Abstract
Background
Emphasis on prevention of healthcare-associated infections (HAI) including ventilator-associated pneumonia (VAP) has increased as hospitals are beginning to be held financially accountable for such infections. HAIs are often represented as being avoidable; however, the literature indicates that complete preventability may not be possible. The vast majority of research on risk factors for VAP concerns individual level factors. No studies have investigated the role of the patient's environment prior to admission. In this study we aim to investigate the potential role pre-hospital environment plays in VAP etiology.
Methods
In a retrospective cohort study, a sample of 5,031 trauma patients treated with mechanical ventilation between 1996–2010 was analyzed to determine the effect of neighborhood on the probability of developing VAP. We evaluated the effect of zip code using multilevel logistic regression analysis adjusting for individual level factors associated with VAP.
Results
We identified three zip codes with rates of ventilator-associated pneumonia that differed significantly from the mean. Logistic regression indicated that zip code, age, gender, race, injury severity, paralysis, head injury, and number of days on the ventilator were significantly associated with VAP. However, median zip code income was not.
Conclusions
Spatial factors that are independent of health care quality may potentiate the likelihood of a patient developing VAP and possibly other types of healthcare acquired infections. Un-modifiable environmental patient characteristics may predispose certain populations to developing infections in the setting of trauma.
Level of Evidence
III
Background
While the Centers for Medicare and Medicaid Services (CMS) has recently backed away from a proposal to include non-payment for ventilator-associated pneumonia (VAP) on its list of “never events”, the basic premise underpinning the idea remains (1). CMS reasoned that hospitals should be held accountable for healthcare associated infections (HAI) because such infections are preventable using current evidence-based infection control measures. However, the evidence with regard to the preventability of VAP is mixed at best (2–5). There is even controversy regarding how VAP is diagnosed prompting the Centers for Disease Control to implement a new surveillance algorithm that includes more objective data to define ventilator associated events (6). These varying results suggest that underlying factors independent of quality of clinical care may play a role in VAP development.
Individual level risk factors for VAP include antibiotic exposure, pre-existing medical co-morbidities, chest injury burden, increasing age, and gender, among other factors, have all been associated with development of VAP (7,8). Pre-injury environmental exposures may also play a role in development of VAP following injury. The rise of Geographic Information Systems (GIS) advanced mapping and analytic techniques and the field of epigenetics has made it possible to explore the impact of environmental and neighborhood exposures on health (9). There is growing evidence that the environment in which people live can shape subsequent health events, beyond those predicted by their individual demographic or health histories (10–15). Air pollution, indoor allergens from pests, income and education and even neighborhood violence are associated with asthma (11, 16–20). Given that such exposures have the propensity to cause damage to the respiratory system, it is plausible that they may also increase an individual's risk for acquiring VAP after injury. In this study, we investigated spatial variation in VAP incidence rates across the geographic region served by The Presley Memorial Trauma Center (PMTC) in Memphis, TN. We hypothesized that pre-injury neighborhood would be associated with variable rates of VAP and that pre-injury neighborhood would be independently associated with post-injury development of VAP.
Methods
Study Setting and Study Cohort
The PMTC is located in Shelby County, Tennessee in the city of Memphis and is the only Level I trauma center for an approximately 150-mile radius. The PMTC catchment area includes western Tennessee, northern Mississippi and eastern Arkansas. Persons with moderate to severe injuries or that potentially have moderate to severe injuries based on mechanism of injury are directed to PMTC under triage guidelines and significantly injured persons in the catchment area are customarily transferred to PMTC for definitive care. Therefore, nearly all moderate to severely injured patients in the PMTC catchment area are treated at the PMTC making it possible to estimate VAP rates in trauma patients from this population. The PMTC trauma registry (NTRACS, Digital Innovations, Forrest Hill Maryland) was used for this study. Patients admitted from 1996 – 2010 and who were on the ventilator for 2 or more days were included in the study. Patients who were burned, victims of drowning, bites/stings, overexertion, poisoning and suffocation were excluded. Patients without a valid address were also excluded.
