Abstract
Summary
The risk of transfusion-transmitted infections has been greatly reduced by improvements in donor screening and testing. However, newly recognized blood-borne infectious agents can be threats to blood safety. In order to evaluate the prevalence some of these agents in blood donors, a systematic review was conducted. Data were obtained from published papers related to HGV, Torque Teno virus (TTV), HTLV, West Nile virus (WNV) and SEN virus (SEN-V). Based on these studies, the prevalence of HGV varied from 1 to 8.6% for anti-E2 and from 0 to 4.8% for HGV RNA. The prevalence of TTV DNA and HTLV-I varied from 2.7 to 79.5% and from 0.013 to 2.3%, respectively. The WNV-specific IgM antibody and WNV RNA are negative in blood donors. Prevalence rates of SEN-V in Iranian blood donors range from 23 to 90.8%. Consequences of these infectious agents for blood safety are different. Thus, the need to perform laboratory screening as well as effectiveness and efficiency of laboratory tests depend on pathogenicity level and epidemiological conditions of emerging infections. However, being prepared based on the current level of risk and interventions to reduce the risk can be effective in reducing the potential threat for blood supply.
KeyWords: Infectious diseases, Emerging, Blood supply, Iran
Introduction
Currently, blood transfusion has become a substantial part of medical practice. Every second, someone in the world needs blood for surgery, trauma, severe anemia, or complications of pregnancy [1]. In other words, without blood transfusion, life-saving medical treatments, such as surgical procedures, pregnancy-related complications, the treatment of thalassemic and other multitransfused patients, cancer treatment, organ transplants, and bone marrow transplants would not be possible. Therefore, it is necessary that sufficient blood supplies are available within a very short notice.
The safest blood donors are voluntary, non-remunerated blood donors. The number of blood donations is more than 1.7 million units annually in Iran, and 100% of our donations are voluntary and non-remunerated. In Iran 40% of all blood donations were collected from regular blood donors during the year 2007 [2].
Testing of all donated blood for hepatitis B surface antigen (HBsAg), HIV-land −2 antigen-antibody, HCV antibody, syphilis and HTLV-I/II (being mandatory in three provinces based on the local epidemiological evidence) is one of the main strategies for protecting against serious transfusion-transmitted infections (TTIs) in blood recipients. Therefore, in recent years the risk of transfusion-transmitted infections has been greatly reduced by improvements in donor screening and testing so that today the blood supply is safer. However, given that emerging and re-emerging infections (including also infectious diseases) are considered as important factors of mortality and morbidity in different populations [3], conditions for blood centers are becoming more complex. Of the identified virulent pathogens, including viruses, bacteria, fungi, protozoa, and helminthes, approximately 175 species are considered emerging pathogens [4]. Emerging infections are defined to be those infectious diseases whose incidence has increased within the past 2 decades or threatens to increase in the near future [5].
Several factors are involved in the appearance of emerging diseases. These infections may result from ecologie changes or emanate from genetic, biological, social, and economic factors.
The total effect of these factors will lead to the development of emerging diseases.
Emerging and re-emerging microorganisms, like other microbial agents, can threaten blood safety. Epidemiology of newly emerged pathogens differs according to socioeconomic, geographic, and cultural conditions. Geographically, Iran is situated in the northern temperate zone with a variety of climate types and varied in social, economic, cultural, and health aspects. Iran also neighbors the countries with various economic and health conditions. Thus, travelling across borders and vast range of commercial trading (agricultural products and livestock) adds up to the likelihood of transmission of newly emerged pathogens. In this study, the seroepidemiological status of some newly described viruses related to blood transfusion has been investigated in Iran.
Methods
A systematic review was constructed. For this review, data were obtained from published papers by a computerized search of all recorded English and Farsi literature during the years 2000 to 2011. Search in resources was performed through databases such as Medline, Scopus, Proquest, Iranme-dex, and Magiran. The words used in the search were as follows: blood transfusion, Iran, emerging infections, specific vi-ruses (HGV, TTV, HTLV-I/II, WNV, SEN-V). Furthermore, we searched for ongoing or completed studies on this issue in the documents of the Iranian Blood Transfusion Research Center.
