Abstract
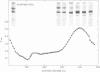
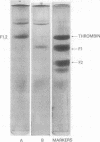
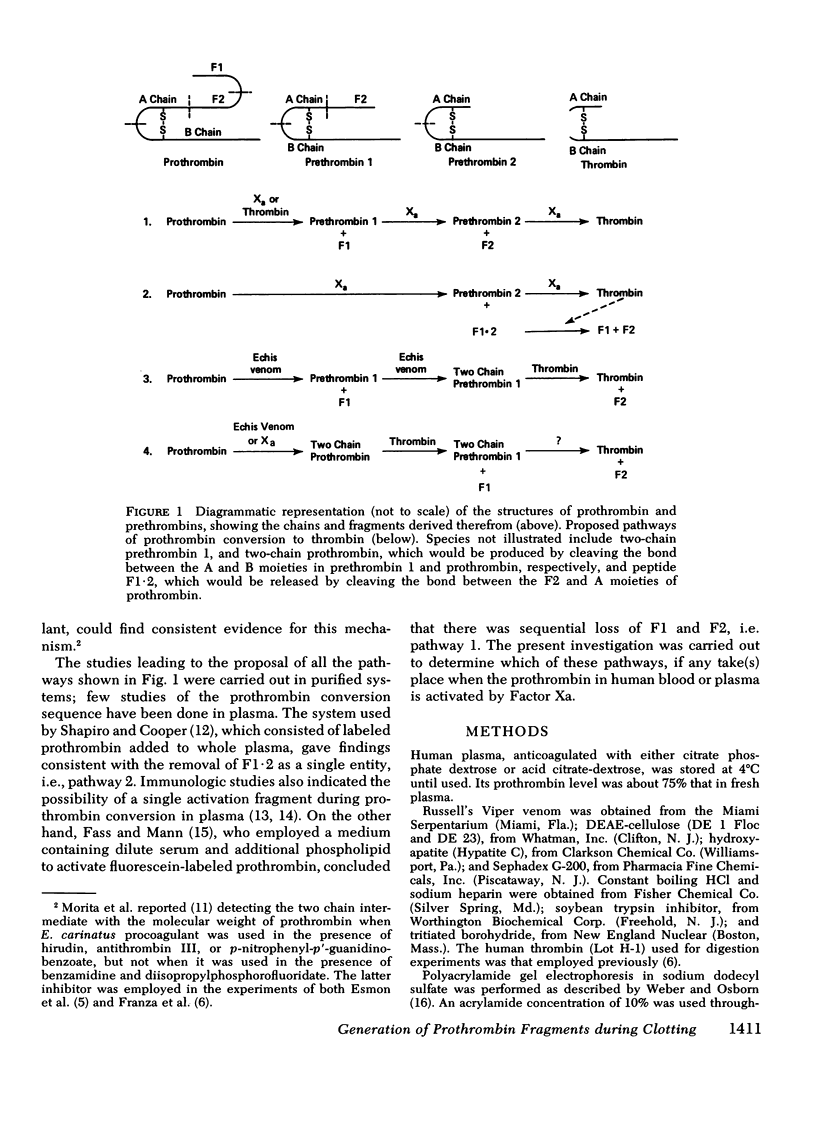
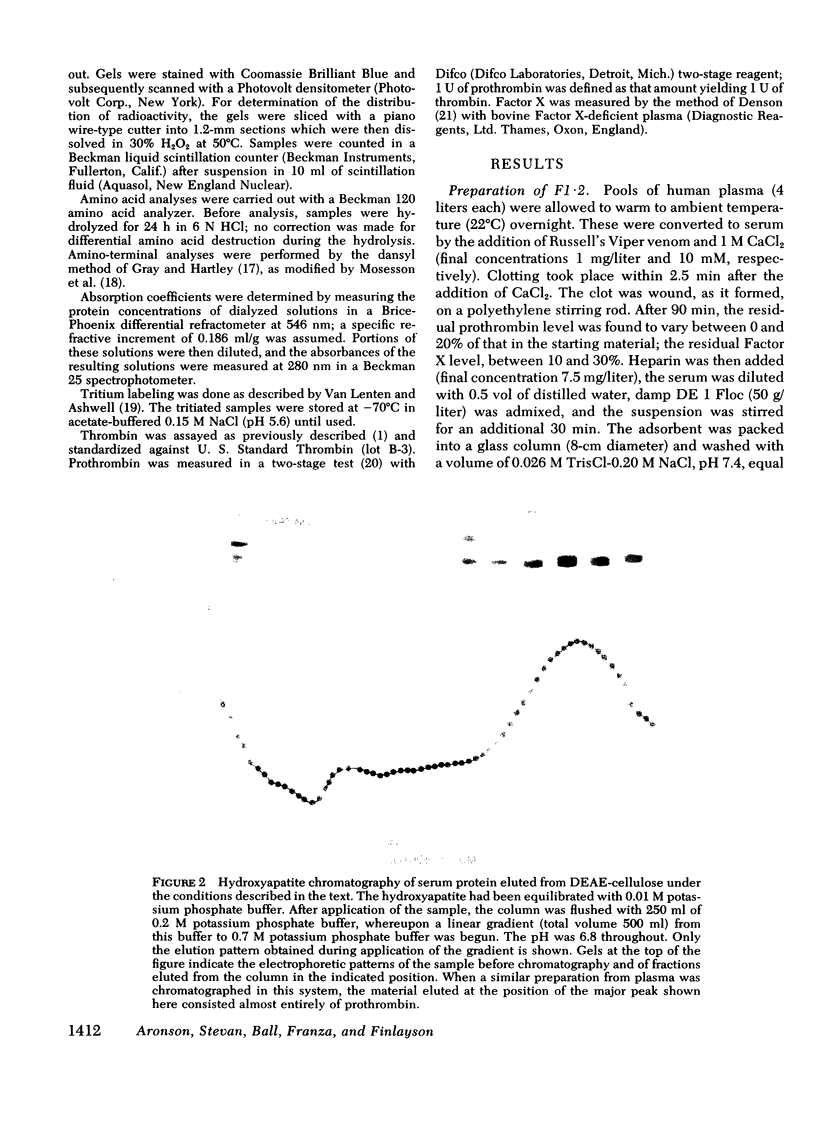
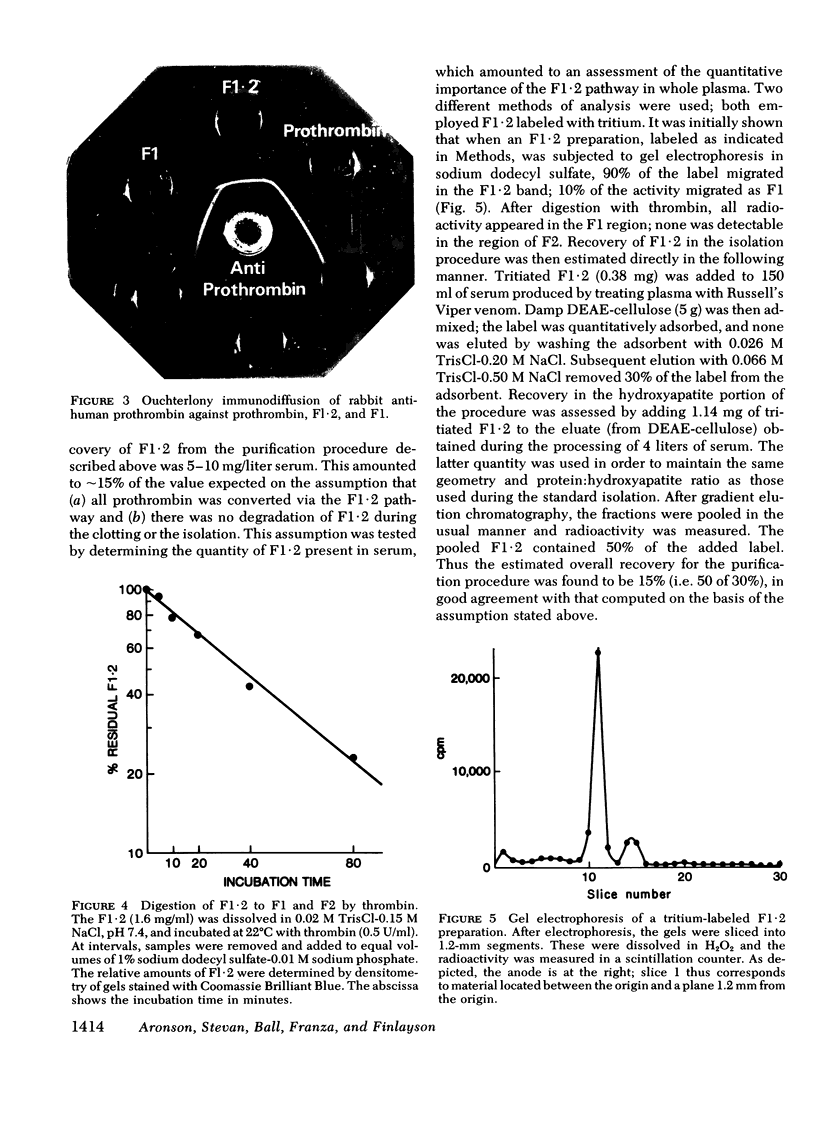
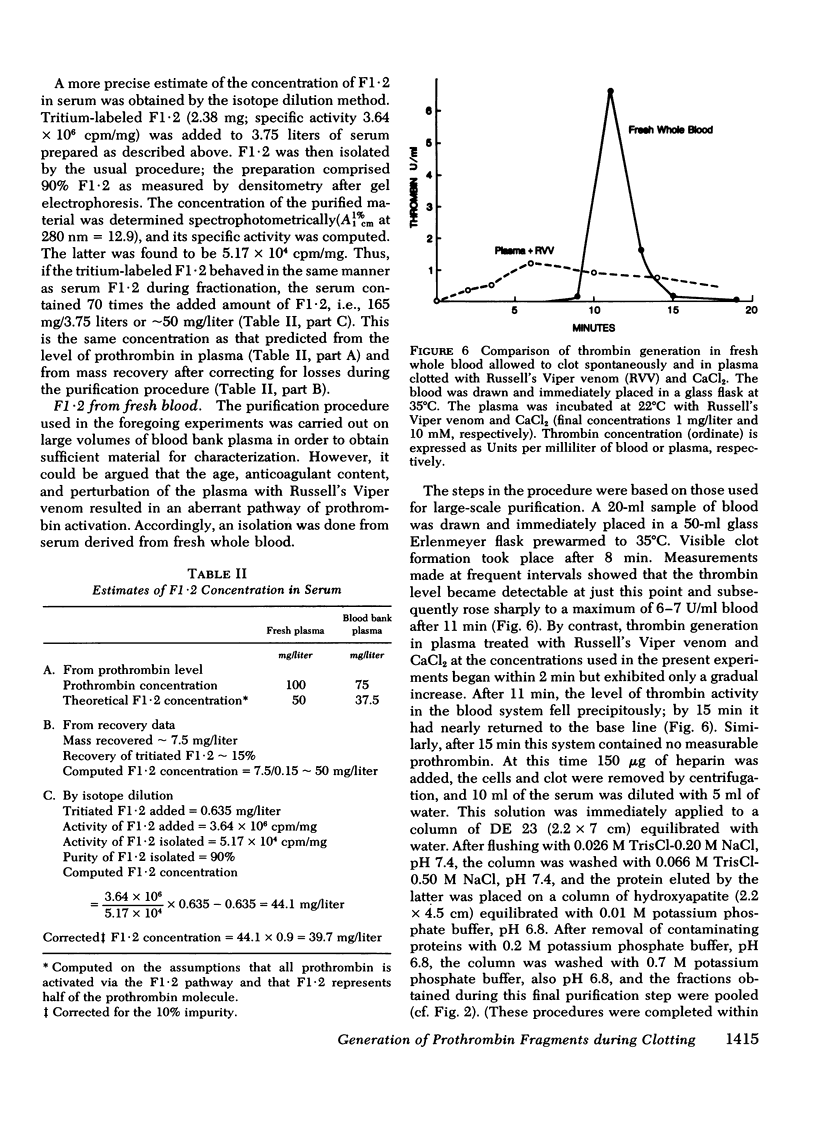
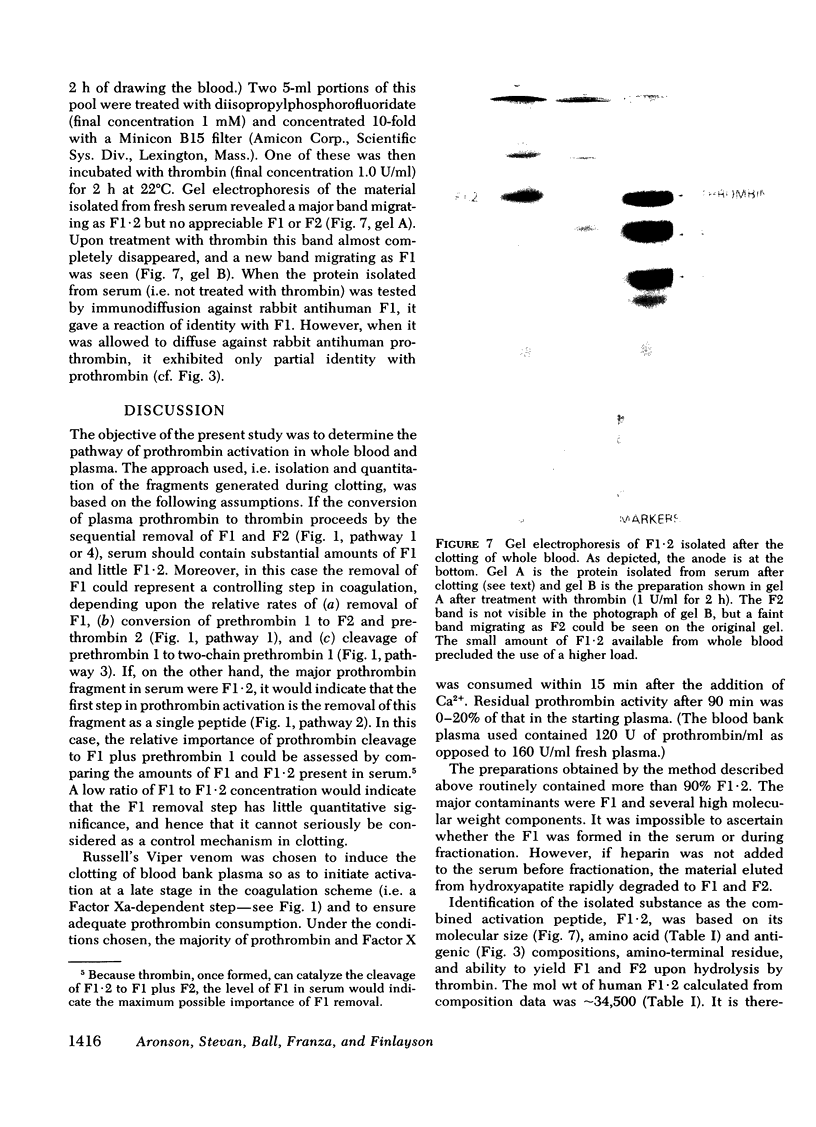
We have investigated the pathway of prothrombin activation in blood and plasma. By means of a rapid purification procedure involving chromatography on DEAE-cellulose and hydroxyapatite, we demonstrated that the major prothrombin fragment in serum is that representing the amino-terminal half of prothrombin (i.e. F1-2). The F1-2 isolated was characterized by its size, amino acid and antigenic compositions, amino-terminal residue, and the peptides (designated F1 and F2, respectively) it yielded upon hydrolysis by thrombin. Measurements by the isotope dilution technique showed that F1-2 could account for the fate of at least 90% of the prothrombin originally present in plasma. By contrast, the serum concentration of the fragment representing the