Abstract
Summary
The detection of antibodies against the human leukocyte antigen (HLA) complex has become indispensable in every clinical practice. The development of solid-phase assays like the Luminex allows the standardized measurement of anti-HLA antibodies (HLAab) with high sensitivity, albeit the relevance for some clinical settings remains a matter of debate. In this review we aim to describe the principle of Luminex-based antibody detection, including two modifications that allow identifying solely complement-activating antibodies. We then describe three applications for Luminex: i) detection of HLAab preceding solid-organ transplantation and monitoring of donor-specific antibodies posttransplant as a risk factor for antibody-mediated rejection; ii) presence of HLAab in recipients as a risk for graft failure in hematopoietic stem cell transplantation, especially in haploidentical or mismatched transplantations; iii) role of HLAab in blood transfusion including refractory thrombocytopenia and selection of suitable platelet donors, transfusion-related lung injury after plasma transfusion, and immunization against HLA after red blood cell transfusion despite leukodepletion. Although the Luminex platform constitutes a potent technology for HLA antibody detection, some drawbacks require the well-educated analysis and interpretation of data in critical cases. In addition, Luminex has become an important tool to identify clinically relevant antibodies.
KeyWords: Luminex, HLA antibodies, Transfusion
Introduction
The recognition of human leukocyte antigens (HLA) as trigger of the alloimmune response started with the initial observation of antibodies (later recognized as being HLA-specific) in sera of polytransfused patients and multiparous women [1, 2] - historically reviewed by Thorsby [3]. However, an understanding of the complexity and significance of the HLA system for histocompatibility was gained later by analyzing the alloimmunization with HLA antibodies (HLAab] following experimental skin transplantation by agglutination assays [4].
Nowadays, the detection of HLAab can be assessed by a diversity of more sophisticated methods including on the one hand cell-based assays like the complement-dependent cytotoxicity test (CDC) or flow cytometry and on the other hand solid-phase assays (SPA) like ELISA and Luminex. However, these methods differ substantially in sensitivity and specificity [5, 6, 7]. In the field of solid-organ transplantation SPA, especially Luminex-based assays, are currently used worldwide most frequently, and HLAab are now widely accepted to be clinically relevant both pre- and posttransplant as comprehensively reviewed in an antibody consensus paper [8]. However, the range of applications for Luminex assays is more widespread.
In this review we aim to describe the Luminex technique as the most sensitive method for assessment of alloimmunization in different clinical settings, including solid-organ transplantation, hematopoietic stem cell transplantation (HSCT), and blood transfusion. Furthermore, we will discuss the pros and cons of this innovative method and provide future perspectives for clinical applications.
Principles of the Luminex Method
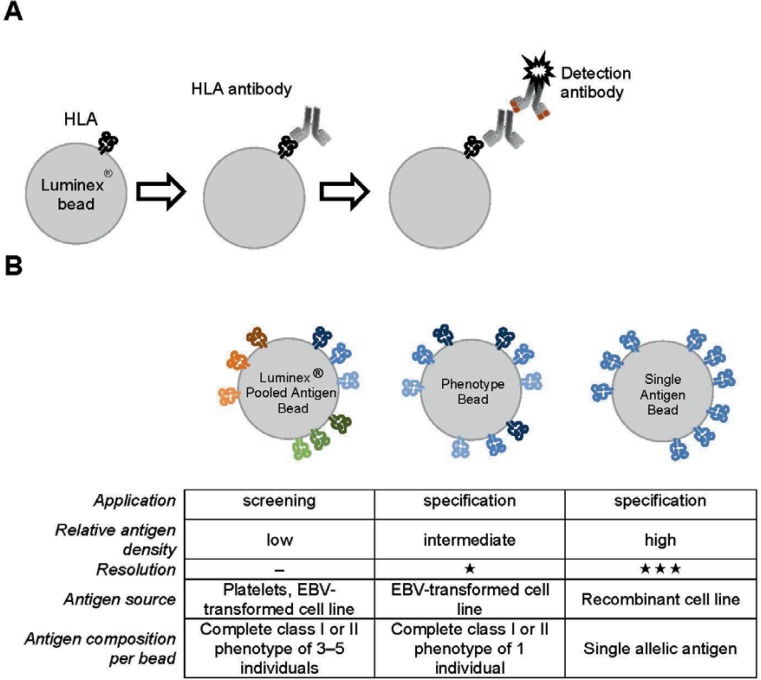
Nowadays, SPA are widely established in histocompatibility laboratories. In contrast to cell-based assays, SPA make use of solubilized HLA molecules immobilized to a solid matrix that could be either a microtiter plate (ELISA) or microbeads (Luminex). HLA may be purified from platelets or from transfected or recombinant lines. The Luminex-based assays utilize polystyrene microbeads impregnated with a unique mixture of two fluorescent dyes that are simultaneously excited by a red laser at 635 nm. The emitted light can be detected at wavelengths of 660 nm (red) and 730 nm (infrared) using a dedicated footprint flow cytometer (Luminex®100/200™). By measuring the composition of the emission intensities for both channels, up to 100 distinct beads with a unique HLA antigen can be identified concomitantly. The detection of HLAab is achieved by using a secondary antibody conjugated with the reporter fluorophore R-phycoerythrin (PE) which is excited by a green laser (532 nm) and detected at 576 nm. The test principle is depicted in figure 1A.
