Abstract
AIM: To compare the overall survival (OS) and progression-free survival (PFS) with associated adverse events (AE) in patients with unresectable hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE) + sorafenib vs TACE alone.
METHODS: In this retrospective cohort study we collected data on all consecutive patients with a diagnosis of unresectable HCC between 2007 and 2011 who had been treated with TACE + sorafenib or TACE alone. We hypothesized that the combination therapy is superior to TACE alone in improving the survival in these patients. Data extracted included patient’s demographics, etiology of liver disease, histology of HCC, stage of liver disease with respect to model of end stage liver disease score and Child-Turcotte-Pugh (CTP) classification and Barcelona Clinic Liver Cancer (BCLC) staging for HCC. Computed tomography scan findings, alpha fetoprotein levels, number of treatments and related AE were also recorded and analyzed.
RESULTS: Of the 43 patients who met inclusion criteria, 13 were treated with TACE + sorafenib and 30 with TACE alone. There was no significant difference in median survival: 20.6 mo (95%CI: 13.4-38.4) for the TACE + sorafenib and 18.3 mo (95%CI: 11.8-32.9) for the TACE alone (P = 0.72). There were also no statistically significant differences between groups in OS (HR = 0.82, 95%CI: 0.38-1.77; P = 0.61), PFS (HR = 0.93, 95%CI: 0.45-1.89; P = 0.83), and treatment-related toxicities (P = 0.554). CTP classification and BCLC staging for HCC were statistically significant (P = 0.001, P = 0.04 respectively) in predicting the survival in patients with HCC. The common AE observed were abdominal pain, nausea, vomiting and mild elevation of liver enzymes.
CONCLUSION: Combination therapy with TACE + sorafenib is safe and equally effective as TACE alone in patients with unresectable HCC. CTP classification and BCLC staging were the significant predictors of survival. Future trials with large number of patients are needed to further validate this observation.
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, Sorafenib, Survival, Adverse events
Core tip: The incidence of hepatocellular carcinoma (HCC) is increasing and there is a need for better treatment modalities. Transarterial chemoembolization (TACE) and sorafenib are the main course of treatment for unresectable HCC. However there is an emphasis to combine them to improve survival. There is very limited data available to compare the effectiveness of TACE alone vs combination with sorafenib. Our results showed equal efficacy for both treatment arms without compromising adverse events. Child-Turcotte-Pugh classification and Barcelona Clinic Liver Cancer staging were significant predictors of survival. This study is the first reported in the literature comparing the outcome when treated with TACE alone vs TACE + sorafenib in United States patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of death from cancer worldwide and ninth leading cause of death from cancer in United States. It accounts for over 12000 deaths per year in the United States. The incidence of HCC is increasing dramatically primarily due to the aging of people infected with the hepatitis C virus (HCV)[1]. During the past two decades, the incidence of HCC in the United States has tripled while the 5-year survival rate for patients who do not have a liver transplant (LT) remains < 12%. The 5-year cumulative risk for the development of HCC in patients with cirrhosis ranges from 5% to 30%, with the highest risk in patients infected with HCV and has decompensated disease[2].
There are several potentially curative or palliative approaches to the treatment of HCC. The choice of treatment is driven by the degree of hepatic dysfunction as calculated by Child-Turcotte-Pugh (CTP) classification (Table 1), cancer stage as per Barcelona Clinic Liver Cancer (BCLC) staging System for HCC (Table 2) and the resources available. When the lesion is small, the patient may be a surgical candidate if there is preservation of liver function. However < 5% of patients are deemed resectable with the acceptable risk[3]. Patients who meet the Milan criteria (1 lesion ≤ 5 cm or 3 lesions ≤ 3 cm each with no vascular invasion) may be listed for LT[4]. However, even with the priority status afforded by the model of end stage liver disease (MELD) system, the wait may be prolonged and complications may include tumor growth[5,6]. Patients with no or compensated cirrhosis and no vascular invasion but with large or multifocal lesions are considered to have intermediate-stage HCC. In these patients, if LT is not possible, local ablative therapy is the next best option[7].
Table 1.