Outcome Determination and Independent Variables
The main outcome for this study was VAP and is diagnosed using quantitative cultures from bronchoalveolar lavage (BAL) effluent obtained during fiberoptic bronchoscopy. Strict diagnostic criteria for diagnosis of VAP are used at the PMTC (21,22). Briefly, patients meeting any three of the following clinical criteria are eligible for BAL to confirm the presence of VAP: abnormal temperature (>101°F or < 96°F), abnormal leukocyte count (>10,000mm3 or < 4,000 mm3), change in character of sputum, new or changing infiltrate on chest radiograph and increasing ventilator requirements. BAL is performed in patients meeting these clinical criteria. A quantitative culture is obtained and ≥ 105 organisms is deemed diagnostic of VAP. No episodes of VAP are diagnosed in the absence of BAL.
Individual level variables included patient demographic information (gender, race, age), injury information (mechanism of injury, injury severity score, paralysis, Glasgow Coma Score, acute lung injury, hypotension) and days on ventilator. Neighborhood level variables included zip code and zip code median income as reported by the 2000 U.S. Census data.
Spatial Analysis
Patient addresses were geocoded using ArcGIS 10.0 (Redlands, CA) and aggregated to the corresponding census tract. Census tracts are small, relatively permanent subdivisions of a county and are designed to be homogeneous with respect to population characteristics, economic status, and living conditions (23). Once points were assigned to the appropriate census tract, VAP rates were calculated as the number of VAP cases per 1,000 ventilator days. Maps depicting the VAP rate (per 1,000 ventilator days), number of patients who were ventilated, and the number of VAP cases were generated. We chose to use census tract rather than zip code as the geographic unit of analysis in the spatial analysis so that we could examine more refined local variations in VAP rates.
In addition to mapping the VAP rate, we also investigated VAP rate patterns using Local Moran's I (LMI). LMI identifies geographic outliers and clusters by testing for randomness in the spatial distribution of data (24,25). LMI scores indicate census tracts that have VAP rates significantly higher or lower than neighboring census tracts. LMI Z scores were also mapped. An LMI Z score that is more than two standard deviations away from the mean is considered to be a cluster or outlier in the case of strongly positive or negative values, respectively.
Statistical Analysis
Descriptive statistics obtained include frequency distributions of all variables and we determined statistical significance with the χ2 test (p<0.05) for categorical variables and Student t test for equality of means for continuous variables. Wilcoxon Rank Sum Test was used to compare continuous variables without a normal distribution. SAS PROC GLIMMIX was used to fit multilevel generalized logistic regression models for the likelihood of developing VAP, assuming a binomial distribution and logit link function (26). This technique accounts for clustering of patients in neighborhoods so that it is possible to separate neighborhood effects from individual level effects when determining their impact on development of VAP. Because the PMTC receives many patients from sparsely populated areas and VAP is a rare outcome, we aggregated observations into zip codes rather than census tracts in order to ensure that a minimum number of observations per geographic unit could be met for the regression analysis. Observations were clustered on the zip code level and only zip codes with a minimum of 5 observations were included in the analysis. Estimates and adjusted odds ratios are reported. Odds ratios from the regression analysis were also mapped and zip codes that significantly differed from the mean of all zip codes are indicated. SAS 9.2 (Cary, NC) was used for statistical calculations.
Results
Description of Study Participants
Table 1 provides a brief summary of characteristics of the study population. Overall, 38.7% of patients developed VAP and the VAP rate was determined to be 31.6 per 1,000 Ventilator-Days. On bivariate analysis, VAP was associated with women and non-whites (Table 1). Other factors that were associated with VAP on bivariate analysis were age, blunt injury, increasing injury severity, neurologic injury, acute lung injury and hypotension at the time of admission (Table 1). Not surprisingly, the median number of days that the patient spent on the ventilator was significantly different in patients who developed VAP. VAP patients spent a mean of 15 days on the ventilator (IQR=9–23) while patients who did not develop VAP spent a mean of 4 days on the ventilator (IQR=2–9) (p<0.001). 17.9% of patients who developed VAP died, and not significantly different from those who did not develop VAP (17.7%, p=0.882). Regarding spatial variables, on bivariate analysis and without taking clustering into account patients of certain zip codes were more likely to develop VAP (p=0.001), however, there was no relationship between a patient's zip code median income and VAP (P=0.253).
