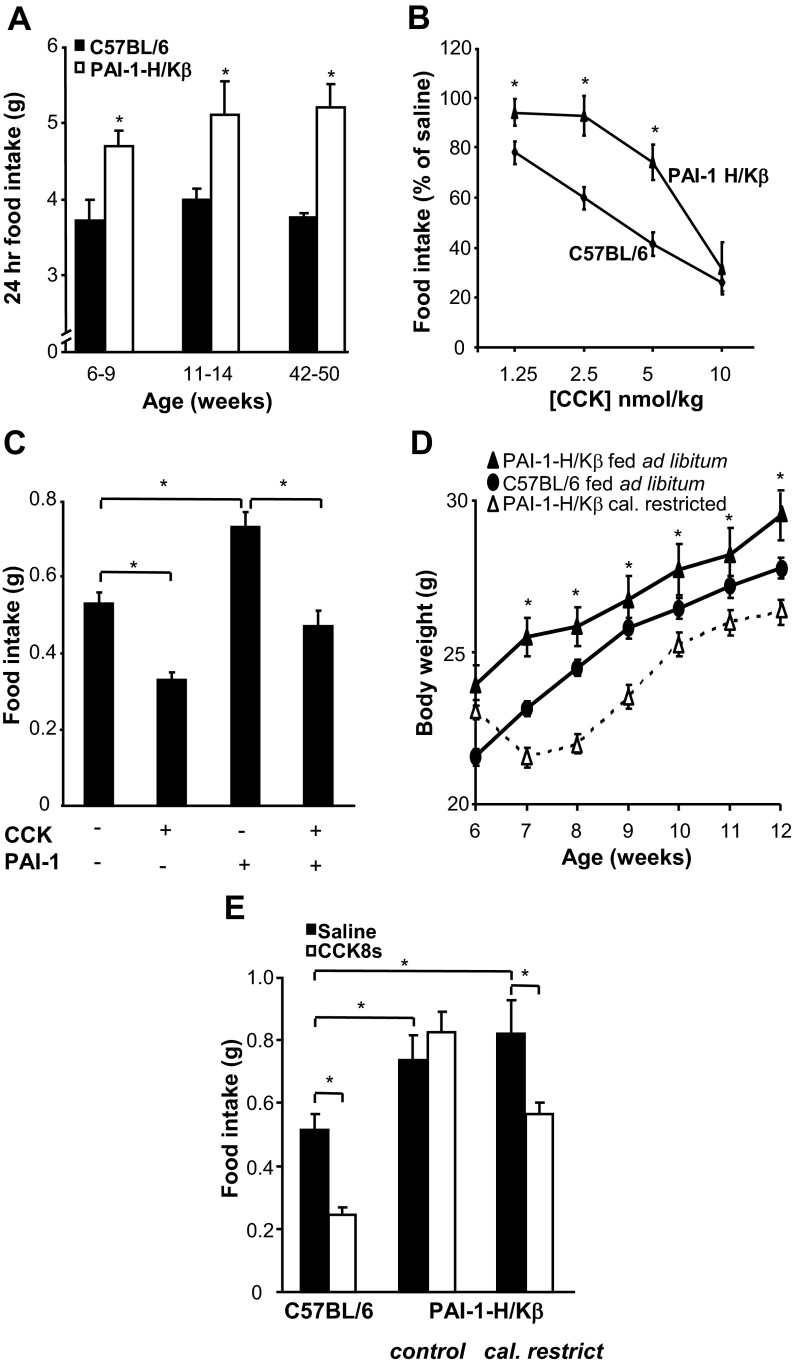
There was approximately 25% higher 24-hour food intake in PAI-1-H/Kβ compared with C57BL/6 mice at ages from 6 to 50 weeks (Figure 2A and Supplemental Figure 1B). Hyperphagia was evident in both male and female mice in the line showing moderate obesity and in males of a second line (Supplemental Figure 1B). The hyperphagia was not considered to be attributable to increased ghrelin expression, because total plasma ghrelin (Figure 1F) and gastric ghrelin mRNA abundance were similar in C57BL/6 and PAI-1-H/Kβ mice (1.0 ± 0.27 vs 1.07 ± 0.25, respectively). In C57BL/6 mice, food intake over 30 min after a 24-hour fast was dose-dependently inhibited by ip CCK8s (1.25–10 nmol/kg). However, in PAI-1-H/Kβ mice 30-minute food intake after fasting was greater than in C57BL/6 mice, the dose-response curve to CCK was shifted to the right, and only the higher doses of CCK reduced food intake (Figure 2B). The data suggest that PAI-1 suppresses the satiety effect of CCK; consistent with this, in C57BL/6 mice, PAI-1 (2.5 nmol/kg, ip) significantly increased food intake over 30 minutes after a 24-hour fast and reduced the satiety action of CCK8s so that when the two were given together, food intake was not significantly different from control (Figure 2C). To determine whether the obese phenotype in PAI-1-H/Kβ mice was a consequence of hyperphagia and CCK resistance, we examined transgenic mice fed the same daily food ration as that consumed by C57BL/6 mice. The PAI-1-H/Kβ mice in this experiment exhibited an initial reduction in body weight, but thereafter, body weight increased but remained significantly less than in PAI-1H/Kβ mice fed ad libitum (Figure 2D). Moreover, PAI-1-H/Kβ mice on the restricted diet retained their hyperphagic phenotype when given ad libitum access to food after an overnight fast; in these mice, CCK modestly reduced food intake by 23.5 ± 14.4% compared with 50.8 ± 5.2% in C57BL/6 mice, although on an absolute basis there was no significant difference between food intake after CCK in diet-restricted PAI-1-H/Kβ mice and saline-treated C57BL/6 mice (Figure 2E).