Child-Turcotte-Pugh scoring system and classification for patients with chronic liver disease
| Measures | 1 point | 2 points | 3 points |
| Serum total bilirubin (mg/dL) | ≤ 2 | 2-3 | > 3 |
| Serum albumin (g/dL) | > 3.5 | 2.8-3.5 | < 2.8 |
| INR | < 1.7 | 1.71-2.30 | > 2.30 |
| Ascites | None | Mild | Moderate to severe |
| Hepatic encephalopathy | None | Grade I-II (or suppressed with medication) | Grade III- IV (or refractory) |
| CTP points | CTP class | One year predicted survival | |
| 5-6 | A | 100% | |
| 7-9 | B | 81% | |
| 10-15 | C | 45% |
INR: International normalized ratio; CTP: Child-Turcotte-Pugh.
Table 2.
Barcelona Clinic Liver Cancer staging system for hepatocellular carcinoma
| Stage of HCC | Tumor features | Child-Pugh classification | Performance status test | Treatment |
| Stage 0 | Single < 2 cm carcinoma in situ | Child-Pugh A | 0 | Resection |
| Stage A | Single < 5 cm or 3 nodules < 3 cm | Child-Pugh A-B | 0 | Liver transplant, percutaneous ethanol injection, radiofrequency ablation |
| Stage B | Single > 5 cm or multi-nodular | Child-Pugh A-B | 0 | TACE |
| Stage C | Portal vein invasion | Child-Pugh A-B | 1-2 | Sorafenib |
| Stage D | Distant metastasis | Child-Pugh A-B | 3-4 | Symptomatic |
HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization.
Loco-regional treatment with transarterial chemoembolization (TACE) is offered to patients awaiting LT or as a palliative therapy to those who do not meet the Milan criteria for LT[8-12]. Treatment with repeated TACE shows significant survival benefits in patients with metastatic HCC who have preserved liver function[13]. A meta- analysis of randomized, controlled trials assessing the use of TACE as primary palliative treatment for HCC showed that it was associated with a 20%-25% improvement in 2-year survival rate vs conservative treatment[14]. The limitation of TACE is the incomplete target lesion necrosis, which requires repeated treatments in many patients. Despite the efficacy in local disease control and symptomatic relief, long-term survival rates in HCC patients after TACE remain low due to local and/or regional recurrence, as well as distant metastasis[15]. Effective systemic chemotherapy for advanced HCC is also needed to improve the overall survival of these patients[16].
Sorafenib, an orally active multikinase inhibitor with effects on tumor-cell proliferation and tumor angiogenesis, was initially identified as a Raf kinase inhibitor that acts by inhibiting the serine-threonine kinase Raf-1 and B-Raf. It also inhibits vascular endothelial growth factor receptors 1, 2 and 3; platelet-derived growth factor receptor β; and receptor tyrosine kinase receptor tyrosine kinases[17]. In a recent randomized, controlled trial (Sorafenib HCC Assessment Randomized Protocol also known as SHARP), patients with advanced HCC who were treated with sorafenib vs placebo had a 37% increase in survival (equivalent to a gain of 2 to 3 mo of life)[18,19]. Another meta-analysis of randomized controlled trials showed that survival rates were higher in patients treated with sorafenib-based vs placebo-based chemotherapy[20].
Since TACE is the most widely used primary treatment of HCC before LT or as a palliative therapy (in patients who are not LT candidates), and sorafenib is the only proven effective systemic treatment for advanced HCC[21], there is a strong rational to combine both treatment modalities[22]. Combining TACE with agents with anti-angiogenic properties is a promising strategy because TACE is thought to cause local hypoxia, resulting in a temporary increase in levels of VEGF, and sorafenib provides anti-angiogenesis activity by inhibiting VEGF levels. In a recent study, plasma VEGF decreased from 93 to 67 ng/L in patients treated with sorafenib + TACE[23].