Results
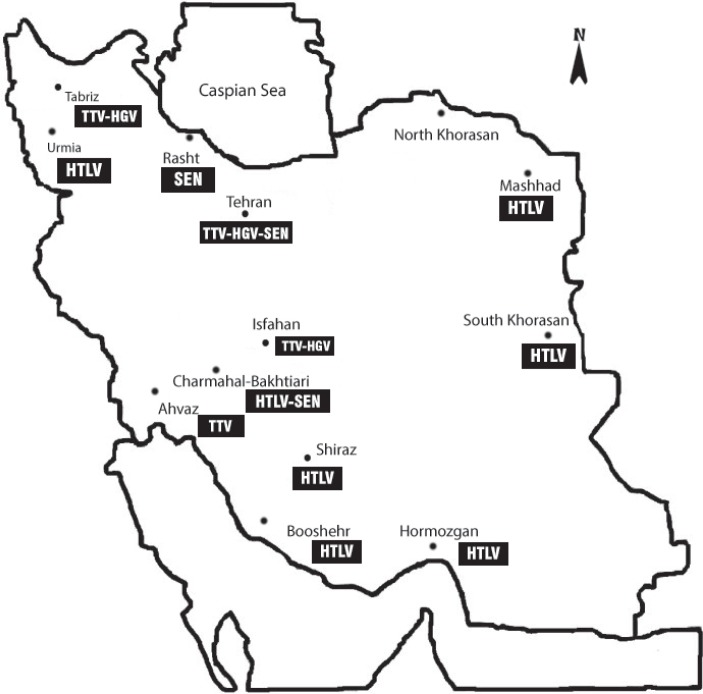
Overall, a total of 23 articles based on keywords used in connection with the emerging infectious agents in Iranian blood donors were identified. These studies were on viruses such as HGV, Torque Teno viruses (TTV), HTLV-I/II, West Nile virus (WNV), and SEN virus (SEN-V). The geographic distribution of some newly described viruses in Iranian blood donors is shown on map (fig. 1).
Fig. 1.
Geographie distribution of some newly described viruses in Iranian blood donors.
HGV
HGV, as non-A-E hepatitis, was discovered in 1996 by different groups of investigators. In the literature the virus was named HGV and GBV-C. Molecular specifications of these agents have shown them to be indeed identical isolates of the same virus [6, 7].
HGV is a single-stranded RNA virus belonging to the Flaviviridae family. According to striking reasons, Stapleton et al. [8] proposed to assign GBV-C/HGV as species within a new genus. HGV RNA is detected by using a reverse transcriptase polymerase chain reaction assay. The antibody to envelope protein E2 of this virus (anti-E2) is available. Anti-E2 is a marker for previous HGV infection and is useful for seroepidemiological studies. It has been demonstrated that GBV-C/HGV has worldwide distribution, and type 2 is highly prevalent in many countries [9]. The clinical significance of HGV infection with respect to acute or chronic hepatitis is not well understood. The available data suggest that HGV does not cause acute non-A-E viral hepatitis, has no pathologic effects on the liver, and is not a significant cause of fulminant hepatitis [10, 11, 12].
Epidemiology of HGV in Iranian blood donors has been investigated by several authors (table 1). All of these studies have been done on voluntary blood donors.
Table 1.
Prevalence of HGV/GBV-C Anti E2 and RNA among Iranian voluntary blood donors
| Reference | City | Total number tested | Anti E2 N (%) | HGV RNA n (%) |
|---|---|---|---|---|
| Ramezani et al. [13] | Tabriz | 478 | 5 (1) | 0 (0) |
| Gharehbaghian et al. [14] | Tehran | 330 | 14 (14.2) | – |
| Amini Kafi-Abad et al. [15] | Tehran | 100 | – | 1 (1) |
| Rezvan et al. [16] | Tehran | 514 | 44 (8.6) | – |
| Salehi et al. [17] | Isfahan | 40 | 2 85) | – |
| Amini Kafl-Abad et al. [18] | Tehran | 400 | – | 19 (4.8) |
| Total | 1,862 | 65 (4.8) | 20 (2) | |
Laboratory studies have been conducted through anti-E2 and HGV RNA detection. Based on these studies, the prevalence of HGV anti-E2 varies from 1 to 8.6%. Overall, the average HGV antibody prevalence in voluntary blood donors is 4.8%. The lowest prevalence was reported in Tabriz and the highest in Tehran (fig. 1). All these cities are among the country's big cities. These studies have also investigated the frequency of viral RNA in apparently healthy donors. The prevalence of HGV RNA in under study donors varies from 0 to 4.8%. The average GBV-C/HGV RNA prevalence rate is 2%.