Figure 2.
PAI-1-H/Kβ mice are hyperphagic and resistant to CCK. A, Increased 24-hour food intake in PAI-1-H/Kβ compared with C57BL/6 mice at 6 to 9, 11 to 14, and 42 to 50 weeks. B, Resistance to the satiating action of ip CCK8s in PAI-1-H/Kβ compared with C57BL/6 mice refed for 30 min after a 24-hour fast (PAI-1-H/Kβ control intake, 0.78 ± 0.06 g/30 min; C57BL/6, 0.53 ± 0.03 g/30 min). C, Administration of ip PAI-1 (2.5 nmol/kg) to C57BL/6 mice stimulates food intake and inhibits the effect of CCK8s (2.5 nmol/kg). D, Reduced weight gain in PAI-1-H/Kβ mice fed the daily food ration consumed by C57BL/6 mice. E, PAI-1-H/Kβ mice pair-fed with C57BL/6 mice (6 weeks), ie, calorie restricted (cal. restrict.) vs control, exhibit hyperphagia and partial sensitivity to CCK8s (2.5 nmol/kg) compared with C57BL/6 mice when subsequently fasted 24 hours and fed for 30 minutes ad libitum. *, P < .05; n = 6–8.
PAI-1 suppresses brainstem neuron activation by CCK and requires uPAR
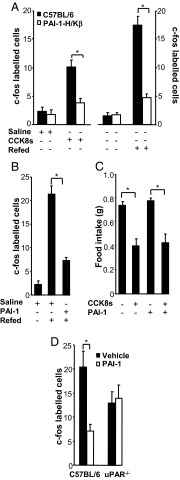
The satiety effect of CCK is mediated via vagal afferent neurons and accompanied by increased expression of c-fos in neurons of the NTS (Supplemental Figure 2). However, in PAI-1-H/Kβ mice, c-fos expression in the NTS was significantly less than in C57BL/6 mice after CCK8s (Figure 3, A and B, and Supplemental Figure 2). In addition, there was significantly reduced c-fos expression in the PAI-1-H/Kβ mice compared with C57BL/6 after refeeding after 24 hours fasting, suggesting that brainstem neuron activation in transgenic mice is reduced in response to endogenous satiety factors as well as exogenous CCK (Figure 3A). Compatible with the idea that PAI-1 inhibits signaling to the NTS, when PAI-1 (2.5 nmol/kg, ip) was administered to C57BL/6 mice immediately prior to refeeding after a 24-hour fast, there was a significant reduction in c-fos–expressing neurons in NTS (Figure 3B). Some actions of PAI-1 are mediated by interaction with the uPAR. To determine the role of the latter in responses to PAI-1, we examined food intake in uPAR−/− mice after ip CCK8s and PAI-1. Body weight and 24-hour food intake in uPAR-null mice were similar to wild-type mice (Supplemental Figure 3), and administration of CCK8s (2.5 nmol/kg ip) also inhibited food intake in uPAR−/− mice; however, PAI-1 (2.5 nmol/kg ip) alone had no effect on food intake and did not suppress the effect of CCK8s (Figure 3C). Moreover, the expression of c-fos in the NTS in uPAR−/− mice after refeeding was not significantly different after administration of PAI-1, consistent with the idea that PAI-1 acts via uPAR (Figure 3D).
Figure 3.