Results from a large phase II randomized, double-blind, placebo-controlled trial (SPACE study) showed that the concurrent administration of TACE and sorafenib (TACE + sorafenib) has a manageable safety profile and suggested that time to progression and time to vascular invasion or extra-hepatic spread may be improved vs treatment with TACE alone[24]. Another study by Pinter et al[25] showed no difference in survival in patients with advanced stage HCC treated with TACE alone vs sorafenib alone (P = 0.377). However, several other studies showed improved progression-free median survival and disease control rate in patients with advanced HCC who were treated with TACE and sorafenib[26-29]. Nevertheless, very few studies have compared overall survival (OS) and progression-free survival (PFS) in patients treated with TACE vs combination therapy with sorafenib. Chung et al[28] from South Korea are conducting a phase II study on the safety, tolerability and efficacy of TACE and sorafenib in patients with HCC (START trial). The study is currently ongoing and an interim analysis revealed that the disease control rate was 91.2% while the overall response rate was 52.4%; the authors concluded that combination therapy is safe and effective with no unexpected side effects.
A recently published retrospective observational study by Qu et al[30] conducted in China showed that median survival time was significantly longer in patients with HCC treated with sorafenib and TACE vs TACE alone (27 mo vs 17 mo, P = 0.001). Despite the positive outcomes reported with the combined therapy, a clinical trial that enrolled a small number of patients was stopped prematurely due to adverse events (AE) and safety concerns with the combination therapy of high-dose doxorubicin-based TACE regimen and sorafenib[31].
Hypothesis
Due to the limited data regarding survival in patients with HCC - particularly those in the United States - treated with these different treatment modalities, we performed a retrospective cohort study of patients with unresectable HCC who were treated with TACE alone or TACE + sorafenib. The primary aim of the study was to compare the efficacy including benefits and harms of TACE alone vs combination therapy with sorafenib in patients with unresectable, non-transplantable HCC. We hypothesized that the combination therapy with TACE + sorafenib is superior to TACE alone in improving the survival in patients with advanced HCC. The secondary aim of the study was to find out the significant predictors of survival in these patients.
MATERIALS AND METHODS
A retrospective cohort study was conducted at the James Haley VA hospital after IRB approval (IRB Pro 000005448). Data was collected on all consecutive patients with a diagnosis of unresectable HCC from January 1, 2007 through December 31, 2011.
Criteria
Inclusion criteria: (1) patients age above 18 with unresectable biopsy-proven HCC who were not a candidate for LT; (2) patients who had been treated with TACE alone or TACE + sorafenib; and (3) patients with Child’s A and B cirrhosis.
Exclusion criteria: (1) patients with CHILD’s C cirrhosis and BCLC stage D for HCC; (2) liver transplant recipients; (3) patients with prior liver resection for HCC; and (4) patients who did not receive TACE as primary therapy.
Outcome measures
The primary outcome was OS and mortality. The secondary outcomes were PFS (where progression was defined as an increase in tumor size and MELD score), and AE associated with two treatments modalities. Treatment-related AE were assessed using the Common Terminology Criteria for AE (CTCAE) version 4.0.
Data abstraction
Data extracted included patient’s demographics, etiology of liver disease, histology of HCC, stage of liver disease with respect to MELD score, 3.78 [Ln serum bilirubin (mg/dL)] + 11.2 (Ln INR) + 9.57 [Ln serum creatinine (mg/dL)] + 6.43, CTP classification and BCLC staging for HCC[32]. CT scan findings (pre and post treatment), alpha fetoprotein (AFP) levels during the treatment, number of TACE or TACE + sorafenib treatments, and treatment AE were also recorded. Data on patient status (alive vs deceased vs progression) was collected periodically until the last follow-up which was November 30th, 2012.
Description of treatments
TACE: Hepatic artery obstruction was performed during an angiographic procedure and is combined with the injection into the hepatic artery of chemotherapeutic agents, mixed with lipiodol. Hepatic angiography was initially done to identify all arteries feeding the tumor by intervention radiologist. After the tumoral arterial supply was assessed, the catheter was introduced into the target artery. The catheter was then advanced to interrupt the blood flow as close to tumor as possible to minimize necrosis of the surrounding area. Particulate used for TACE was drug eluting microspheres (LC beads) about 300-500 micron in size. Chemotherapeutic agents (Doxorubicin 75-150 mg with a mean of 125 mg as per treating physician’s discretion) were adsorbed on the particulate bead and then injected into the tumor through the microcatheter.