Epidemiological investigations in healthy blood donors in some other areas are shown in table 2. GBV-C/HGV RNA prevalence rates range from 0 to 4% in India, UK, Japan, USA, Germany, Turkey, Scotland, Norway, Indonesia, France, Taiwan, and Sweden. It has also been reported at varying ranges (5–15.8%) in Tunisia, Central Brazil, and China (table 2). The 2% prevalence of GBV-C/HGV RNA and the 4.8% prevalence of GBV-C/HGV antibody (anti-2) in Iranian blood donors are the same as those in healthy blood donors in many countries around the world.
Table 2.
Reported prevalence of HGV/GBV-C infection in healthy blood donors in some published studies
| Reference | Country | Total number tested | Anti E2 n (%) | HGV RNA n (%) |
|---|---|---|---|---|
| Desai et al. [19] | India | 120 | – | 0 (0) |
| Minton et al. [20] | UK | 100 | – | 1 (1) |
| Saitoh et al. [21] | Japan | 157 | 4 (2.5) | 2 (1.3) |
| Roth et al. [22] | Germany | 1,408 | – | 14 (1.34) |
| Alter et al. [23] | USA | 500 | – | 7 (1.4) |
| Seifried et al. [24] | Germany | 5,733 | – | 90 (1.6) |
| Hanci et al. [25] | Turkey | 100 | 3 (3) | 2 (2) |
| Blair et al. [26] | Scotland | 1,020 | – | 23 (2.25) |
| Nordbo et al. [27] | Norway | 1,001 | 105 (10.5) | 25 (2.5) |
| Handajani et al. [28] | Indonesia | 150 | – | 4 (2.7) |
| Mercier et al. [29] | France | 2,739 | – | 93 (3.4) |
| Yu et al. [30] | Taiwan | 500 | 36 (7.2) | 17 (3.4) |
| Björkman et al. [31] | Sweden | 458 | 62 (13.6) | 18 (3.9) |
| Kar et al. [32] | India | 50 | – | 2 (4) |
| Loiseau et al. [33] | France | 500 | – | 21 (4.2) |
| Mastouri et al. [34] | Tunisia | 600 | – | 32 (5.3) |
| Oliveira et al. [35] | Central Brazil | 241 | – | 17 (7.1) |
| Yan et al. [36] | China | 203 | – | 31 (15.8) |
| Mastouri et al. [34] | Tunisia | 912 | 44 (4.9) | – |
| Mercier et al. [29] | France | 2,219 | 210 (10) | – |
HGV transmission by blood transfusion has been conclusively recognized, but its role in causing hepatitis is not well understood. Stapleton et al. [10] showed that the viral replication in peripheral blood cells occurs but replication of HGV in liver cells has not been observed.
Because GBV-C/HGV does not cause any significant diseases, currently blood products are not screened for this virus. However, further studies need to be done to take decisions about the necessity of screening on blood and blood products.
TTV
Transfusion-transmitted virus or TTV was identified in Japanese patients (TT for the initials of the patient in whom the virus was isolated for the first time) in 1997 [37]. TTV is anon-enveloped single-stranded DNA virus. Currently, the International Committee on Taxonomy of Viruses classified TTV into genus Anellovirus [38]. The virus was first identified in humans; however, recent data showed that these viruses are prevalent in non-human primates and mammalians such as chimpanzees, cats, dogs, and pigs [39].
TTV DNA has been detected in blood and body fluids such as semen, saliva, vaginal secretion, breast milk, and tears, and it can be transmitted through parenteral, sexual, and mother-to-child routes [40, 41, 42]. Transmission by transfusion of blood and blood products is an identified way of transmission.
TTV DNA is detected by using a reverse transcriptase polymerase chain reaction assay. However, because of genetic variability of the virus and technical aspects, the results of PCR assays can be different [43].