Suppressed stimulation of c-fos in NTS neurons in PAI-1-H/Kβ mice in response to CCK8s and role of uPAR. A, Quantification of NTS c-fos–labeled neurons in response to CCK8s (2.5 nmol/kg ip) in PAI-1-H/Kβ and C57BL/6 mice. B, Increased c-fos labeling in C57BL/6 mice after refeeding and inhibition by exogenous PAI-1 (2.5 nmol/kg, ip). C, In uPAR−/− mice, CCK8s (2.5 nmol/kg, ip) inhibits food intake, but this is not blocked by PAI-1 (2.5 nmol/kg; cf C57BL/6 mice, Figure 2C). D, In uPAR−/− mice, PAI-1 (2.5 nmol/kg, ip) does not influence the number of NTS neurons expressing c-fos after refeeding, whereas in C57BL/6 mice, PAI-1 decreases fos labeling. *, P < .05; n = 5–8.
Similar susceptibility to diet-induced obesity in PAI-1-H/Kβ and wild-type mice
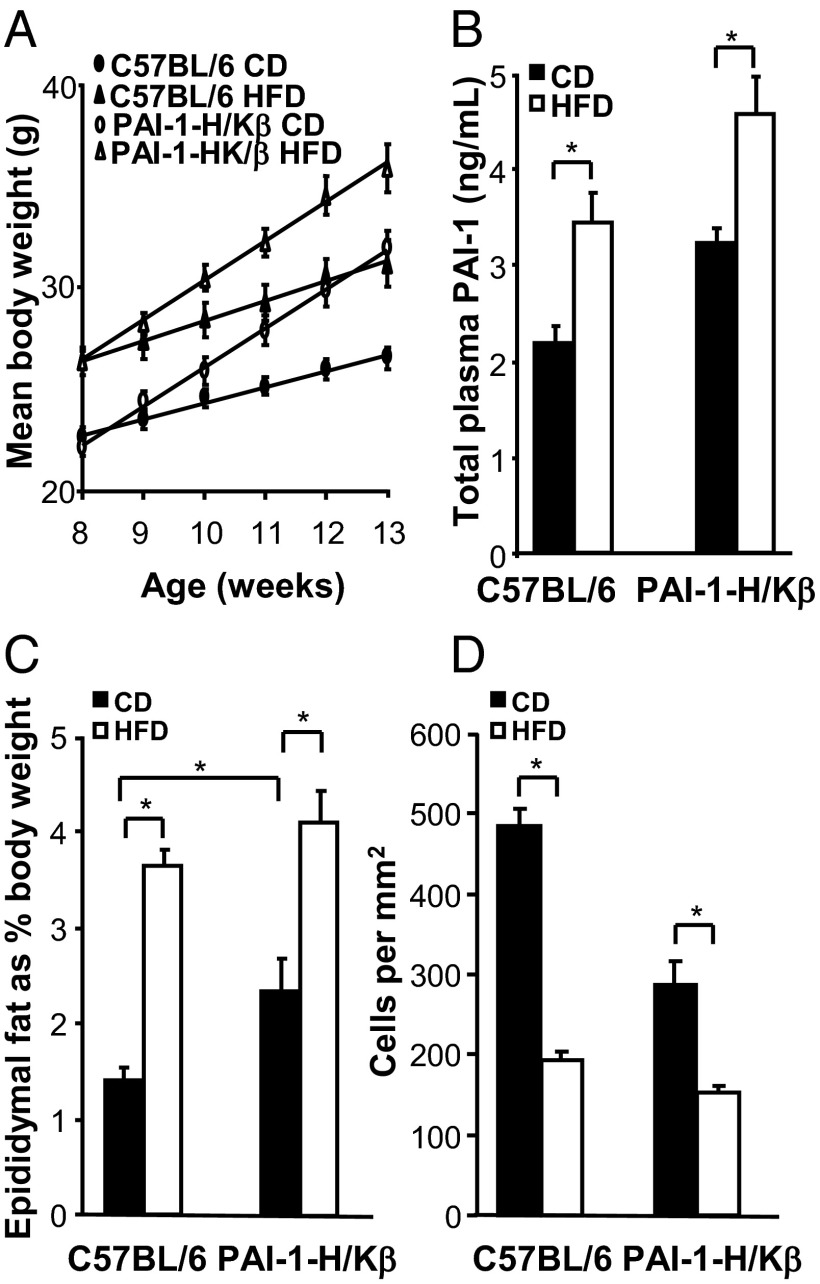
When PAI-I-H/Kβ mice were fed a diet with 45% calories as fat, there was increased weight gain that was proportionately similar to that in C57BL/6 mice (Figure 4A). Plasma PAI-1 was moderately elevated in both C57BL/6 and PAI-I-H/Kβ mice fed a high-fat diet (Figure 4B), and there was increased adiposity as indicated by increased epididymal fat pad mass and adipocyte cell volume (Figure 4, C and D). Conversely, PAI-1-null mice on a C57BL/6 background are resistant to diet-induced obesity (24), and this was confirmed using the present experimental protocol (Supplemental Figure 4).