Sorafenib: An oral starting dose of 200 mg twice daily was initiated by the treating oncologist and increased to 400 mg twice daily in the majority of patients. The decision to continue or stop the treatment because of AE was made by the oncologist.
Statistical analysis
OS was calculated from the first day of initial treatment with TACE or TACE + sorafenib to status at last contact (dead vs alive). PFS was calculated similarly except that we noted the progression as either increase in tumor size and MELD score or death at last contact. Treatment outcomes of the TACE vs TACE + sorafenib groups were compared. Time-to-event data analysis was estimated by the Kaplan-Meier survival method and compared by the Log-rank test. Differences in treatment effect between the TACE and TACE + sorafenib groups, including OS and PFS, were also assessed using the Cox proportional hazard model and summarized as HR along with 95%CI. All statistical tests were two-sided, and a P value ≤ 0.05 was considered statistically significant. Differences in treatment effects for dichotomous outcomes were compared using Fisher exact test. All the analyses were done using STATA statistical analysis[33,34].
RESULTS
Patients and treatment characteristics
Forty-three consecutive patients were eligible for inclusion. All patients were male and underwent liver biopsy prior to treatment to confirm the diagnosis of HCC. At diagnosis, the MELD score ranged from 6 to 22 (mean 9.5). There were no statistically significant differences in any of the baseline characteristics (age, etiology of liver disease, histology of HCC, CTP classification, MELD score, AFP, tumor size) between the two treatment groups with the exception of the BCLC stage C (Table 3). The maximum number of TACE sessions per patient was 6 with an average of 1.9 sessions per patient. The average time from the first TACE treatment to the initiation of sorafenib was 8 mo. The mean duration of sorafenib treatment was 11.7 mo (range 1.9-42 mo), and the mean follow-up duration was 23 mo (range 3-56 mo). None of the patients were lost to follow up and all the clinical encounters were completed and recorded.
Table 3.
Comparison of demographic and disease characteristics of patients with hepatocellular carcinoma in both treatment groups
| Characteristic | TACE + sorafenib (n = 13) | TACE alone (n = 30) | P value |
| Age (yr) | 61.4 ± 7.5 | 59.2 ± 7.4 | 0.39 |
| Etiology | 0.18 | ||
| Alcohol | 2 (15.4) | 1 (3.4) | |
| Hepatitis C | 6 (46.1) | 17 (56.6) | |
| Hepatitis C and alcohol | 3 (23.1) | 11 (36.6) | |
| Non-alcohol/non-hepatitis C | 2 (15.4) | 1 (3.4) | |
| HCC histology | 0.86 | ||
| Poorly differentiated | 1 (7.6) | 3 (10.0) | |
| Moderately differentiated | 7 (53.8) | 13 (43.3) | |
| Well differentiated | 5 (38.6) | 14 (46.7) | |
| CTP classification | 0.69 | ||
| A | 11 | 23 | |
| B | 2 | 7 | |
| BCLC staging for HCC | 0.004 | ||
| A | 6 | 22 | |
| B | 2 | 8 | |
| C | 5 | 0 | |
| BCLC staging for HCC (excluding stage C) | 0.98 | ||
| A | 6 | 22 | |
| B | 2 | 8 | |
| MELD score | 8.8 ± 2.3 | 9.8 ± 2.9 | 0.29 |
| AFP (ng/mL) | 6.6 (2.3-745) | 8.1 (1.9-6000) | 0.96 |
| Tumor size seen on CT with the largest diameter (cm) | 4 (1.5 -16.7) | 3.1 (1.4 -5.8) | 0.58 |
Data are summarized as the mean ± SD or median (range). HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; CTP: Child-Turcotte-Pugh; BCLC: Barcelona Clinic Liver Cancer; MELD: Model of end stage liver disease; AFP: Alpha fetoprotein; CT: Computed tomography.