Epidemiology of TTV in Iranian voluntary healthy blood donors has been investigated by several authors. Based on these studies, the prevalence of TTV DNA varies from 2.7 to 79.5% (table 3). All, except two, of these studies used the primers NG059, NG063, and NG061. Bouzari et al. [48, 49] used the primers from the untranslated region (T801, T935) in their studies. The average TTV DNA prevalence rate in voluntary blood donors is 28.5%. Prevalence of TTV in healthy blood donors in some other areas are shown in table 4. The reported prevalence rates of TTV infection in healthy blood donors in different countries range from 1 to 75%.
Table 3.
Prevalence of TTV among Iranian voluntary blood donors
| Reference | City | Total number tested | TTV DNA n (%) |
|---|---|---|---|
| Khoshbaten et al. [44] | Tabriz | 407 | 11 (2.7) |
| Pourshams et al. [45] | Tehran | 312 | 70 (22.4) |
| Jalali Far et al. [46] | Ahwaz | 253 | 60 (23.7) |
| Zandie et al. [47] | Tehran | 250 | 103 (41) |
| Bouzari et al. [48] | Tabriz | 100 | 65 (65) |
| Bouzari et al. [49] | Isfahan | 132 | 105 (79.5) |
| Total | 1,454 | 414 (28.5) | |
Table 4.
Reported prevalence of TTV infection in healthy blood donors in some published studies
| Reference | Country | Total number tested | TTV DNA n (%) |
|---|---|---|---|
| Charlton et al. [50] | USA | 100 | 1(1) |
| Werno et al. [51] | New Zealand | 413 | 13 (3.15) |
| Handa et al. [52] | USA | 250 | 21 (8.4) |
| Ho et al. [53] | Taiwan | 244 | 29 (11.9) |
| de Castro Amarante et al. [54] | Brazil | 270 | 32 (11.9) |
| Nakano et al. [55] | Korea | 100 | 14 (14) |
| Kalkan et al. [56] | Turkey | 125 | 21 (16.8) |
| Urwijitaroon et al. [57] | Northeast Thailand | 101 | 28 (28) |
| Hashish et al. [58] | Egypt | 95 | 46 (48.4) |
| Alfaresi et al. [59] | United Arab Emirates | 100 | 75 (75) |
TTV infection in blood donors is common around the world. On the other hand, the cases of transmission through blood transfusion have been proven. However, the ability of TTV to induce primary hepatitis, its effect on severity of hepatitis due to other viruses, and its pathogenicity for acute or chronic liver disease are controversial [51, 60, 61]. Due to the above-mentioned reasons, currently blood products are not screened for this virus.
HTLV
HTLV-I/II is a single-stranded RNA virus belonging to Retroviridae family. HTLV-I was identified in 1979 and was reported on in 1980 [62]. This virus is endemic in many geographic regions around the world such as parts of Africa, South America, and southwestern Japan. Currently, approximately 10–20 million people around the world are infected [63]. The virus is also endemic in some geographical regions of Iran such as Khorasan provinces (fig. 1). The first report of a serological survey of the virus in Iran showed a prevalence rate of 2.3% in Mashhad blood donors [64].
Diagnostic methods have relied upon serological screening by using an enzyme-linked immunoassay. If the result is positive, as a confirmatory testing a Western blot analysis or a PCR assay may be carried out. Since the available diagnostic kits cannot clearly distinguish these two types of viruses, they may be reported as HTLV-I/II.
There are several known modes of transmission, including mother to child, mainly through breastfeeding, sexual contact, and parenteral transmission by blood transfusion, or by sharing of needles and syringes.
HTLV-I is associated with several diseases such as adult T-cell leukemia/lymphoma and HTLV-I-associated myelopathy/ tropical spastic paraparesis. Moreover; this virus is likely linked to diseases such as uveitis, infective dermatitis, synovitis, and thyroiditis.