Figure 4.
Adiposity in PAI-1-H/Kβ and C57BL/6 mice on a high-fat diet. A, Body weight of mice fed a control diet (CD) or high fat diet (HFD) (45% calories as fat) for 5 weeks. B, Plasma PAI-1 concentrations are increased in C57BL/6 and PAI-1-H/Kβ mice on HFD. C, Epididymal fat pad mass is increased in C57BL/6 and PAI-1-H/Kβ mice on HFD. D, Adipocyte hypertrophy in C57BL/6 and PAI-1-H/Kβ mice on HFD expressed as cells per square millimeter. *, P < .05; n = 8.
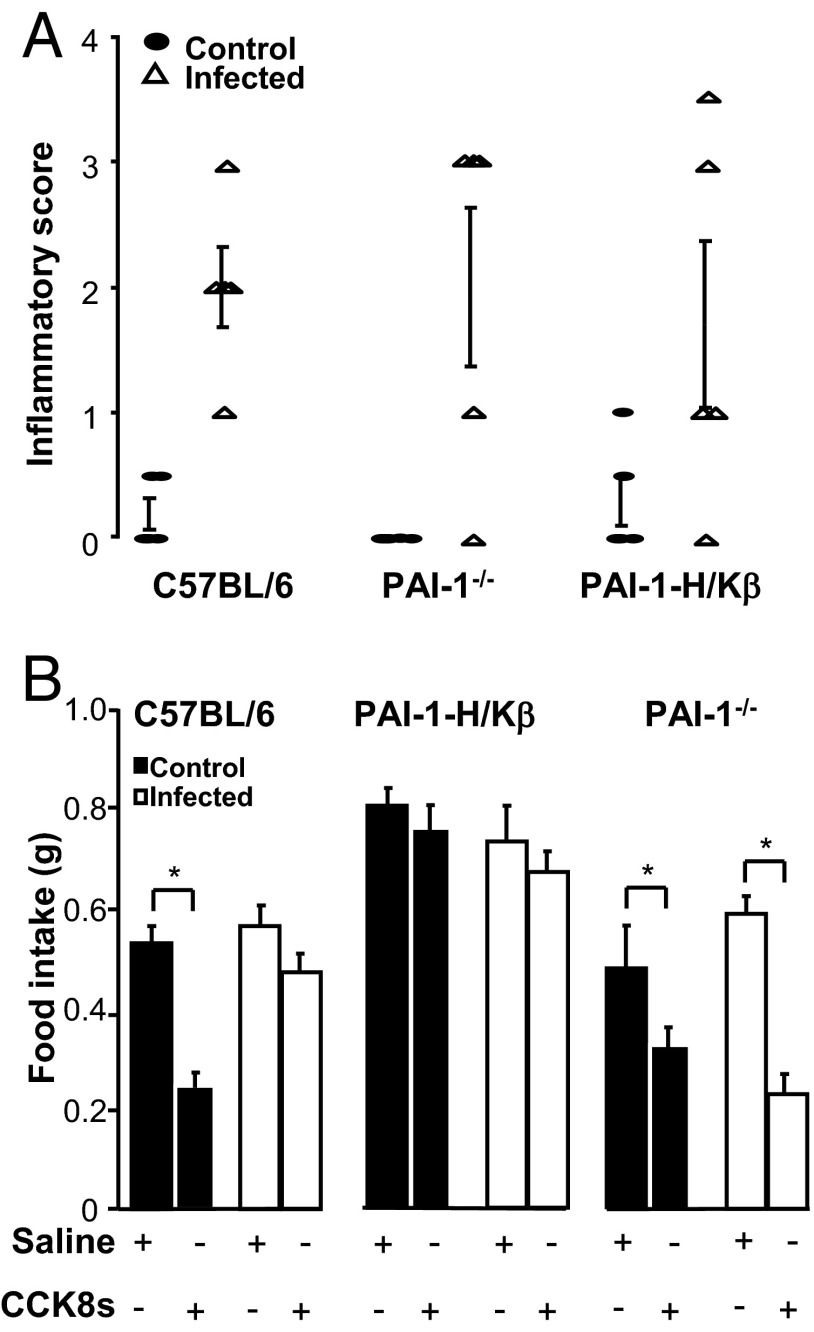
H. felis infection decreases the satiating effects of CCK8s in C57BL/6 but not in PAI-1−/− mice
Because Helicobacter infection increases gastric PAI-1 (3), we then examined food intake in H. felis-infected C57BL/6, PAI-1-H/Kβ, and PAI-1−/− mice. Confirming previous findings, PAI-1 mRNA was approximately 2-fold higher in the gastric corpus of infected compared with control mice (100 ± 3 vs 206 ± 18, P < .05). All three strains were successfully infected as indicated by antral urease tests or antral histology (not shown), and all three strains exhibited similar inflammatory cell infiltrates in the gastric corpus (Figure 5A). We did not detect parietal cell loss in any of the three strains at the time of food intake studies. Food intake over 24 hours was similar in infected and uninfected mice in each strain, although the hyperphagic phenotype of PAI-1-H/Kβ mice was maintained (Supplemental Figure 5A). Body weight was also similar in infected and uninfected mice in each strain up to 6 months of infection; PAI-1-H/Kβ mice retained their moderately obese phenotype, although after 6 months, there was a modest decrease in weight gain in infected mice (Supplemental Figure 5B). Although CCK8s inhibited food intake in uninfected C57BL/6 mice, the response was virtually abolished in infected C57BL/6 mice (Figure 5B). There was no effect of CCK8s on food intake in either infected or uninfected PAI-1-H/Kβ mice. Importantly, however, in PAI-1−/− mice, the dose-response curve for inhibition of food intake by CCK8s was closely similar to that of C57BL/6 mice, exogenous PAI-1 increased food intake as in wild-type mice (Supplemental Figure 6), and the satiety response to CCK persisted in infected mice (Figure 5B).