Outcomes
OS and PFS: Thirty patients (70%) were treated with TACE alone and 13 (30%) with combination therapy (TACE + sorafenib). Overall HCC-related mortality was 74% (32/43 patients). Of the 32 patients who died, 23 (72%) received TACE alone and 9 (28%) received combination therapy (P = 0.70). There was no significant difference in median survival time between groups: 20.6 mo (95%CI: 13.4-38.4) for the TACE + sorafenib group and 18.3 mo (95%CI: 11.8-32.9) for the TACE group (P = 0.72). There was no statistically significant difference in OS between the 2 treatment groups (Figure 1A). The HR for OS was 0.82 (95%CI: 0.38-1.77), which indicated an 18% hazard reduction in mortality with TACE + sorafenib vs TACE, however, this difference was not statistically significant (P = 0.61). The HR for PFS was also not statistically significant 0.93 (95%CI: 0.45-1.89; P = 0.83) (Figure 1B).
Figure 1.
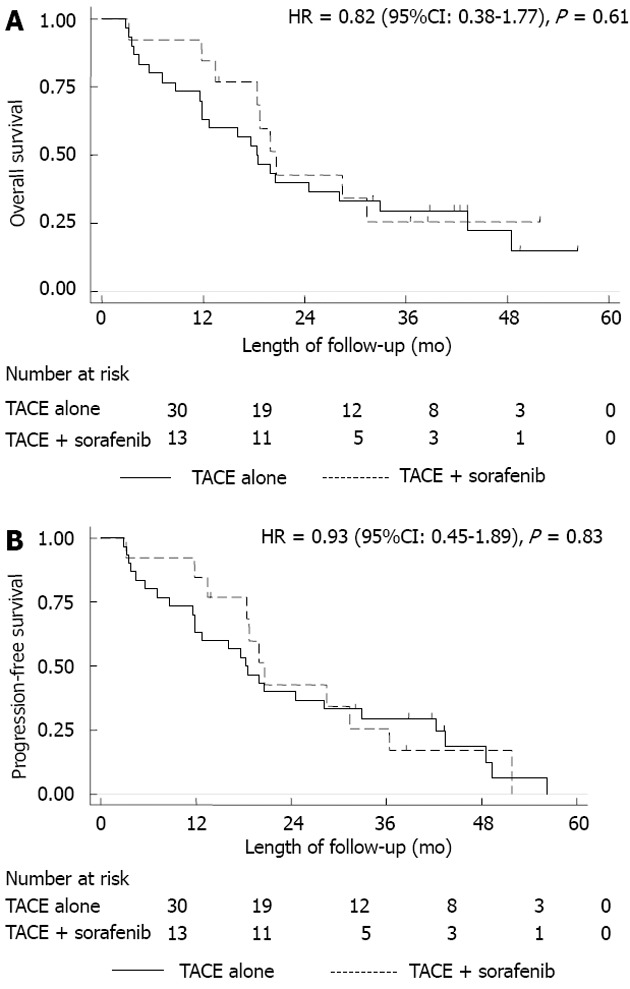
Kaplan-Meier survival. A: Kaplan-Meier overall survival for transarterial chemoembolization (TACE) alone and TACE + sorafenib; B: Kaplan-Meier progression-free survival for TACE alone and TACE + sorafenib.
The HR for OS was 0.56 (95%CI: 0.21-1.47; P = 0.24) and for PFS was 0.70 (95%CI: 0.3-1.6; P = 0.41) after excluding BCLC stage C patients. There was a decrease in hazard in favor of the combination therapy (after excluding BCLC stage C patients), but the difference was not statistically significant.
AFP levels ranged from 1.86 to 6000 (mean 85.9). Four patients had AFP levels above 400 and one patient with AFP of 6000 in TACE alone group (BCLC stage B with no vascular invasion). However there was no statistically significant difference of OS with HR of 0.81 (95%CI: 0.36-1.86; P = 0.63) and PFS with HR of 0.93 (95%CI: 0.44-1.97; P = 0.85), after removing these patients.
CTP classification of severity of liver disease (class B) and BCLC staging for HCC (stage C) were statistically significant (P = 0.001, P = 0.04 respectively) when analyzed by univariate Cox regression model in predicting the outcome and OS in patients with HCC (Table 4). This observation emphasizes the fact that patients with advance liver disease and higher stage of HCC have the worst outcome. Age, etiology of liver disease, tumor size and histology, MELD score and AFP level did not impact the OS in our cohort of patients.