Fig. 1.
A Principle of the Luminex assay to detect HLA antibodies. Each specific bead impregnated with two fluorophores is coated with HLA from cell lines and platelets or recom-binant HLA. In case the test serum contains antibodies directed against the specific HLA it will bind to the appropriate bead. The binding is then detected by a PE-conjugated secondary antibody specific for human IgG. The combination of the fluorescence signals from each bead indicating the HLA specificity and the secondary reagent indicating bound HLA-specific antibodies is acquired by the Luminex system using appropriate lasers and detectors. B Comparison of the characteristics of the three distinct bead preparations commercially available.
In principle there are three types of panels used for Luminex beads which can be distinguished based on the composition of target antigens: i) pooled antigen panels have bead populations coated with either affinity-purified HLA class I (HLA-A, -B, -C) or HLA class II (HLA-DR, -DQ, -DP) antigens which have been pooled from multiple individuals and are used for qualitative HLAab detection; ii) phenotype beads are coated with either the HLA class I or class II phenotype antigens of a single individual; and iii) single antigen panels comprise bead populations coated each with a single allelic, recombinant HLA molecule (fig. IB). Screening for HLAab can be achieved appropriately by using pooled antigen panels that are relatively inexpensive and provide information on the presence or absence of antibodies belonging to a particular class of HLA. Moreover, these panels are useful for tracking changes in the breadth and/or level of antibody binding in a series of sequential sera. Determination of antibody specificity with highest sensitivity and degree of resolution to the allelic antigen level is achieved by using single antigen beads (SAB).
Although bead-based immunoassays are approved only for qualitative assignment of HLAab specificities, several publications demonstrate to some extent correlations of the mean fluorescence intensity (MFI) of the detection antibody as measured in Luminex bead arrays with cross-match results using CDC or flow cytometry as well as clinical outcomes [9, 10]. Nevertheless, MFI measures cannot directly be converted into antibody titers as the MFI simply represents a surrogate marker for the amount of bound antibody and is affected by several factors, including antibody concentration in the serum but also density, conformation and orientation of the antigen, as well as by the antibody avidity toward the respective antigen.
Technical Issues
Despite major advantages of the Luminex technology, there are important critical factors that need consideration when analyzing the data [11]. Therefore, this section is devoted to technical issues with respect to the application of these assays.
A recently described issue inherent with the Luminex technology is the potential underestimation of HLAab due to weak or even false-negative reactions. Similar to the prozone or Hook phenomenon, well known from precipitation immunoassays [12], the serum appears negative or weak when tested neat and becomes more strongly reactive upon dilution, especially in the case of highly sensitized patients [13]. Dilution or hypotonic dialysis of the serum as well as the addition of dithiothreitol and treatment with EDTA or heat inactivation were described to overcome this phenomenon [14, 15, 16]. The cause of the prozone-like effect using Luminex beads is not well understood. Both blocking IgM and complement component Cl which interferes with the detection antibody have been discussed in the literature [13,17].
Furthermore, the immobilization procedure of the HLA molecules to the beads alters the tertiary structure of the molecules exposing neoepitopes which may partly lead to deviant reactions. First described by Morales-Buenrostro et al. [18], HLAab specificities were detected among healthy males without classical alloimmunizing events. These antibodies, referred to as ‘natural’ HLAab, could be shown by epitope analyses to react with polymorphic amino acid residues usually not exposed to the molecular surface [19]. Cryptic epitopes are accessible only on dissociated antigens and comprise amino acids buried by the peptide within the MHC binding groove. Other epitopes can be found within the alpha-3 domain of HLA class I antigens in close proximity to the transmembrane domain and are thus in the native orientation of the molecule inaccessible by antibodies due to steric hindrance. The trigger of these antibodies is still unknown. However, it is suspected that heterologous immunity may lead to antibodies which are directed against peptides derived from viral or bacterial pathogens and bearing HLA-mi-metic epitopes. Key features of ‘natural HLAab as detectable by Luminex are the relative low level (1,000–5,000 MFI) and specificities against less frequent HLA as listed in table 1.
Table 1.