Due to complications attributed to this virus, its transmission through blood transfusion, especially in patients undergoing repeated blood transfusions, is an important issue. In this study 9 authoritative articles were identified related to the prevalence of HTLV-I/II in Iranian blood donors. There are 2 articles in Farsi language about healthy blood donors that are related to prevalence of HTLV-I which are searchable by using Farsi keywords only. These studies have been conducted in regions such as Bushehr and Hormozgan. In these areas, the frequency rate was 0.013% (3/22,740) and 0.18% (2/1,100), respectively. In addition, we found 7 articles in English language related to the frequency of virus in blood donors (table 5). In these studies (Farsi and English), laboratory screening tests have been conducted on 347,850 healthy blood donors, with HTLV-I/II antibody being confirmed in 2,784 donors. Based on these studies, the prevalence rate of HTLV-I/II varies from 0.013 to 2.3%. The lowest prevalence rate was reported in Bushehr and the highest in Mashhad. Differences in diagnostic kits, geographical conditions, and demographic status of the subjects can affect the results of studies in different parts of the country. The seroprevalence rates of HTLV infections in blood donors in some countries are as follows: 0% in Turkey (Izmir) [71] and Lebanon [72], 0.005% in the UK [73], 0.002% in Sweden [74], 0.01–0.03% in the USA and Canada, 0.002% in Norway, 0.0056% in Greece [75], 1.9% in India [76], and 0.08–8% in Japan [77]. Blood banks of some countries, such as those of the UK, the USA, Canada, and Japan, routinely screen all blood donations for HTLV-I/II.
Table 5.
Prevalence of HTLV-1 among Iranian voluntary blood donors
| Reference | City | Total number tested | HTLV-1 n (%) |
|---|---|---|---|
| Ghafouri et al. [65] | South Khorasan | 42,652 | 18 (0,042) |
| Rezvan et al.[66] | multicenter | 15,866 | 47 (0.29) |
| Khameneh et al. [67] | Urmia | 2,046 | 7 (0.34) |
| Tarhini et al. [68] | Mashhad | 232,648 | 1,054 (0.45) |
| Karimi et al. [69] | Charmahal-Bakhtiari | 800 | 5 (0.62) |
| Abbaszadegan et al. [70] | Mashhad | 28,487 | 219 (0.77) |
| Farid et al. [64] | Mashhad | 1,511 | 35 (2.3) |
Khorasan provinces (Khorasan Razavi, Southern Khorasan, and Northern Khorasan) located in the north-east region of Iran are known as endemic areas (fig. 1). The town of Mashad as a religious center hosts people both as residents and pilgrims with different economic, social, cultural, and health backgrounds. For this reason laboratory screening has started routinely since 1995 in these regions [78]. Dispersion of the virus has been reported in other areas among donors. But given the fact that data from blood donors should not be generalized to other groups for making decision to perform routine screening tests in other parts of the country, there is a need for more evidence with regard to the prevalence of the virus in the general population.
WNV
WNV is a single-stranded RNA virus belonging to the family Flaviviridae. The virus was first isolated in the West Nile district of Uganda in 1937. Distribution of WNV has been reported from many geographic locations in Africa, Europe, Asia, and America [79].
The virus is transmitted to humans by mosquitoes. Different species of mosquitoes and different species of birds as a reservoir of the virus are involved in the transmission of the virus [80, 81].
Presence of birds as the reservoir and of mosquitoes capable of transmitting the virus has been reported in various parts of Iran [82]. Studies also indicated that there is seropositivity in some parts of the country in the general population. A sero-epidemiological survey in preschool children from the north of Iran (Caspian Sea) showed a 10% prevalence rate [83]. In another study, a seroprevalence rate of 26.6% (186/698) has been reported in the general population in some provinces, with a higher rate in central and southwestern parts [84]. Clinical manifestation of infection with this virus ranges from an asymptomatic infection to severe central nervous system involvement. In mild and moderate forms, sign and symptoms such as fever, headaches, malaise, muscle and joint pains, lymphadenopathy, and skin rash occur. Meningoencephalitis as a lethal complication is located at the end of the clinical manifestation spectrum [85].
Subsequent to the clinical findings related to WNV, including fever and meningoencephalitis in four cases of organ transplantation, investigations related to the possibility of virus transmission through blood transfusion were done. Finally, 21 cases of posttransfusion WNV was confirmed by the Centers of Disease Control and Prevention in 2002, and WNV was recognized as an emerging pathogen transmitted through blood transfusion [85].
Different prevalence rates of the virus in blood donors from different countries have been reported.
Frequencies of WNV RNA in the USA were 1.49 and 0.44 per 10,000 donations in 2003 and 2004, respectively [86]. Prevalence of anti-WNV antibodies was 6.8 per 1,000 sera and for WNV RNA 17.5 per 100,000 donations among blood donors in north-east Italy [87]. Moreover, 0.56% of blood donors in Central Anatolia (Turkey) were WNV-seropositive [88], but 0% (0/500) in the United Arab Emirates [89].