Table 4.
Statistical analysis for each covariate in univariate cox regression model predicting overall survival
| Characteristic | HR (95%CI) | P value |
| Age (yr) | 0.99 (0.95-1.04) | 0.79 |
| Etiology | 1.04 (0.66-1.63) | 0.86 |
| Alcohol | ||
| Hepatitis C | ||
| Hepatitis C and alcohol | ||
| Non-alcohol/non-hepatitis C | ||
| HCC histology | 0.73 (0.43-1.25) | 0.26 |
| Poorly differentiated | ||
| Moderately differentiated | ||
| Well differentiated | ||
| CTP classification | 3.84 (1.74-8.51) | 0.001 |
| A | ||
| B | ||
| BCLC staging for HCC | 1.58 (1.02-2.46) | 0.04 |
| A | ||
| B | ||
| C | ||
| BCLC staging for HCC (excluding stage C) | 1.5 (0.66-3.43) | 0.34 |
| A | ||
| B | ||
| MELD score | 1.1 (0.98-1.23) | 0.08 |
| AFP (ng/mL) | 0.99 (0.82-1.20) | 0.98 |
| Tumor size seen on CT with the largest diameter (cm) | 1.06 (0.93-1.20) | 0.41 |
HCC: Hepatocellular carcinoma; CTP: Child-Turcotte-Pugh; BCLC: Barcelona Clinic Liver Cancer; MELD: Model of end stage liver disease; AFP: Alpha fetoprotein; CT: Computed tomography.
AE: The most common AE observed in our cohort of patients were abdominal pain, nausea, vomiting and mild elevation of liver enzymes. Specifically 2 patients had hand-foot skin reaction syndrome secondary to sorafenib after increasing the dose from 200 mg twice daily to 400 mg twice daily. The treatment with sorafenib was then stopped for few weeks and then re-introduced with a lower dose (200 mg twice daily) without any side effects. None of the side effects secondary to both treatments were life-threatening. There was no statistically significant difference in the AE between the two treatment groups (Table 5). No grade 4 or above AE as per CTCAE version 4.0 were observed with either TACE or sorafenib.
Table 5.
Adverse events attributed to both treatment arms
| Adverse events (CTCAE grades 1-3) | TACE alone | TACE + sorafenib | P value |
| Hand foot skin reaction | 0 | 2 | 0.554 |
| Diarrhea | 0 | 1 | |
| Hypertension (mild) | 0 | 1 | |
| Abdominal pain (mild) | 6 | 1 | |
| Nausea, vomiting | 3 | 0 | |
| Elevated liver enzymes (< 2 times of normal limits) | 1 | 2 |
TACE: Transarterial chemoembolization; CTCAE: Common terminology criteria for adverse events.
DISCUSSION
To our knowledge this is the first study to compare the outcome of patients with HCC treated with two different modalities (TACE alone vs TACE + sorafenib) in United States patients. Survival was slightly better in the TACE + sorafenib group than the TACE group, as demonstrated by an 18% hazard reduction in mortality, however this difference was not statistically significant most likely because of small sample size. The median survival was slightly prolonged in patients treated with TACE + sorafenib vs TACE alone (20.6 mo vs 18.3 mo). The observed effect of TACE + sorafenib compared with TACE alone was seen without any significant differences in AE. Furthermore, the patient’s data was not compromised as none of the patients were lost to follow up and all the clinical encounters were completed. Both treatment groups were comparable in terms of disease processes and prognostic factors. The AEs related to treatment with TACE and sorafenib were comparable to those reported in the literature[27]. CTP classification of severity of liver disease and BCLC staging for HCC were the only significant predictors of survival in our patients when analyzed in a univariate cox regression model.
Our findings support the findings of a recently published phase III study in which Japanese and Korean patients with advanced HCC were randomized to receive sorafenib or placebo (1:1) after TACE therapy. Median times to progression (TTP) in the sorafenib and placebo groups were 5.4 and 3.7 mo, respectively (HR = 0.87, 95%CI: 0.70-1.09; P = 0.252). The HR in sorafenib/placebo for overall survival was 1.06 (95%CI: 0.69-1.64; P = 0.790) and they concluded that combination therapy with TACE + sorafenib is not superior to TACE alone[35].