List of the most frequent ‘natural HLA class I antibody specificities detected among 424 healthy blood donors without classical HLA alloimmunizing events [18] opposed to the antigen frequency among the US population with European descent [67] revealed the pardoxon between low frequent antigens but fairly high prevalence of antibodies
| Specific A locus | Antibody frequency among blood donors, % | Caucasian antigen frequency, % | Specific B locus | Antibody frequency among blood donors, % | Caucasian antigen frequency, % |
|---|---|---|---|---|---|
| A*30:02 | 18.9 | 0.9 | B*15:12 | 11.1 | 0 |
| A*31:01 | 11.3 | 2.3 | B*82:01 | 10.4 | 0 |
| A*80:01 | 8.5 | 0 | B*15:16 | 9.9 | 0 |
| A*34:01 | 6.8 | 0 | B*37:01 | 7.8 | 1.3 |
| A*66:02 | 6.6 | 0 | B*44:02 | 6.1 | 9.0 |
| A*43:01 | 5.9 | 0 | B*45:01 | 5.9 | 0.4 |
| A*66:01 | 5.9 | 0.2 | B*81:01 | 4.7 | 0 |
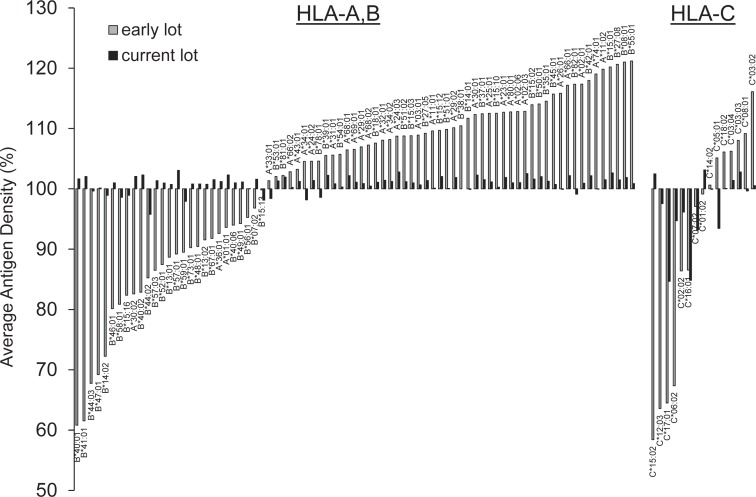
The relative density of a particular antigen differs substantially between pooled antigen, phenotype and SAB. Especially, HLA-C but also -DQ and -DP on SAB are characterized by a higher relative antigen density as compared to phenotype beads and human cells. The relatively low expression of HLA-C as compared to HLA-A and -B on human cells was shown to be regulated by a designated non-coding region leading to faster degradation of mRNA. In contrast, cDNA-transfected cells, the source of recombinant single antigens for SAB, revealed equal surface expression [20]. As a consequence, HLAab directed against these naturally low-expressed antigens run the risk of being detectable exclusively by SAB. This fact may contribute to the controversy on the clinical relevance of HLAab as there is an eminent discrepancy in antigen density between the antibody detection assay and the surface of endothelial cells as the antibody target of a renal allograft. Consequently, HLA-DQ antibodies detected exclusively by SAB reveal only a low immunological risk for renal transplant [21]. Disparities in relative antigen quantity exist not only across different bead formats but also among SAB. Since the advent of SAB, continuous improvements in the manufacturing process and quality assurance measures have contributed to a more uniform antigen density across all beads from lotto lot (fig. 2).
Fig. 2.
Decreasing variability of the relative antigen density on HLA class I SAB of one vendor comparing an early and the most current lot. Relative antigen density was assessed by the monoclonal antibody clone W6/32 which binds a conformational epitope carried by all HLA-A, -B and -C native antigens. Variability of antigen density could be significantly reduced from an early (LAB-Screen lot#003) to a current lot (lot#007). HLA-C antigen density still reveals the highest variability.
Modifications of Luminex Assays
SPA including the Luminex platform are well established to screen for HLA IgG antibodies or to specify for class I or class II. The Luminex assay has the great advantage of allowing the use of a variety of secondary detection antibodies to assess immunoglobulin isotypes [22] or subclasses [23] as well as complement components [24].
In 2005 Wahrmann et al. [25] published the first cell-independent in vitro assay to detect complement-binding HLAab. The authors applied HLA-coated microbeads to detect C4d as the alloantibody-triggered complement split product deposit by the use of a standard flow cytometer. A similar method pub-lished by Smith et al. [26] detects C4d-fixing HLAab on Luminex SAB. The identification of the initial complement component Clq which directly binds to alloantibodies on Luminex SAB was first demonstrated by Chin et al. [27]. Both the C4d and Clq method have recently been shown to be equivalent in detecting complement-fixing antibodies in vitro [28]. However, the marked difference between those assays is that the latter requires the addition of purified Clq protein instead of a complex human complement preparation, eliminating potential inhibitory effects by serum proteins, and thus resulted overall in much higher MFI values. The Clq assay combines the sensitivity and specificity of the Luminex technique with the ability to detect the complement-binding potential of antibodies all in one single assay.
Although the Luminex technology was first introduced to and is most abundantly used in the setting of solid-organ transplantation, its current use is much more widespread. Thus, further applications in the context of HSCT and blood product transfusions will be discussed in the subsequent sections.
HLA Antibodies and Solid-Organ Transplantation
In the late 1960s Terasaki and colleagues impressively demonstrated that preformed cytotoxic donor-specific HLAab (DSA) can immediately deteriorate the transplant within minutes after reperfusion (i.e., by hyperacute antibody-mediated rejection) and introduced the pretransplant CDC cross-match into the clinical consideration, a measure which is still the decisive test preceding kidney transplantation [29]. Despite this early recognition of the power of HLAab detection, the focus in the field of solid-organ transplantation was on the cellular immune response indicated by the immunosuppressive treatment posttransplant which can successfully control cellular immunity but leaves humoral reactivity almost unappreciated [30]. Without doubt, the introduction of powerful immunosuppressive drugs, including the family of calcineurin inhibitors as well as the anti-metabolite azathioprine, significantly improved transplant outcome [31]. It is also undisputable that humoral immunity is dependent on co-stimulation by T helper cells; however, the missing second defensive line targeting humoral immunity potentially could make the overall immunosuppressive regime partly ineffective. In fact, in an elegant study by Sellarés et al. [32] it was shown that the risk for the development of antibody-mediated rejection significantly increases over time.