Figure 5.
H. felis infection decreases satiating effects of CCK8s in C57BL/6 but not PAI-1−/− mice. A, Inflammatory cell infiltrates are increased in the stomach of infected (▵) compared with control (●) C57BL/6, PAI-1-H/Kβ, and PAI-1−/− mice. B, Food intake (30 minutes) in C57BL/6, PAI-1-H/Kβ, and PAI-1−/− mice in response to CCK8s (2.5 nmol/kg, ip) in control and H. felis-infected animals. In infected C57BL/6 mice, CCK8s does not inhibit food intake, whereas in PAI-1−/− mice, the response is preserved. CCK has no effect in either infected or uninfected PAI-1-H/Kβ mice. *, P < .05; n = 5–8.
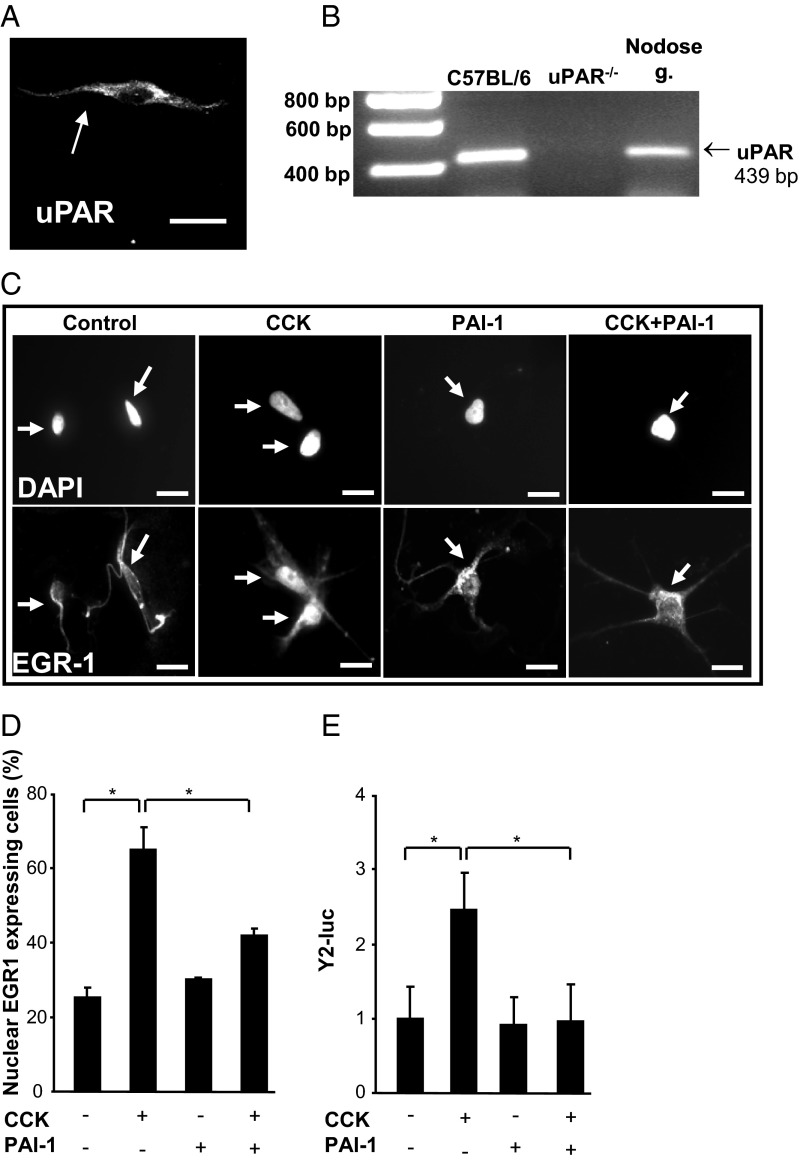
PAI-1 inhibits nodose neuron responses to CCK
The data suggest that PAI-1 inhibits the action of CCK on vagal afferent neurons and that uPAR is required for this effect. Immunohistochemical studies revealed the expression of uPAR in cultured vagal afferent neurons (Figure 6A), and this was confirmed by RT-PCR (Figure 6B). Stimulation of these neurons with CCK8s (10nM) caused redistribution of the immediately-early gene EGR-1 to the nucleus (Figure 6, C and D) as previously reported (11), and treatment with PAI-1 (40nM) inhibited the response (Figure 6, C and D). A marker of transcriptional responses to CCK in these neurons is increased expression of Y2 receptors (23), and using a Y2R-luc construct, we found that PAI-1 completely inhibited CCK stimulation of Y2R-luc expression (Figure 6E).
Figure 6.
Expression of uPAR in nodose neurons and inhibition of nodose neuron responses to CCK by PAI-1. A, Immunohistochemical localization of uPAR in a cultured nodose neuron. B, RT-PCR showing a band of the predicted size in rat nodose ganglion and in a positive control tissue (C57BL/6 mouse spleen) but not control tissue from uPAR−/− mice. C, Nuclear localization of EGR-1 in response to CCK8s (10nM) in cultured rat vagal afferent neurons is suppressed by PAI-1 (40nM): top, 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei; bottom, EGR-1 localization. Nuclei are indicated by arrows. D, Quantification of neurons exhibiting nuclear EGR-1 in response to CCK8s and reversal by PAI-1. E, Expression of Y2-luc in transiently transfected rat nodose ganglion neurons is increased by CCK8s (10nM), and this is inhibited by PAI-1 (40nM); *, P < .05; n = 4–8.