Table 1.
Characteristics of persons admitted to a Level I trauma center in Shelby County, Tennessee, 1996–2010 (N=5,195).
| Characteristics | Patients without VAP | Patients with VAP | p-value* |
|---|---|---|---|
| Patients n (%) | 3180 (61.1) | 2015 (38.7) | |
| Age, Median (IQR) | 37.5 (25–53) | 41.4 (27–56) | <0.001 |
| Days on Ventilator, Median (IQR) | 4 (2–9) | 15 (9–23) | <0.001 |
| Dead n (%) | 564 (17.7) | 361 (17.9) | 0.882 |
| Zip Code (n=212) | 0.001 | ||
| Zip Code Median Income, Mean (SD) | $33,279 (12,849) | $33,445 (12,963) | 0.253 |
| Gender n (%) | <0.001 | ||
| Female | 893 (17.2) | 438 (8.4) | |
| Male | 2287 (44.0) | 1577 (30.4) | |
| Race/Ethnicity n (%) | <0.001 | ||
| White | 1617 (31.1) | 1217 (23.4) | |
| Non-White | 1563 (30.1) | 798 (15.4) | |
| Mechanism of Injury n (%) † | <0.001 | ||
| Penetrating | 652 (12.6) | 243 (4.7) | |
| Blunt | 2528 (48.7) | 1772 (34.1) | |
| Injury Severity (ISS) n (%) ‡ | <0.001 | ||
| Mild (1–6) | 136 (2.6) | 26 (0.5) | |
| Moderate (8–13) | 432 (8.3) | 131 (2.5) | |
| Severe (14–20) | 614 (11.8) | 283 (5.4) | |
| Critical (21 and greater) | 1960 (37.7) | 1569 (30.2) | |
| Paralysis n (%) | <0.001 | ||
| Not Paralyzed | 3026 (58.2) | 1744 (33.6) | |
| Paralyzed | 154 (3.0) | 271 (5.2) | |
| Glasgow Coma Score n (%) | <0.001 | ||
| 14 and above | 1535 (30.4) | 774 (15.3) | |
| 4 to 13 | 949 (18.8) | 754 (14.9) | |
| 3 or less | 592 (11.7) | 447 (8.8) | |
| Acute Lung Injury n (%) | <0.001 | ||
| No Lung Injury | 3154 (60.7) | 1969 (37.9) | |
| Lung Injury | 26 (0.5) | 46 (0.9) | |
| Hypotension n (%) § | 0.091 | ||
| Not Hypotensive | 2766 (54.8) | 1744 (34.6) | |
| Hypotensive | 308 (6.1) | 228 (4.5) |
Continuous variables were analyzed using Student t test and categorical variables were analyzed using χ2
Mechanism of injury was determined from ICD-9 E-codes recorded during hospital admission.
Injury severity score was used to classify the injury as either mild (1–6), moderate (8–13), severe (14–20) and critical (21 and greater).
Hypotension was defined as systolic blood pressure < 90 mmHg.
Variation in VAP Rates across Neighborhoods
Mapping of census tract VAP rates showed that census tracts of greater distance from PMTC had higher VAP rates (Figure 1). Within Shelby County, census tracts in the southern and eastern portions of the county tended to have higher VAP rates. Overall, census tracts with high VAP rates did not tend to be clustered together, but rather were neighbored by census tracts with low VAP rates. Local Moran's I analysis indicated that 19 census tracts had significantly higher rates of VAP than neighboring census tracts and 2 had two significantly lower rates of VAP (Figure 2). Census tracts in the northwest region of the PMTC service area with high rates of VAP tended to be clustered, as indicated by their strongly positive LMI Z score. Within Shelby County, there were 7 tracts that were found to have significantly higher VAP rates than their contiguous tracts. These tracts had strongly negative z scores, indicating they were significantly dissimilar from the surrounding area, and were therefore considered to be outliers. The LMI analysis indicates that VAP rates vary significantly at the local scale. Overall the LMI analysis demonstrated that census tracts with high VAP rates do not tend to be clustered or segregated to specific areas of the county. This may indicate that the neighborhood environment varies significantly between neighboring census tracts.
Figure 1.