With implementation of the more sensitive, second generation of SPA in the late 1990s based on the Luminex technology, the focus of transplantation immunology was shifted towards the humoral immune response. Presence of HLAab could be associated with episodes of rejections and led Terasaki [33] to revive the humoral theory of transplantation in which he described several lines of evidence showing that HLAab: i) lead to complement (C4d) deposits associated with early kidney graft failures, ii) are an appropriate indicator of presensitization leading to early acute rejections, iii) were present in 96% of patients who rejected a kidney graft, and iv) are associated with chronic rejection in 33 studies of kidney, heart, lung, and liver grafts. A recent antibody consensus paper from clinicians and immunogeneticists comprehensively presents data on the clinical relevance of HLAab both pre- and posttransplant [8].
The standard Luminex assay detecting IgG HLAab is currently the most widespread assay for antibody assessment in histocompatibility testing; however, its modifications to detect exclusively complement-binding antibodies as mentioned earlier gain increasing attention.
Pretransplant DSA as detected by the C4d assay could be associated with a trend towards adverse 5-year graft survival among 338 renal allograft recipients [34]. Similarly, pretransplant Clq-binding HLAab revealed a negative impact on 10-year graft survival (35 vs. 60%) which did not reach statistical significance due to the low number of cases, but indicates the power of this assay to identify a group of recipients with a negative CDC cross-match but Clq DSA being associated with an increased risk of graft rejection [35].
Nowadays, increasing efforts are made to tailor the maintenance immunosuppressive therapy to the individual allograft recipient, based on surrogate markers for rejection and tolerance [36]. Detection of de novo DSA using Luminex constitutes a suitable non-invasive biomarker to identify patients with increased risk for antibody-mediated rejection (AMR) [37]. Conversely, when AMR occurs, monitoring of the DSA level provides to some extent a prediction of the outcome: In a series of 10 patients treated for refractory AMR with the proteasome inhibitor bortezomib the sustained reduction of the strongest DSA as indicated by the MFI was predictive for improved 18-month allograft survival and reversal of AMR [38]. The Clq assay, however, has been shown to be superior to standard IgG Luminex in evaluating the effect of desensitization by plasmapheresis or immunoglobulins [24].
These findings emphasize the versatile applications of the standard and modified Luminex assays not only for pretransplant risk stratification and prediction of rejection but also for monitoring of therapeutic interventions in solid-organ transplantation.
HLA Antibodies and Hematopoietic Stem Cell Transplantation
The tremendous increase in the number of mismatched HSCT in recent years - including the unparalleled boost of cord blood units - is accompanied by the use of reduced intensive pretransplantation conditioning (RIC) regimens, as very recently shown by Brand et al. [39]. Although this approach has led to a shift in the clinical paradigm stressing the necessity of HLA antibody detection and the performance of pretransplant cross-matches, the realization in clinical practice has not yet been accomplished. This has in part been due to the lack of highly sensitive diagnostic tools, although already in 1977 Storb and coworkers [40] have recognized a higher prevalence of HSCT graft rejection in pretransplant transfused patients with acquired platelet refractoriness. In contrast to solid-organ transplantations where HLA antibody monitoring posttransplant provides essential information for graft rejection, in HSCT current data suggest that HLA antibody detection of de novo DSA is restricted to the increased risk of graft failure. There is an increased body of evidence that the significance of preformed DSA for graft failure differs markedly between malignant and non-malignant disorders requiring HSCT [41].
The impressive meta-analysis of Brand et al. [42] distinguished between different clinial settings with distinct origin of the stem cells, such as bone marrow, peripheral blood, or umbilical cord blood, and the different regimens of myeloablative and non-myeloablative conditioning. The authors show a detrimental effect of DSA on graft failure as detected by a positive CDC cross-match. Nevertheless, even antibodies solely identified by standard Luminex technology that were not able to fix or deposit complement have been shown to be a risk factor for the outcome of HSCT [39]. It is worth mentioning that especially antibodies directed against HLA-DP antigens, which can reliably be detected only by Luminex SAB, could be associated with an increase in graft rejection (up to 38% in a cohort of 20 patients harboring exclusively HLA-DP antibodies). A recent report of patients undergoing haploidentical HSCT described that 3 of 5 patients with high levels of DSA encountered graft failure [43]. Also stem cell donor-derived HLAab can influence the graft outcome. Tanguchietal. [44] found a frequency of 22% parous female stem cell donors to be HLAab-positive compared to 0% in non-parous females and 1.8% in males. Four out of 7 patients that received stem cells from HLAab-positive donors were found with a similar set of antibodies, most likely due to co-transplanted plasma B cells. The clinical significance, however, remains unclear.
Taken together these findings emphasize independently the clinical potency of complement binding Luminex assays like Luminex-Clq or -C4d, which have a sufficient high sensitivity to detect those clinically relevant antibodies that remain otherwise undetected by CDC cross-matches [28].
HLA Antibodies and Blood Transfusion
It is commonly acknowledged that patients receiving red blood or platelet concentrates might become immunized against residual leukocytes. After the introduction of leukocyte-reduced red blood cell concentrates the immunization rate dropped down from 13.5 to 3–4% [45]. Nevertheless, the generation of transfusion-based HLAab still harbors the risk of severe unwanted side effects including platelet refractoriness and transfusion-related lung injury (TRALI).