amino-terminal third of prothrombin (viz. F1) was less than 10% that of F1-2. These results demonstrated that the major route of prothrombin conversion in blood or plasma involves the removal of the combined activation fragment (F1-2) as a single peptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson D. L., Ménaché D. Chromatographic analysis of the activation of human prothrombin with human thrombokinase. Biochemistry. 1966 Aug;5(8):2635–2640. doi: 10.1021/bi00872a022. [DOI] [PubMed] [Google Scholar]
- DENSON K. W. The specific assay of Prower-Stuart factor and factor VII. Acta Haematol. 1961 Feb;25:105–120. doi: 10.1159/000206523. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. III. The factor Xa-catalyzed activation of prothrombin. J Biol Chem. 1974 Dec 25;249(24):7782–7790. [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G., Jackson C. M. The conversion of prothrombin to thrombin. II. Differentiation between thrombin- and factor Xa-catalyzed proteolyses. J Biol Chem. 1974 Jan 25;249(2):606–611. [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G., Jackson C. M. The conversion of prothrombin to thrombin. V. The activation of prothrombin by factor Xa in the presence of phospholipid. J Biol Chem. 1974 Dec 25;249(24):7798–7807. [PubMed] [Google Scholar]
- Fass D. N., Mann K. G. Activation of fluorescein-labeled prothrombin. J Biol Chem. 1973 May 10;248(9):3280–3287. [PubMed] [Google Scholar]
- Franza B. R., Jr, Aronson D. L., Finlayson J. S. Activation of human prothrombin by a procoagulant fraction from the venom of Echis carinatus. Identification of a high molecular weight intermediate with thrombin activity. J Biol Chem. 1975 Sep 10;250(17):7057–7068. [PubMed] [Google Scholar]
- Ganrot P. O., Niléhn J. E. Prothrombin fragmentation during coagulation of whole blood and plasma. Scand J Clin Lab Invest. 1969 Aug;24(1):15–21. doi: 10.3109/00365516909080126. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O., Stenflo J. Prothrombin derivatives in human serum. Isolation and some properties of the non-thrombin fragments. Scand J Clin Lab Invest. 1970 Sep;26(2):161–168. doi: 10.3109/00365517009049229. [DOI] [PubMed] [Google Scholar]