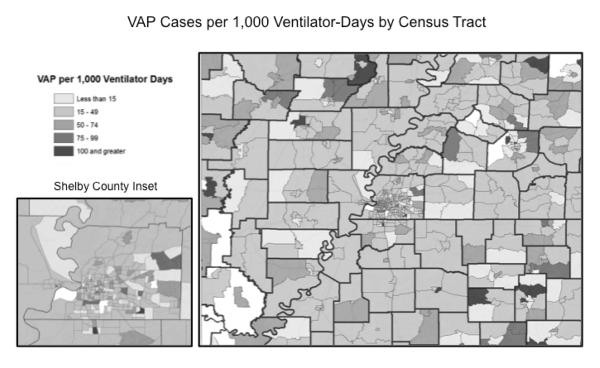
Ventilator-associated pneumonia rate by census tract. Darker shades indicate higher VAP rates. White areas have no data.
Figure 2.
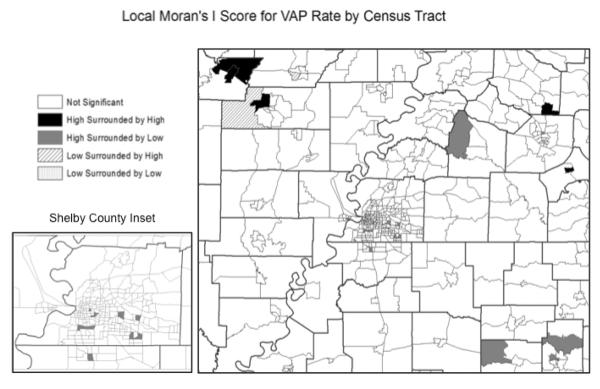
Census tracts with VAP rates that differ significantly from neighboring tracts. Black indicates a cluster of census tracts with VAP rates that are higher than surrounding tracts. Gray indicates tracts with rates significantly higher than neighboring tracts that are not clustered.
Multilevel Generalized Logistic Regression Modeling
Accounting for clustering by neighborhood at the zip code level using multi-level analysis, age, gender, race/ethnicity, injury severity, neurologic injury and days on the ventilator were associated with VAP (Table 2). Even after accounting for individual level variables, three zip codes were identified to vary significantly from the mean. Two had significantly higher probability of developing VAP (OR 1.59, 1.02–2.48; 1.49, 1.02–2.20) and one had a decreased likelihood of developing VAP (OR 0.67, 0.45–0.98). These zip codes tended to be in the most distant service areas of PMTC. Also, these zip codes tended to be in areas where LMI found census tracts with high VAP rates that were clustered together. Overall, many of the 212 zip codes trended towards significance and the final model estimated the p value to be 0.0852. We also incorporated zip code median income into the model and tested for interactions between income and mechanism of injury and race. Median income was not significant in any of the models tested. Fit statistics indicated that both median income and acute lung injury could be removed from the final model.
Table 2.
Logistic Regression Analysis of Ventilator-Associated Pneumonia
| Variable | OR (95% C.I.) | %Change | p-Value |
|---|---|---|---|
| Mechanism of Injury | |||
| Blunt | Reference | ||
| Penetrating | 0.93 (0.90,0.96) | <.0001 | |
| Gender | |||
| Male | Reference | ||
| Female | 0.91 (0.88,0.94) | <.0001 | |
| Race/Ethnicity | |||
| White | Reference | ||
| Non-White | 0.94 (0.91,0.97) | <.0001 | |
| Injury Severity | |||
| Mild | Reference | ||
| Moderate | 1.06 (0.98,1.15) | 0.1298 | |
| Severe | 1.11 (1.03,1.20) | 0.0075 | |
| Critical | 1.19 (1.11,1.28) | <.0001 | |
| Paralysis | |||
| Not Paralyzed | Reference | ||
| Paralyzed | 1.13 (1.07,1.18) | <.0001 | |
| Glasgow Coma Score | |||
| 14 and above | Reference | ||
| 4 to 13 | 1.08 (1.05,1.11) | <.0001 | |
| 3 | 1.05 (1.02,1.09) | 0.0028 | |
| Age (years) | 0.01 | 0.0232 | |
| Days on Ventilator | 1.01 | <.0001 | |
| Zip Code | 0.0852 |
Discussion
The results of this study, the first to link pre-injury neighborhood exposure and VAP in injured patients, adds to the existing data and extends the controversy surrounding VAP preventability. There was clear clustering of VAP in patients after injury based on pre-injury neighborhood. The neighborhood effect, while modest, was independent of strong individual level effects. While this is the first study to demonstrate a relationship between pre-injury neighborhood and post-injury outcomes, there is ample evidence in other diseases that the environment can play a significant role in disease risk and outcomes.