Because of the clinical importance of this virus and reported cases of WNV transmission through blood transfusion, in some countries, routine screening is done at present [90].
Only one study was performed in Iran to evaluate the frequency of WNV in blood donors. This study, which was conducted on 500 healthy blood donors in Tehran, showed that all donors were negative for WNV-specific IgM antibody and WNV RNA. 5% (25\500) were positive for WNV-IgG antibody [91]. It is necessary to emphasize that this study was performed on Tehran blood donors. Therefore, it could not be generalized to all geographic areas, and more comprehensive studies should be conducted.
SEN-V
SEN-V (SEN for the initials of the patient in whom the virus was isolated for the first time) is a non-enveloped and single-stranded circular DNA virus belonging to the family of TTV-related viruses. To date, phylogenetic analysis shows nine SEN-V isolates, named SEN-V-A-I [92]. SEN-V is endemic throughout the world, but its prevalence differs by geographic region. The prevalence rates in healthy persons in the USA, Taiwan, Thailand, Greece and Italy have been reported to be 1.8%, 15%, 5%, 24% and 13%, respectively; moreover, prevalence rates of 8–17% and of 10–22% have been reported in Germany and Japan, respectively [93].
SEN-V is usually detected by using a reverse transcriptase PCR assay. With respect to primers used, the sensitivity of this test can be different. This difference in sensitivity might be an explanation for the differences in the reported prevalences of the SEN-V [94].
SEN-V transmission occurs through parenteral and non-par-enteral routes. In fact, transmission in intravenous drug users as well as via iatrogenic, vertical transmission and blood transfusion has been reported [94].
In order to investigate the epidemiology of this virus, three different studies have been conducted on blood donors in Iran. Prevalence rates of SEN-V in Iranian blood donors range from 23% (60/260) [95] over 23.33% (14/60) [96] to 90.8% (109/120) [97]. These studies have been conducted in three different cities (fig. 1). The higher frequency belongs to Rasht in the northern Iran. This significant difference might be related to laboratory methods used or geographical differences.
Umemura et al. [98] strongly suggest that SEN-V is transmitted through blood transfusions, and they showed that acute infection with SEN-V transmitted by blood transfusion can lead to hepatitis. In addition, there is a relation between the number of blood units transfused and SEN-V infection [98]. On the other hand, Shibata et al. [99] did not find any significant association with SEN-V as a causative agent of non A-C hepatitis or liver cirrhosis.
In conclusion, despite the high prevalence of SEN-V in healthy blood donors, and given these controversies and the lack of clinical significance of infection with the virus, screening tests are not performed in blood centers.
Conclusion
The safety of blood supply is a multifactorial process. Donor screening and testing are two mainstays in blood processing centers. With respect to all measures taken, the blood supply is safer than any time, and in comparison to other medical interventions the risks associated with blood transfusion are extremely low. Due to the nature of the factors associated with emerging infections, identification of the epidemic and pandemic status of newly recognized or emerging blood-borne infectious agents in a community is an important issue.
Out of the viruses studied in the present research, HTLV and WNV are known factors threatening health of blood recipients. Considering the epidemiological map of the viruses in Iran, HTLV is currently the greatest concern. Routine screening tests are being conducted in a few provinces with high prevalence. But if a prospective comprehensive epidemiological study suggests a countrywide screening test, test sensitivity and specificity might be challenging and also government funds will be necessary.
As far as other emerging or re-emerging pathogens are concerned, it is imperative for another comprehensive epidemiological study to be conducted on the basis of newly formulated strategies.
It is worthwhile to apply pathogen inactivation methods, use leukocyte reduction techniques, establish hemovigilance system, and implement look-back programs to aim at ensuring blood safety and reducing the risk of transmission of emerging infections down to near zero.
Disclosure Statement
The authors declared no conflct of interest.
References
- 1.World Health Organization Universal Access to Safe Blood Transfusion, www.who.int/bloodsafsty/publi-cations/UniversalAccesstoSafeBT.pdf (last accessed April 25, 2013).