- Heldebrant C. M., Butkowski R. J., Bajaj S. P., Mann K. G. The activation of prothrombin. II. Partial reactions, physical and chemical characterization of the intermediates of activation. J Biol Chem. 1973 Oct 25;248(20):7149–7163. [PubMed] [Google Scholar]
- Kornalik F., Blombäck B. Prothrombin activation induced by Ecarin - a prothrombin converting enzyme from Echis carinatus venom. Thromb Res. 1975 Jan;6(1):57–63. doi: 10.1016/0049-3848(75)90150-4. [DOI] [PubMed] [Google Scholar]
- Lanchantin G. F., Friedmann J. A., Hart D. W. On the occurrence of polymorphic human prothrombin. Electrophoretic and chromatographic alterations of the molecule due to the action of thrombin. J Biol Chem. 1968 Feb 10;243(3):476–486. [PubMed] [Google Scholar]
- Morita T., Iwanaga S., Suzuki T. Activation of bovine prothrombin by an activator isolated from Echis carinatus venom. Thromb Res. 1976 May;8(2 Suppl):59–65. doi: 10.1016/0049-3848(76)90048-7. [DOI] [PubMed] [Google Scholar]
- Morita T., Iwanaga S., Suzuki T. The mechanism of activation of bovine prothrombin by an activator isolated from Echis carinatus venon and characterization of the new active intermediates. J Biochem. 1976 May;79(5):1089–1108. doi: 10.1093/oxfordjournals.jbchem.a131150. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Galanakis D. K. The essential covalent structure of human fibrinogen evinced by analysis of derivatives formed during plasmic hydrolysis. J Biol Chem. 1973 Nov 25;248(22):7913–7929. [PubMed] [Google Scholar]
- Rosenberg J. S., Beeler D. L., Rosenberg R. D. Activation of human prothrombin by highly purified human factors V and X-a in presence of human antithrombin. J Biol Chem. 1975 Mar 10;250(5):1607–1617. [PubMed] [Google Scholar]
- Stenn K. S., Blout E. R. Mechanism of bovine prothrombin activation by an insoluble preparation of bovine factor X a (thrombokinase). Biochemistry. 1972 Nov 21;11(24):4502–4515. doi: 10.1021/bi00774a012. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]