Since Dr. John Snow used mapping techniques to determine the source of a series of cholera cases in central London over 150 years ago there has been a realization that environment impacts health outcomes (27). The role of the environment in health is evident in the spatial clustering of many types of health data. Studies utilizing GIS have demonstrated that spatial trends vary significantly in regards to both infectious disease, such as Methicillin-Resistant Staphylococcus Aureus infection, and chronic diseases such as asthma (28, 29). The development of both chronic lung disease and acute respiratory infection has been associated with environment and other spatial correlates (27). The findings in the current study are similar to these previous reports. Pre-injury environment appears to influence the risk of VAP in the post-injury period independent of individual level risk factors.
In this study, we attempted to control for patient factors known to be associated with VAP to isolate the role environment and, potentially, socioeconomic factors play in the probability of developing VAP. The LMI analysis indicated that census tracts with significantly high rates of VAP tend to be randomly dispersed and are outliers relative to neighboring census tracts. An environmental component at the local level could explain this observation.. A potential example of how this could occur may be that a group of homes share a common attribute such as mold or a specific building material and this common environmental exposure triggers an epigenetic change, making individuals more susceptible to pneumonia.
As the site of gas exchange, the lungs are exposed to a multitude of environmental stressors which may produce a normal immune response. However, when individuals are frequently exposed to environmental stressors, chronic inflammation and airway remodeling may result, indicating that epigenetic changes may have taken place (30,31). Asthma, along with chronic bronchitis, pulmonary fibrosis, chronic obstructive pulmonary disease and certain lung cancers have all been shown to be at least partially attributable to an environmental component separate from individual level factors (32,33). Additionally, exposure to air pollutants has also been shown to increase susceptibility to influenza infections by modifying epithelium in the human airway (34). This indicates that a distinct, biological mechanism exists that mediates the relationship between an individual's environment and their proneness to respiratory infection.
The results of the regression analysis, which used zip code as the geographic unit of analysis, demonstrated that areas further from the study site tended to have more extreme outcomes. This was also similar to the LMI analysis, which showed greater clustering of census tracts with high rates as compared to the metropolitan areas. Because of the relatively rare outcome of VAP at the neighborhood level, it is difficult to investigate the potential reasons for this rural versus urban difference in the current data. Several factors could be in play including variation in injury mechanisms, differences in transport times, exposure to other hospitals prior to arrival at the PMTC, or other environmental factors.
One environmental variable that could be associated with high VAP rate is the median year housing structures were built. Both of the zip codes that had significantly higher rates of VAP were in counties that had median home ages in the oldest quartile within the PMTC service area. Both of these counties had a median build year of 1969. The zip code with a significantly lower rate of VAP was contained in a county that was in the quartile with the newest homes with a median build year of 1989 (35). It is possible that trends in methods of home construction or building materials could partially account for this association. Another explanation may be that older homes have accumulated more indoor pollutants over time and therefore their inhabitants are exposed to higher amounts of mold or other respiratory irritants.
While decreasing neighborhood socioeconomic status is linked to increased risk of blunt and penetrating injury, there does not appear to be a link between neighborhood socioeconomic status and VAP risk in the post-injury period in this study (36). Median zip code income was no different between those who developed VAP and those who did not in either the descriptive or regression analyses. Geographic prevalence of co-morbidities may be more likely to account for the variation seen in VAP rates across zip codes. Both zip codes with significantly high VAP rates were in counties with smoking prevalence (45.4% and 30.0%) and asthma prevalence (13.6% and 11%) higher than the national rate (19% and 8.2%, respectively) (37–40). It is possible that spatial trends in VAP rates are associated with the prevalence of behavioral risk factors.