However, our findings are in contrast with those from a recent study assessing the effectiveness of TACE + sorafenib vs TACE in Chinese patients; Qu et al[30] reported a statistically significant improvement in median survival time with the combination therapy when compared to TACE alone (27 mo vs 17 mo, P = 0.001). The primary reason for the positive results observed in the study by Qu et al[30] may be attributed to their larger sample size (90 patients) in their study compared with our study with 43 patients.
Furthermore, the study by Qu et al[30] is limited by a relatively uneven duration of patient follow-up for the compared treatment modalities (25 mo for TACE alone and 46 mo for combination therapy). In contrast, our study has even and longer duration of follow-up (56 mo for TACE alone and 52 mo for combination therapy). Nevertheless, the results from both studies need to be confirmed in a randomized, placebo-controlled prospective study. A similar phase III study (SPACE trial) that is currently ongoing will evaluate differences in outcome between the 2 treatment groups[24]. Three hundred and seven patients with unresectable HCC and CHILD’s A cirrhosis were enrolled. Preliminary data showed statistically significant advantage of sorafenib + TACE over placebo + TACE in time to progression of HCC (TTP median 169 d, HR = 0.797, 95%CI: 0.588-1.080; P = 0.072).
Limitations of our study include its retrospective study design, the small number of patients included, and a patient population from a single institute. In addition, the decision to treat with TACE vs TACE + sorafenib was made by the treating physicians who might be prone to selection bias due to their belief in the superiority of one of the treatments. The benefit difference by looking at the survival curves, occurred early during the course of treatment suggesting the possibility of selection bias. There is also no data available on quality of life differences between these 2 cohorts of patients. A major strength of this study is the long duration of follow-up post-therapy (longest follow up of 56 mo) which enabled us to capture most treatment-related events and no lost to follow up. This is also the first reported study from the United States comparing the effectiveness of these two treatment modalities in patients with HCC.
In conclusion, combination therapy with TACE + sorafenib is safe and equally effective as TACE alone without any unexpected AE, in patients’ with unresectable HCC. The median survival time was prolonged by 2 mo in the combination treatment group, but it was not statistically significant. CTP classification and BCLC staging for HCC were the only significant predictors of survival emphasizing the fact that patients with advance liver disease and higher stage of HCC have the worst outcome. Future trials with large number of patients are needed to further validate this observation.
ACKNOWLEDGMENTS
Special thanks to Dr. Richter for providing the analytical review of the manuscript.
COMMENTS
Background
The incidence of hepatocellular carcinoma (HCC) is increasing especially in patients with chronic hepatitis C and there is a need for better treatment modalities to improve the overall survival. Transarterial chemoembolization (TACE) and sorafenib are the main course of treatment for unresectable HCC in patients who are not candidates for liver transplantation. However there is an emphasis to combine these two treatment modalities to improve the overall survival. To date, there is very limited data available especially in United States patients, to compare the effectiveness of TACE alone vs TACE + sorafenib.
Research frontiers
This study looked at the outcome of patients with unresectable HCC treated with TACE alone vs TACE + sorafenib. The primary outcome was to assess the overall and progression-free survival among the two groups.
Innovations and breakthroughs
This study showed equal efficacy for both treatment arms without compromising adverse events. The median survival time was prolonged by 2 mo in the combination treatment group, but it was not statistically significant. Child-Turcotte-Pugh (CTP) classification and Barcelona Clinic Liver Cancer (BCLC) staging for HCC were the only significant predictors of survival in these patients. This study is the first reported in the literature comparing the outcome of patients with HCC when treated with TACE alone vs TACE + sorafenib in United States patients.
Applications
Combination therapy is safe and effective in patients with unresectable HCC. CTP classification and BCLC stage accurately predicted the survival in these patients.
Terminology
Combination therapy with TACE and sorafenib is available for patients with unresectable, non-transplantable HCC. Adverse events secondary to both treatments are not unexpected and are comparable to what is reported in the literature.