Red Blood Cell Transfusions
Red blood cells virtually lack the expression of HLA class I and II. In contrast, passenger leukocytes that express high levels of HLA are a potent trigger for alloimmunization. Despite substantial advances in leukodepletion, the remaining leukocytes (less than 1 χ 106/unit) still constitute a potential immunogen, especially in polytransfused patients [46]. In addition, there is still a body of evidence that even residual HLA class I expression on erythrocytes can contribute to alloimmunization [47,48]. Although it would thus be desirable to provide recipient HLA-matched red blood cell transfusions - at least in selected cases [49] -, this is not the case in every day's clinical practice due to several important facts: i) limited availability of HLA-matched products, ii) time- and cost-consuming selection processes, iii) non-practical logistics in emergency situations.
In some indications, including renal anemia, stimulation of endogenous erythropoiesis has been made possible by introduction of recombinant erythropoietin and its mimetics and has been established as the best, ‘biosafe alternative for red blood cell transfusions, reducing the risk of iron overload. However, in recent years new concerns regarding the use of these drugs in chronic kidney disease patients arose, critically discussing the life-threatening side effects like stroke that might outweigh the beneficial rise in erythropoietin-induced hemoglobin [50]. If these studies hold up in the future, an increase in the use of red blood cell products which is associated with the risk of sensitization could be awaited. In addition to the mere presence of alloimmunizing leukocytes - leading to de novo HLAab production - increased production of cytokines (including proinflammatory IL-6 and IL-10) might lead to modulation of the immune response, as recently shown for the immediate postoperative period after cardiac surgery [51].
In order to detect the degree of sensitization, SPA like the Luminex single antigen assay are among the most appropriate methods. Due to their high sensitivity, they allow to detect even low or transient HLAab levels that are commonly observed following transfusions. So far, it is not yet known to which degree alloimmunizations triggered by pregnancies, transplantations, or transfusions contribute to the generation of clinically relevant HLAab [52].
Platelet and Plasma Transfusions
Platelet refractoriness is caused by a variety of factors including splenomegaly, fever, sepsis [53] or alloimmunizations against HLA or - less commonly - platelet antigens (HPA) [54]. In highly immunized patients the chance of finding fully compatible blood donors is markedly diminished. However, as mentioned above, complement-based Luminex modifications can be applied to exclusively detect cytotoxic HLAab allowing to assess those antibodies that are most likely clinically relevant with the aim to increase the pool of potential donors [55].
To our knowledge the only larger study applying such an approach was that by the group of Tyan and coworkers [57]. They found that the panel reactivity identified by the Clq assay was on average lower than by Luminex standard IgG (60 vs. 94%), which increases the fraction of potential donors. In addition, they conclude that consideration of clinically relevant HLAab only might be a better way to identify those platelet units that will result in acceptable platelet increments and might be thus an effective and rapid approach to supply patients with refractory thrombocytopenia [56]. The complement-binding HLAab are best detected by cross-matches before platelet transfusions or by Clq- or C4d-based Luminex.
Another topic where HLAab play a role in transfusion medicine are events of non-hemolytic transfusion reactions. The clinical signs are mild urticarial, febrile reactions up to TRALI [58]. While typically preformed antibodies present in the recipient are the source of immune reactions, in TRALI antibodies present in the blood donor are passively co-transferred and cause the adverse immune reaction. While most antibodies are directed against HLA class II, there are also antibodies recognizing human neutrophil antigens (HNA), mostly HNA-3a, that are often found in multi-parous women [59]. Some HNA antibodies can be detected by a separate Luminex LABScreen™ approach. Since the implementation of the Luminex platform for detection of HLAab and HNA antibodies (HNAab), there are numerous reports providing a body of evidence that this higher sensitivity allows the better verification of suspected cases of TRALI [60, 61, 62]. TRALI is an underreported rare event but the leading cause of transfusion-related morbidity and mortality [63]. HLAab and HNAab turned out to be the only independent risk factor for TRALI in a large study on 668 patients, when adjusted for other known patient risk factors (OR = 14.2; 95% CI = 1.5–132) [61].
In order to reduce the cases of TRALI without generally excluding all females from donating plasma, an antibody prescreening in the UK identified donors positive for HLAab or HNAab and excludes only those from donating [64, 65]. This practice was confirmed by a recent study by the Canadian Blood Services [66].
Conclusion
Taking all aspects mentioned above into consideration, the Luminex technology is a sophisticated technique that requires interpreters with educated experience and expertise. Nevertheless, application of the appropriate Luminex assays allows both detection of all HLAab with high sensitivity and the exclusive recognition of those HLAab that are clinically relevant due to their complement-activating capacity. Luminex thus provides valuable information for bona fide decision making in a variety of clinical settings.
Disclosure Statement
The authors declared no conflct of interest. NL is a consultant of BmT GmbH.