Another possibility for the differences in VAP rates across census tracts and zip codes may be that a certain pathogens associated with VAP are concentrated within particular communities. Studying the spatial distribution of VAP-causing pathogens could help hospitals identify a common source of pathogen exposure.
Providers could apply results from spatial analysis in two parts. The first requires investigating which potential risk factors are associated with spatial trends in their data and the second is determining how to alter care according to those risk factors. For example, a census tract with a high infection rate may contain a public housing complex. The high infection rate could possibly occur because public housing residents are living in crowded conditions that promote transmission of VAP-causing pathogens. Based on this information, clinicians could assume that people living within the same area are likely to be exposed to the same strain of VAP-causing pathogen due to their close proximity to the complex. This information could be used to predict which patients are likely to develop VAP and attempt to prevent infection or to direct empiric antibiotic choice for VAP.
As with all retrospective studies that demonstrate an association, this one has limitations. Because the findings from the LMI analysis demonstrated that census tracts with significantly high rates of VAP tended to be outliers rather than clusters, and were bordered by census tracts with significantly lower VAP rates, there may be issues with aggregating VAP rate data to the zip code level. Coarser geographic units of analysis allow for greater statistical power, however, they may lose sensitivity in respect to local variations. Another issue when investigating geographic variation in prevalence of certain health outcomes is that it is difficult to adjust for individual level factors in small areas. Often cases are not spread evenly across the area of interest and it is difficult to differentiate individual level effects from neighborhood effects due to the small number of patients residing in an area. The population size can severely limit the number of individual level characteristics that can be reliably analyzed. A larger population size in this study may have allowed us to consider more co-morbidities in the analysis. Another limitation is that the data set spans a long range of time and it is difficult to determine spatio-temporal variations in data. Characteristics of neighborhoods can change significantly over the period of 15 years and this may have been problematic to identify in this analysis. Further, since this study only included one site, we were able to determine that VAP cases did form clustering patterns; however, we did not have the power to determine which neighborhood characteristics were associated with increases in susceptibility to VAP. Data from multiple sites would be helpful in determining whether or not certain neighborhood living conditions, housing materials, sociodemographic statuses, or environmental characteristics can explain spatial clustering patterns. Unfortunately, much of the spatial data pertinent to explaining our findings is not available and is not reported at sub-county levels.
Despite these limitations, our results indicate that spatial factors independent of health care quality may potentiate the likelihood of a patient developing VAP after injury. Environmental factors that are difficult or impossible to modify may predispose certain populations to developing infections in the setting of trauma. Further, there is evidence in the acutely ill surgical population, including trauma patients, that patients may present already colonized to develop early VAP, often within 48 hours of admission. This leaves little time for preventive efforts to become effective.
Acknowledgments
This work was supported by grant number K23GM084427 from the NIGMS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Presented at the 71st Annual Meeting of the American Association for the Surgery of Trauma as a Poster Abstract.
The authors have no conflicts of interest to declare.
Author Contributions
Zarzaur – literature review, study design, data analysis, data interpretation, critical revisions
Bell – literature review, data analysis, data interpretation, writing
Croce – data analysis, critical revisions
Fabian – data analysis, critical revisions
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Medicare and Medicaid Services [Accessed January, 2010];CMS Proposes Additions to List of Hospital-Acquired Conditions for Fiscal Year 2009. 2008 Available at http://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=3042.
- 2.Macleod JB. Broadening never events: is it a plausible road to improved patient safety? Arch Surg. 2010;145:151–2. doi: 10.1001/archsurg.2009.278. [DOI] [PubMed] [Google Scholar]
- 3.Pronovost P, Goeschel C, Wachter R. The Wisdom and Justice of Not Paying for “Preventable Complications”. JAMA. 2008;299:2197–2199. doi: 10.1001/jama.299.18.2197. [DOI] [PubMed] [Google Scholar]
- 4.Werner R, Goldman LE, Dudley RA. Comparison of Change in Quality of Care Between Safety-Net and Non-Safety-Net Hospitals. JAMA. 2008;299:2180–2187. doi: 10.1001/jama.299.18.2180. [DOI] [PubMed] [Google Scholar]
- 5.Harbarth S, Sax H, Gastmeier P. The preventable proportion of nosocomial infections: an overview of published reports. Journal of Hospital Infection. 2003;54:258–266. doi: 10.1016/s0195-6701(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control [Accessed January 22, 2013];Device-associated Module: Ventilator-Associated Event Protocol. 2013 Available at http://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf.