Discussion
Gastrointestinal epithelial cells secrete both anorexigenic and orexigenic factors, many of which influence food intake by acting on vagal afferent neurons. In addition to the physiological mechanisms by which gut signals influence food intake, it is also clear that interactions between the gut microbiota and immune and nervous systems can profoundly influence energy balance. We now report that PAI-1, which is increased in plasma in obesity (5, 6), and in stomach with Helicobacter infection (3, 4), acts as a gastric factor suppressing satiety signals. Specifically, the data suggest that PAI-1 suppresses gut-brain signaling by CCK, thereby increasing food intake. These actions are relevant to understanding how homeostatic mechanisms controlling food intake are disrupted in obesity and with infection and inflammation.
Transgenic mice with targeted overexpression of PAI-1 to gastric parietal cells using the H+/K+ ATPase β-subunit promoter (17), have moderately elevated plasma PAI-1, but in general, plasma concentrations are lower than those reported in other PAI-1 transgenic mice (25–27). An obese phenotype has not been described in other transgenic mice in which PAI-1 expression is targeted to cells outside the GI tract; indeed, targeted expression to adipocytes is reported to induce resistance to diet-induced obesity (26), which was not found in PAI-H/Kβ mice. We suggest, therefore, that it is local, ie, paracrine actions of PAI-1 within the stomach that are functionally relevant in the present model. It is worth noting that other phenotypes ascribed to PAI-1 transgenic mice, eg, reduction in tail length due to thrombosis and tail ischemia (28), have not been noted in PAI-1-H/Kβ mice. Neither do they exhibit a diabetic phenotype (at least up to 12–14 weeks) or reduced viability or fertility.