Peer review
TACE alone or in combination with sorafenib is effective for the treatment of HCC. No statistical difference in survival was seen in the two treatment arms. No difference in outcome was seen after excluding patients with BCLC stage C and alpha fetoprotein > 400.
Footnotes
P- Reviewers Kagawa T, Kaplan DE, Lesmana CRA S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, Rodés J, Bruix J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 7.Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, Cicorelli A, Tumino E, Federici G, Cioni R, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–1178. doi: 10.1016/j.ejrad.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 10.Varga M, Valsamis A, Matia I, Peregrin J, Honsová E, Safanda M, Oliverius M. Transarterial chemoembolization in hepatocellular carcinoma. Rozhl Chir. 2009;88:434–438. [PubMed] [Google Scholar]
- 11.Biolato M, Marrone G, Racco S, Di Stasi C, Miele L, Gasbarrini G, Landolfi R, Grieco A. Transarterial chemoembolization (TACE) for unresectable HCC: a new life begins? Eur Rev Med Pharmacol Sci. 2010;14:356–362. [PubMed] [Google Scholar]
- 12.Yamanaka K, Hatano E, Kitamura K, Iida T, Ishii T, Machimito T, Taura K, Yasuchika K, Isoda H, Shibata T, et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol. 2012;47:343–346. doi: 10.1007/s00535-011-0511-x. [DOI] [PubMed] [Google Scholar]
- 13.Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, Sung KB, Lee JL, Kang YK, Lim YS, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26:145–154. doi: 10.1111/j.1440-1746.2010.06341.x. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Mäkisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg. 2011;100:22–29. doi: 10.1177/145749691110000105. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503–509. doi: 10.1053/j.seminoncol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, Wang D, Gao H. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21:326–332. doi: 10.1097/CAD.0b013e3283350e26. [DOI] [PubMed] [Google Scholar]
- 21.Copur MS. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498; author reply 2498–2499. [PubMed] [Google Scholar]
- 22.Hoffmann K, Glimm H, Radeleff B, Richter G, Heining C, Schenkel I, Zahlten-Hinguranage A, Schirrmacher P, Schmidt J, Büchler MW, et al. Prospective, randomized, double-blind, multi-center, Phase III clinical study on transarterial chemoembolization (TACE) combined with Sorafenib versus TACE plus placebo in patients with hepatocellular cancer before liver transplantation - HeiLivCa. BMC Cancer. 2008;8:349. doi: 10.1186/1471-2407-8-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dufour JF, Hoppe H, Heim MH, Helbling B, Maurhofer O, Szucs-Farkas Z, Kickuth R, Borner M, Candinas D, Saar B. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist. 2010;15:1198–1204. doi: 10.1634/theoncologist.2010-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. J Clin Oncol. 2012;30(Suppl 4):abstr LBA154. [Google Scholar]
- 25.Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, Stauber R, Grünberger B, Müller C, Kölblinger C, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 26.Duan F, Wang MQ, Liu FY, Wang ZJ, Song P. Clinical observation of the treatment with combination of transcatheter arterial chemoembolization and sorafenib for hepatocellular carcinoma with lung metastasis. Zhonghua Zhongliu Zazhi. 2009;31:716–718. [PubMed] [Google Scholar]
- 27.Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C, Morelli G, Clark V, Suman A, George TJ, Nelson DR. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205–213. doi: 10.1111/j.1365-2036.2011.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448–2458. doi: 10.1002/ijc.27925. [DOI] [PubMed] [Google Scholar]
- 29.Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–1342. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. doi: 10.1186/1471-2407-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieghart W, Pinter M, Reisegger M, Müller C, Ba-Ssalamah A, Lammer J, Peck-Radosavljevic M. Conventional transarterial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: a pilot study. Eur Radiol. 2012;22:1214–1223. doi: 10.1007/s00330-011-2368-z. [DOI] [PubMed] [Google Scholar]
- 32.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 33.Abadie A, Drukker D, Herr JL, Imbens GW. Implementingmatching estimators for average treatment effects in Stata. The Stata Journal. 2004;4:290–311. [Google Scholar]
- 34.Stata Corporation. Stata program. 11 version. College Station: 2010. [Google Scholar]
- 35.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]