- 7.Pascale GD, Pennisi MA, Raggi V, Piervincenzi E, Bernini V, Occhionero A, De Santis P, Moccaldo A, Cicconi S, Maviglia R, Tumbarello M, Antonelli M. Clinical and epidemiological risk factors for ventilator-associated pneumonia in a cohort of critically ill patients. Critical Care. 2012;16(Suppl 1):P73. [Google Scholar]
- 8.Magnotti L, Croce MA, Fabian TC. Is Ventilator-Associated Pneumonia in Trauma Patients an Epiphenomenon or a Cause of Death? Surgical Infections. 2004;5:237–242. doi: 10.1089/sur.2004.5.237. [DOI] [PubMed] [Google Scholar]
- 9.Nuckols J, Ward MH, Jarup L. Using Geographic Information Systems for Exposure Assessment in Environmental Epidemiology Studies. Environ Health Perspect. 2004;112:1007–1015. doi: 10.1289/ehp.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellen IG, Mijanovich T, Dillman K. Neighborhood Effects on Health: Exploring the Links and Assessing the Evidence. Journal of Urban Affairs. 2001;23:391–408. [Google Scholar]
- 11.Ahluwalia S, Matsui E. The indoor environment and its effects on childhood asthma. Current Opinion in Allergy and Clinical Immunology. 2011;11:137–143. doi: 10.1097/ACI.0b013e3283445921. [DOI] [PubMed] [Google Scholar]
- 12.Delfino RJ, Chang J, Wu J, Ren C, Tjoa T, Nickerson B, Cooper D, Gillen DL. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Ann Allergy Asthma Immunol. 2009;102:138–44. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira SE, Stein RT, Ferraro AA, Pastro LD, Pedro SS, Lemos M, da Silva ER, Sly PD, Saldiva PH. Urban Air Pollutants are Significant Risk Factors for Asthma and Pneumonia in Children: The Influence of Location on the Measurement of Pollutants. Arch Bronconeumol. 2012 Jul 2; doi: 10.1016/j.arbres.2012.05.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Karvala K, Toskala E, Luukkonen R, Uitti J, Lappalainen S, Nordman H. Prolonged exposure to damp and moldy workplaces and new-onset asthma. Int Arch Occup Environ Health. 2011;84:713–21. doi: 10.1007/s00420-011-0677-9. [DOI] [PubMed] [Google Scholar]
- 15.Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–71. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch T, Weiland S, Von Mutius E, Safeca A, Gräfe H, Csaplovics E, Duhme H, Keil U, Leupold W. Inner city air pollution and respiratory health and atopy in children. European Respiratory Journal. 1999;14:669–677. doi: 10.1034/j.1399-3003.1999.14c29.x. [DOI] [PubMed] [Google Scholar]
- 17.Cesaroni G, Farchi S, Davoli M, Forastiere F, Perucci C. Individual and area-based indicators of socioeconomic status and childhood asthma. Eur Respir J. 2003;22:619–624. doi: 10.1183/09031936.03.00091202. [DOI] [PubMed] [Google Scholar]
- 18.Tzivian L. Outdoor air pollution and asthma in children. J Asthma. 2011;48:470–81. doi: 10.3109/02770903.2011.570407. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 20.Apter A, Garcia L, Boyd R, Wang X, Bogen D, Have T. Exposure to community violence is associated with asthma hospitalizations and emergency department visits. J Allergy Clin Immunol. 2010;126:552–7. doi: 10.1016/j.jaci.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croce MA. Diagnosis of acute respiratory distress syndrome and differentiation from ventilator-associated pneumonia. Am J Surg. 2000;179(2A Suppl):26S–29S. [PubMed] [Google Scholar]
- 22.Croce MA, Fabian TC, Mueller EW, Maish GO, 3rd, Cox JC, Bee TK, Boucher BA, Wood GC. The appropriate diagnostic threshold for ventilator-associated pneumonia using quantitative cultures. J Trauma. 2004;56:931–4. doi: 10.1097/01.ta.0000127769.29009.8c. [DOI] [PubMed] [Google Scholar]
- 23.United States Census Bureau [Accessed July 17, 2012];Census Tracts and Block Numbering Areas. 2000 Available at http://www.census.gov/geo/www/cen_tract.html.