The moderate obesity exhibited by PAI-1-H/Kβ mice was reversed by feeding the same daily food ration as that consumed by C57BL/6 mice. We therefore attribute the obesity in PAI-1-H/Kβ mice to their hyperphagia when fed ad libitum. In addition, PAI-1-H/Kβ mice exhibited adiposity that was exacerbated on a high-fat diet, although the increased body weight was proportionately similar to that of C57BL/6 mice on the same diet. There is some evidence that within adipose tissue, PAI-1 plays a role in adipocyte differentiation and angiogenesis (29, 30). However, we suggest that gastric expression of PAI-1 is not adipogenic per se but rather acts to blunt satiety signaling, thereby increasing food intake and promoting an obese phenotype.
Both gastric expression of PAI-1 and administration of PAI-1 to C57BL/6 mice were associated with resistance to the satiety effect of CCK. Because PAI-1 is a protease inhibitor, we think it unlikely that the insensitivity to CCK is attributable to increased metabolism of the latter. In keeping with this, plasma CCK concentrations were similar in C57BL/6 and PAI-H-Kβ mice fed ad libitum. It is well established that CCK inhibits food intake via stimulation of vagal afferent neurons that is potentiated by leptin (11, 31). In obesity, there is resistance to CCK that involves changes in vagal afferent sensitivity as well as changes in leptin sensitivity (32–35). The present data suggest that PAI-1 acts in the opposite direction to leptin by suppressing vagal afferent responses to CCK. Although insensitivity to CCK in PAI-1-H/Kβ mice might be argued to reflect changes seen in many models of obesity, this cannot account for the observations that (1) PAI-1 acutely inhibits the effects of CCK on food intake and brainstem fos labeling in C57BL/6 mice, (2) hyperphagia and relative resistance to CCK persist in pair-fed PAI-1-H/Kβ mice in which body weight is comparable to control mice, and (3) PAI-1 acutely inhibits CCK-stimulated translocation of EGR-1 and induction of Y2 expression in isolated nodose ganglion neurons. Because plasma PAI-1 is elevated in obesity, we suggest that it should now be considered as one of the factors contributing to vagal afferent insensitivity to CCK.
In the last decade, ghrelin has provided a useful model for understanding the role of gastric orexigenic factors. Ghrelin stimulates appetite, mediates stress-activated feeding, promotes adiposity, and inhibits the effect of CCK on vagal afferent neurons (2, 36–39). However, the present data do not implicate the ghrelin system in responses to gastric PAI-1. Instead, they indicate that PAI-1 might function as a second gastric factor that is involved in maintaining food intake in response to gastric infection and inflammation.
Interactions between ingested nutrients, the gut microbiota, and the immune and nervous systems exert long-term effects on energy intake and expenditure (40–43). In the case of gastric infection with Helicobacter, a link with disrupted feeding patterns in mice has been recognized (44), and in humans, infection is associated with decreased circulating ghrelin (45). We suggest that just as there is a balance between orexigenic and anorexic signals from the upper GI tract in physiological circumstances, there is also a balance in infection and inflammation between anorexic mediators such as IL1β and those stimulating food intake of which PAI-1 provides a clear example. Thus, although production of proinflammatory cytokines augments CCK effects and so limits food intake, our data indicate that gastric expression of PAI-1 acts to counterbalance this and so maintains food intake overall. We therefore attribute the hyperphagic/obese phenotype in PAI-1-H/Kβ mice, but not infected C57BL/6 mice exhibiting similar increases in gastric PAI-1 mRNA, to the presence of proinflammatory cytokines exerting satiety effects in infected C57BL/6 mice. Together, the data suggest that the functional links between adipokines and the GI tract are stronger than hitherto supposed and indicate novel orexigenic mechanisms modulating vagal afferent signaling and controlling food intake that provide new candidates for intervention in the treatment of feeding disorders.
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust.
All authors contributed to experimental design, data collection, data interpretation, and preparation of the manuscript.
Disclosure Summary: The authors disclose no conflicts.
Footnotes
- CCK
- cholecystokinin
- EGR-1
- early growth response protein 1
- GI
- gastrointestinal
- NTS
- nucleus tractus solitarius
- PAI
- plasminogen activator inhibitor
- tPA
- tissue plasminogen activator
- uPA
- urokinase plasminogen activator
- uPAR
- urokinase plasminogen activator receptor
- Y2R
- neuropeptide Y type 2 receptor.