References
- 1.Dausset J. Iso-leuko-antibodies (in French) Acta Haematol. 1958;20:156–166. doi: 10.1159/000205478. [DOI] [PubMed] [Google Scholar]
- 2.Van Rood JJ, Eernisse JG, Van Leeuwen A. Leucocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–1736. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 3.Thorsby E. A short history of HLA. Tissue Antigens. 2009;74:101–116. doi: 10.1111/j.1399-0039.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 4.Dausset J, Rapaport FT, Colombani J, Feingold N. A leucocyte group and its relationship to tissue histo-compatibility in man. Transplantation. 1965;3:701–705. doi: 10.1097/00007890-196511000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Worthington JE, Robson AJ, Sheldon S, Langton A, Martin S. A comparison of enzyme-linked immuno-absorbent assays and flow cytometry techniques for the detection of HLA specific antibodies. Hum Immunol. 2001;62:1178–1184. doi: 10.1016/s0198-8859(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 6.Zachary AA, Vega RM, Lucas DP, Leffell MS. HLA antibody detection and characterization by solid phase immunoassays: Methods and pitfalls. Meth Mol Biol. 2012;882:289–308. doi: 10.1007/978-1-61779-842-9_17. [DOI] [PubMed] [Google Scholar]
- 7.Altermann WW, Seliger B, Sei S, Wendt D, Schlaf G. Comparison of the established standard complement-dependent cytotoxicity and flow cytometric crossmatch assays with a novel ELISA-based HLA crossmatch procedure. Histol Histopathol. 2006;21:1115–1124. doi: 10.14670/HH-21.1115. [DOI] [PubMed] [Google Scholar]
- 8.Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR, Coates PT, Colvin RB, Cozzi E, Doxiadis II, Fuggle SV, Gill J, Glotz D, Lachmann N, Mohanakumar T, Suciu-Foca N, Sumitran-Holgersson S, Tan-abe K, Taylor CJ, Tyan DB, Webster A, Zeevi A, Opelz G. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 9.Mizutani K, Terasaki P, Hamdani E, Esquenazi V, Rosen A, Miller J, Ozawa M. The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant. 2007;7:1027–1031. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya S. Clinical importance of anti-human leukocyte antigen-specific antibody concentration in performing calculated panel reactive antibody and virtual crossmatches. Transplantation. 2008;85:1046–1050. doi: 10.1097/TP.0b013e318168fdb5. [DOI] [PubMed] [Google Scholar]
- 11.Schönemann C. Management falsch positiver XMAP-Antikörperbefunde bei der Bestimmung von HLA-Antikörpern. Transfusionsmedizin. 2012;2:192–196. [Google Scholar]
- 12.Butch AW. Dilution protocols for detection of hook effects/prozone phenomenon. Clin Chem. 2000;46:1719–1721. [PubMed] [Google Scholar]
- 13.Kosmoliaptsis V, Bradley JA, Peacock S, Chaudhry AN, Taylor CJ. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation. 2009;87:813–820. doi: 10.1097/TP.0b013e318199c581. [DOI] [PubMed] [Google Scholar]
- 14.Zachary AA, Lucas DP, Detrick B, Leffell MS. Naturally occurring interference in Luminex assays for HLA-specific antibodies: characteristics and resolution. Hum Immunol. 2009;70:496–501. doi: 10.1016/j.humimm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Schnaidt M, Weinstock C, Jurisic M, Schmid-Horch B, Ender A, Wernet D. HLA antibody specification using single-antigen beads - a technical solution for the prozone effect. Transplantation. 2011;92:510–515. doi: 10.1097/TP.0b013e31822872dd. [DOI] [PubMed] [Google Scholar]
- 16.Kosmoliaptsis V, O'Rourke C, Bradley JA, Taylor CJ. Improved Luminex-based human leukocyte antigen-specific antibody screening using dithiothrei-tol-treated sera. Hum Immunol. 2010;71:45–49. doi: 10.1016/j.humimm.2009.09.358. [DOI] [PubMed] [Google Scholar]
- 17.Weinstock C, Schnaidt M. The complement-mediated prozone effect in the Luminex single-antigen bead assay and its impact on HLA antibody determination in patient sera. Int J Immunogenet2012; 10.1111/J.1744–313X.2012.01147.x. [DOI] [PubMed]
- 18.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, Lee J-H, El-Awar N, Alberu J. ‘Natural human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 19.El-Awar N, Terasaki PI, Nguyen A, Sasaki N, Morales-Buenrostro LE, Saji H, Maruya E, Poli F. Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood. Hum Immunol. 2009;70:844–853. doi: 10.1016/j.humimm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 20.McCutcheon JA, Gumperz J, Smith KD, Lutz CT, Parham P. Low HLA-C expression at cell surfaces correlates with increased turnover of heavy chain mRNA. J Exp Med. 1995;181:2085–2095. doi: 10.1084/jem.181.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CJ, Kosmoliaptsis V, Summers DM, Bradley JA. Back to the future: Application of contemporary technology to long-standing questions about the clinical relevance of human leukocyte antigen-specific alloantibodies in renal transplantation. Hum Immunol. 2009;70:563–568. doi: 10.1016/j.humimm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Arnold ML, Heinemann FM, Horn P, Ziemann M, Lachmann N, Muhlbacher A, Dick A, Ender A, Tham-manichanond D, Fischer GF, Schaub S, Hallensleben M, Mytilineos J, Hitzler WE, Seidl C, Doxiadis II, Spriewald BM. 16(th) IHIW: Anti-HLA alloantibodies of the of IgA isotype in re-transplant candidates. IntJ Immunogenet. 2013;40:17–20. doi: 10.1111/iji.12032. [DOI] [PubMed] [Google Scholar]