- 24.Anselin L. Local indicators of spatial association: LISA. Geog Anal. 2009;27:93–115. [Google Scholar]
- 25.Soljak M, Samarasundera E, Indulkar T, Walford H, Majeed A. Variations in cardiovascular disease under-diagnosis in England:national cross-sectional spatial analysis. BMC Cardiovascular Disorders. 2011;11:12. doi: 10.1186/1471-2261-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai J, Li Z, Rocke D. [Accessed January 22, 2013];Hierarchical Logistic Regression Modeling with SAS GLIMMIX. Available at http://www.lexjansen.com/wuss/2006/analytics/ANL-Dai.pdf.
- 27.Brody H, Rip MR, Vinten-Johansen P, Paneth N, Rachman S. Map-making and myth-making in Broad Street: the London cholera epidemic, 1854. Lancet. 2000;356:64–8. doi: 10.1016/S0140-6736(00)02442-9. [DOI] [PubMed] [Google Scholar]
- 28.Tirabassi MV, Wadie G, Moriarty KP, Garb J, Konefal SH, Courtney RA, Sachs BF, Wait R. Geographic information system localization of community-acquired MRSA soft tissue abscesses. J Pediatr Surg. 2005;40:962–5. doi: 10.1016/j.jpedsurg.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Nuvolone D, Della Maggiore R, Maio S, Fresco R, Baldacci S, Carrozzi L, Pistelli F, Viegi G. Geographical information system and environmental epidemiology: a cross-sectional spatial analysis of the effects of traffic-related air pollution on population respiratory health. Environ Health. 2011;10:12. doi: 10.1186/1476-069X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–83. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- 31.Berntsen P, Park CY, Rothen-Rutishauser B, Tsuda A, Sager TM, Molina RM, Donaghey TC, Alencar AM, Kasahara DI, Ericsson T, Millet EJ, Swenson J, Tschumperlin DJ, Butler JP, Brain JD, Fredberg JJ, Gehr P, Zhou EH. Biomechanical effects of environmental and engineered particles on human airway smooth muscle cells. J R Soc Interface. 2010;7(Suppl 3):S331–40. doi: 10.1098/rsif.2010.0068.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136:716–25. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 33.De Matteis S, Consonni D, Lubin JH, Tucker M, Peters S, Vermeulen RCh, Kromhout H, Bertazzi PA, Caporaso NE, Pesatori AC, Wacholder S, Landi MT. Impact of occupational carcinogens on lung cancer risk in a general population. Int J Epidemiol. 2012;41:711–21. doi: 10.1093/ije/dys042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesic MJ, Meyer M, Bauer R, Jaspers I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS One. 2012;7:e35108. doi: 10.1371/journal.pone.0035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Census Bureau American Community Survey, 2005–2009, generated using ESRI Community Analyst. 2013 Jan 22; [Google Scholar]
- 36.Zarzaur BL, Croce MA, Fabian TC, Fischer P, Magnotti LJ. A population-based analysis of neighborhood socioeconomic status and injury admission rates and in-hospital mortality. J Am Coll Surg. 2010;211:216–23. doi: 10.1016/j.jamcollsurg.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control Behavioral Risk Factor Surveillance System, 2000–2006. Available at http://www.cdc.gov/BRFSS/
- 38.Arkansas Department of Health [Accessed January 22, 2013];County Health Surveys. Available at http://www.healthy.arkansas.gov/programsServices/healthStatistics/Brfss/Pages/CountyHealthSurveys.aspx.
- 39.Missouri Department of Health and Senior Services [Accessed January 22, 2013];Community Data Profiles. Available at http://health.mo.gov/data/CommunityDataProfiles/
- 40.Tennessee Department of Health [Accessed January 22, 2013];Behavioral Risk Factor Surveillance System. Available at http://health.state.tn.us/statistics/brfss.htm.