References
- 1. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 2. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenny S, Duval C, Sammut SJ, et al. Increased expression of the urokinase plasminogen activator system by Helicobacter pylori in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G431–G441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keates AC, Tummala S, Peek RM, Jr, et al. Helicobacter pylori infection stimulates plasminogen activator inhibitor 1 production by gastric epithelial cells. Infect Immun. 2008;76:3992–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landin K, Stigendal L, Eriksson E, et al. Abdominal obesity is associated with an impaired fibrinolytic activity and elevated plasminogen activator inhibitor-1. Metabolism. 1990;39:1044–1048 [DOI] [PubMed] [Google Scholar]
- 6. Shimomura I, Funahashi T, Takahashi M, et al. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2:800–803 [DOI] [PubMed] [Google Scholar]
- 7. Norsett KG, Steele I, Duval C, et al. Gastrin stimulates expression of plasminogen activator inhibitor-1 in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G446–G453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97:531–536 [DOI] [PubMed] [Google Scholar]
- 9. Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol. 1997;273:R833–R837 [DOI] [PubMed] [Google Scholar]
- 11. de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology. 2010;151:3589–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters JH, Karpiel AB, Ritter RC, Simasko SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology. 2004;145:3652–3657 [DOI] [PubMed] [Google Scholar]
- 13. Date Y, Toshinai K, Koda S, et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–3525 [DOI] [PubMed] [Google Scholar]
- 14. Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaige S, Abou E, Abysique A, Bouvier M. Effects of interactions between interleukin-1β and leptin on cat intestinal vagal mechanoreceptors. J Physiol. 2004;555:297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorenz RG, Gordon JI. Use of transgenic mice to study regulation of gene expression in the parietal cell lineage of gastric units. J Biol Chem. 1993;268:26559–26570 [PubMed] [Google Scholar]
- 18. Berkenpas MB, Lawrence DA, Ginsburg D. Molecular evolution of plasminogen activator inhibitor-1 functional stability. EMBO J. 1995;14:2969–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dockray GJ, Hamer C, Evans D, Varro A, Dimaline R. The secretory kinetics of the G cell in omeprazole-treated rats. Gastroenterology. 1991;100:1187–1194 [PubMed] [Google Scholar]
- 20. McLaughlin J, Grazia LM, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53 [DOI] [PubMed] [Google Scholar]
- 21. Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715 [DOI] [PubMed] [Google Scholar]
- 22. de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burdyga G, de Lartigue G, Raybould HE, et al. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008;28:11583–11592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Taeye BM, Novitskaya T, Gleaves L, Covington JW, Vaughan DE. Bone marrow plasminogen activator inhibitor-1 influences the development of obesity. J Biol Chem. 2006;281:32796–32805 [DOI] [PubMed] [Google Scholar]
- 25. Nordstrom SM, Carleton SM, Carson WL, Eren M, Phillips CL, Vaughan DE. Transgenic over-expression of plasminogen activator inhibitor-1 results in age-dependent and gender-specific increases in bone strength and mineralization. Bone. 2007;41:995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lijnen HR, Maquoi E, Morange P, et al. Nutritionally induced obesity is attenuated in transgenic mice overexpressing plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:78–84 [DOI] [PubMed] [Google Scholar]
- 27. Lijnen HR, Van HB, Umans K, Collen D. Neointima formation and thrombosis after vascular injury in transgenic mice overexpressing plasminogen activator inhibitor-1 (PAI-1). J Thromb Haemost. 2004;2:16–22 [DOI] [PubMed] [Google Scholar]
- 28. Erickson LA, Fici GJ, Lund JE, Boyle TP, Polites HG, Marotti KR. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nature. 1990;346:74–76 [DOI] [PubMed] [Google Scholar]
- 29. Lijnen HR. Murine models of obesity and hormonal therapy. Thromb Res 2011;127(Suppl)3:S17–S20 [DOI] [PubMed] [Google Scholar]
- 30. Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol. 2007;18:240–245 [DOI] [PubMed] [Google Scholar]
- 31. Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Covasa M. Deficits in gastrointestinal responses controlling food intake and body weight. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1423–R1439 [DOI] [PubMed] [Google Scholar]
- 34. Hayes MR, Skibicka KP, Leichner TM, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Lartigue G, Barbier dlS, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301:E187–E195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128 [DOI] [PubMed] [Google Scholar]
- 37. Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiology Gastrointest Liver Physiol. 2006;290:G1289–G1297 [DOI] [PubMed] [Google Scholar]
- 38. Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107:7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chuang JC, Perello M, Sakata I, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014 [DOI] [PubMed] [Google Scholar]
- 41. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDermott JR, Leslie FC, D'Amato M, Thompson DG, Grencis RK, McLaughlin JT. Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut. 2006;55:492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bercik P, Verdu EF, Foster JA, et al. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009;296:R587–R594 [DOI] [PubMed] [Google Scholar]
- 45. Nweneka CV, Prentice AM. Helicobacter pylori infection and circulating ghrelin levels: a systematic review. BMC Gastroenterol. 2011;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.