- 23.Honger G, Hopfer H, Arnold ML, Spriewald BM, Schaub S, Amico P. Pretransplant IgG subclasses of donor-specific human leukocyte antigen antibodies and development of antibody-mediated rejection. Transplantation. 2011;92:41–47. doi: 10.1097/TP.0b013e31821cdf0d. [DOI] [PubMed] [Google Scholar]
- 24.Tyan DB. New approaches for detecting complement-fixing antibodies. Curr Opin Organ Transplant. 2012;17:409–415. doi: 10.1097/MOT.0b013e328355fb9b. [DOI] [PubMed] [Google Scholar]
- 25.Wahrmann M, Exner M, Haidbauer B, Schillinger M, Regele H, Kormoczi G, Bohmig GA. [C4d]FlowPRA screening - a specific assay for selective detection of complement-activating anti-HLA alloantibodies. Hum Immunol. 2005;66:526–534. doi: 10.1016/j.humimm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, Luminex binding antibodies - a new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7:2809–2815. doi: 10.1111/j.1600-6143.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 27.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, Rosenthal D, Reinhartz O, Tyan D. Clinical usefulness of a novel Clq assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatrie heart transplant patients. J Heart Lung Transplant. 2011;30:158–163. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Lachmann N, Todorova K, Schulze H, Schonemann C. Systematic comparison of four cell- and Luminex-based methods for assessment of complement-activating HLA antibodies. Transplantation. 2013;95:694–700. doi: 10.1097/TP.0b013e31827b3dc3. [DOI] [PubMed] [Google Scholar]
- 29.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 30.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 31.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 32.Sellarés J, Freitas DGd, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 33.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 34.Wahrmann M, Bartel G, Exner M, Regele H, Körmöczi GF, Fischer GF, Böhmig GA. Clinical relevance of preformed C4d-fixing and non-C4d-fixing HLA single antigen reactivity in renal allograft recipients. Transplant Int. 2009;22:982–989. doi: 10.1111/j.1432-2277.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 35.Otten HG, Verhaar MC, Borst HPE, Hené RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant. 2012;12:1618–1623. doi: 10.1111/j.1600-6143.2011.03985.x. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie A, Lee IJ. Biomarkers in renal transplantation. Biomark Med. 2008;2:603–612. doi: 10.2217/17520363.2.6.603. [DOI] [PubMed] [Google Scholar]
- 37.Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schönemann C, Zukunft B, Illigens P, Schmidt D, Wu K, Rudolph B, Neumayer H-H, Budde K. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant. 2012;12:1192–1198. doi: 10.1111/j.1600-6143.2011.03961.x. [DOI] [PubMed] [Google Scholar]
- 38.Waiser J, Budde K, Schütz M, Liefeldt L, Rudolph B, Schönemann C, Neumayer H-H, Lachmann N. Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant. 2012;27:1246–1251. doi: 10.1093/ndt/gfr465. [DOI] [PubMed] [Google Scholar]
- 39.Brand A, Doxiadis IN, Roelen DL. On the role of HLA antibodies in hematopoietic stem cell transplantation. Tissue Antigens. 2013;81:1–11. doi: 10.1111/tan.12040. [DOI] [PubMed] [Google Scholar]
- 40.Storb R, Prentice RL, Thomas ED. Treatment of aplastic anemia by marrow transplantation from HLA identical siblings. Prognostic factors associated with graft versus host disease and survival. J Clin Invest. 1977;59:625–632. doi: 10.1172/JCI108680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horan J, Wang T, Haagenson M, Spellman SR, Dehn J, Eapen M, Frangoul H, Gupta V, Hale GA, Hurley CK, Marino S, Oudshoorn M, Reddy V, Shaw P, Lee SJ, Woolfrey A. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for non-malignant disorders. Blood. 2012;120:2918–2924. doi: 10.1182/blood-2012-03-417758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, Wang X, Thall PF, Champlin RE, Fernan-dez-Vina M. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, Fujioka T, Tamaki H, Ikegame K, Okada M, Soma T, Hayashi K, Fujii N, Onuma T, Kusunoki Y, Saji H, Ogawa H. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47:508–515. doi: 10.1038/bmt.2011.131. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi K, Yoshihara S, Maruya E, Ikegame K, Kaida K, Hayashi K, Kato R, Inoue T, Fujioka T, Tamaki H, Okada M, Onuma T, Fujii N, Kusunoki Y, Soma T, Saji H, Ogawa H. Donor-derived HLA antibody production in patients undergoing SCT from HLA antibody-positive donors. Bone Marrow Transplant. 2012;47:1338–1342. doi: 10.1038/bmt.2012.28. [DOI] [PubMed] [Google Scholar]
- 45.The Trial to Reduce Alloimmunization to Platelets Study Group Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 46.Magee BA, Martin J, Cole MP, Morris KG, Courtney AE. Effects of HLA-matched blood transfusion for patients awaiting renal transplantation. Transplantation. 2012;94:1111–1116. doi: 10.1097/TP.0b013e318271d776. [DOI] [PubMed] [Google Scholar]
- 47.Rivera R, Scornik JC. HLA antigens on red cells. Implications for achieving low HLA antigen content in blood transfusions. Transfusion. 1986;26:375–381. doi: 10.1046/j.1537-2995.1986.26486262749.x. [DOI] [PubMed] [Google Scholar]
- 48.de Villartay JP, Rouger P, Müller JY, Salmon C. HLA antigens on peripheral red blood cells: analysis by flow cytofluorometry using monoclonal antibodies. Tissue Antigens. 1985;26:12–19. doi: 10.1111/j.1399-0039.1985.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 49.Scornik JC, Meier-Kriesche HU. Blood transfusions in organ transplant patients: mechanisms of sensiti-zation and implications for prevention. Am J Transplant. 2011;11:1785–1791. doi: 10.1111/j.1600-6143.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 50.Obrador GT, Macdougall IC. Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol2012; 10.2215/CJN.00020112. [DOI] [PubMed]
- 51.Bilgin YM, van de Watering LM, Versteegh MI, van Oers MH, Brand A. Effects of allogeneic leukocytes in blood transfusions during cardiac surgery on inflammatory mediators and postoperative complications. Crit Care Med. 2010;38:546–552. doi: 10.1097/CCM.0b013e3181c0de7b. [DOI] [PubMed] [Google Scholar]
- 52.Hyun J, Park KD, Yoo Y, Lee B, Han BY, Song EY, Park MH. Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplant Proc. 2012;44:222–225. doi: 10.1016/j.transproceed.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 53.Doughty HA, Murphy MF, Metcalfe P, Rohatiner AZ, Lister TA, Waters AH. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang. 1994;66:200–205. doi: 10.1111/j.1423-0410.1994.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 54.Kekomaki R. Use of HLA- and HPA-matched platelets in alloimmunized patients. Vox Sang. 1998;74(suppl 2):359–363. doi: 10.1111/j.1423-0410.1998.tb05443.x. [DOI] [PubMed] [Google Scholar]
- 55.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142:348–360. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 56.Fontaine MJ, Kuo J, Chen G, Galel SA, Miller E, Seque-ira F, Viele M, Goodnough LT, Tyan DB. Complement (Clq) fixing solid-phase screening for HLA antibodies increases the availability of compatible platelet components for refractory patients. Transfusion. 2011;51:2611–2618. doi: 10.1111/j.1537-2995.2011.03194.x. [DOI] [PubMed] [Google Scholar]
- 57.Wiita AP, Nambiar A. Longitudinal management with crossmatch-compatible platelets for refractory patients: alloimmunization, response to transfusion, and clinical outcomes (CME) Transfusion. 2012;52:2146–2154. doi: 10.1111/j.1537-2995.2012.03593.x. [DOI] [PubMed] [Google Scholar]
- 58.Bux J. Transfusion-related acute lung injury (TRALI): a serious adverse event of blood transfusion. Vox Sang. 2005;89:1–10. doi: 10.1111/j.1423-0410.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 59.Flesch BK, Petershofen EK, Bux J. TRALI - new challenges for histocompatibility and immunogenetics in transfusion medicine. Tissue Antigens. 2011;78:1–7. doi: 10.1111/j.1399-0039.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- 60.Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36:53–58. [PubMed] [Google Scholar]
- 61.Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Kulik W, Korevaar J, de Mol BA, Koopman MM, Porcelijn L, Binnekade JM, Vroom MB, Schultz MJ, Juf-fermans NP. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: A prospective nested case-control study. Blood. 2011;117:4218–4225. doi: 10.1182/blood-2010-10-313973. [DOI] [PubMed] [Google Scholar]
- 62.Imoto S, Kawamura K, Tokumine Y, Araki N, Akita S, Nishimura C, Inaba H, Saigo K, Mabuchi O, Okazaki H. Acute non-hemolytic transfusion reactions and HLA class I antibody: advantages of solid phase assay compared with conventional complement-dependent assay. Transfus Med. 2010;20:95–103. doi: 10.1111/j.1365-3148.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldman M, Webert KE, Arnold DM, Freedman J, Hannon J, Blajchman MA, Panel TC. Proceedings of a consensus conference: towards an understanding of TRALI. Transfus Med Rev. 2005;19:2–31. doi: 10.1016/j.tmrv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Lucas G, Win N, Calvert A, Green A, Griffin E, Ben-dukidze N, Hopkins M, Browne T, Poles A, Chapman C, Massey E. Reducing the incidence of TRALI in the UK: the results of screening for donor leucocyte antibodies and the development of national guidelines. Vox Sang. 2012;103:10–17. doi: 10.1111/j.1423-0410.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- 65.Muller MC, Porcelijn L, Vlaar AP. Prevention of immune-mediated trans fusion-related acute lung injury; from bloodbank to patient. Curr Pharm Des. 2012;18:3241–3248. doi: 10.2174/1381612811209023241. [DOI] [PubMed] [Google Scholar]
- 66.Lin Y, Saw CL, Hannach B, Goldman M. Transfusion-related acute lung injury prevention measures and their impact at Canadian blood services. Transfusion. 2012;52:567–574. doi: 10.1111/j.1537-2995.2011.03330.x. [DOI] [PubMed] [Google Scholar]
- 67.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]