- 2.Maghsudlu M, Nasizadeh S, Abolghasemi H, Ahmad-yar SH. Blood donation and donor recruitment in [ran from 1998 through 2007: ten years experience. Transfusion. 2009;49:2346–2351. doi: 10.1111/j.1537-2995.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization The Global Burden of Disease: 2004 Update. www.who.int/heaìthinfo/gìobaì_burden_disease/2004_report_update/en/index.html (last accessed April 25, 2013).
- 4.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philos Trans R Soc LondB. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederberg J, Shope ER, Oaks SC. Factors in emergence. In: Lederberg J, Shope ER, Oaks SC, editors. Emerging Infections: Microbial Threats to Health in the United States. Washington: National Academy Press; 1992. p. 34. [PubMed] [Google Scholar]
- 6.Linnen J, Wages J, Jr, Zhang-Keck ZY, Fry KE, Krawc-zynski KZ, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih JW, Young L, Piatak M, Jr, Hoover C, Fernandez J, Chen S, Zou JC, Morris T, Hyams KC, Ismay S, Lif-son JD, Hess G, Foung SK, Thomas H, Bradley D, Mar-golis H, Kim JP. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 7.Simons JN, Leary TP, Dawson GJ, Pilot-Matias TJ, Muerhoff AS, Schlauder GG, Desai SM, Mushahwar IK. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Sim-monds P. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. ] GenVirol. 2011;92:233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naito H, Abe K. Genotyping system of GBV-C: HGV type 1 to type 4 by the polymerase chain reaction using type-specific primers and geographical distribution of viral genotypes. J Virol Methods. 2001;91:3–9. doi: 10.1016/s0166-0934(00)00207-x. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton JT. GB virus type C/hepatitis G virus. Semin Liver Dis. 2003;23:137–148. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 11.Theodore D, Lemon SM. GB virus C, hepatitis G virus, or human orphan flavivirus? Hepatology. 1997;25:1285–1286. doi: 10.1002/hep.510250541. [DOI] [PubMed] [Google Scholar]
- 12.Feucht HH, Zöllner B, Polywka S, Knödler B, Schröter M, Nolte H, Laufs R. Distribution of hepatitis G viremia and antibody response to recombinant proteins with special regard to risk factors in 709 patients. Hepatology. 1997;26:491. doi: 10.1002/hep.510260234. [DOI] [PubMed] [Google Scholar]
- 13.Ramezani A, Gachkar L, Eslamifar A, Khoshbaten M, Jalilvand S, Adibi L, Salimi V, Hamkar R. Detection of hepatitis G virus envelope protein E2 antibody in blood donors. Int Infect Dis. 2008;12:57–61. doi: 10.1016/j.ijid.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Gharehbaghian A,Tavakoli S, Amini Kafiabad S, Zar-nani AH. Seroepidemiologic HGV in blood donors, haemodialysis patients haemophiliacs and major thalassemics with history of liver disease. Sci J Iran Blood Transfus Organ. 2005;2:189–196. [Google Scholar]
- 15.Amini S, Andalibi Mahmoodabadi S, Lamian S, Jou-laie M, Mahmoodi Farahani M. Prevalence of hepatitis G virus (HGV) in high-risk groups and blood donors in Tehran, Iran. Iranian J Pubi Health. 2005;34:41–46. [Google Scholar]
- 16.Rezvan H, Sharafi M, Shams H, Mahmoodian Shoos-htari M. A preliminary report on prevalence of antibody response to GBV-C, E2 protein in Iranian blood donors and multi trans fused patients. Iranian J Pubi Health. 2007;36:6–11. [Google Scholar]
- 17.Salehi H, Khorvash F, Noori V, Alizade M, Taghian E. Seroepidemiology of HGV in blood donors and patients under hemodialysis. Journal of Isfahan Medical School, Special Issue on Health Promotion, 2009;540–548 [Google Scholar]
- 18.Amini Kafi-Abad S, Samiei SH, Talebian A, Maghsud-loo M, Gharehbaghian A. Hepatitis G virus infection in Iranian blood donors and high-risk groups. Hepat Mon. 2009;9:282–286. [Google Scholar]
- 19.Desai MM, Pal RB, Banker DD. GB virus C/hepatitis G virus infection in Indian blood donors and high-risk groups. Transfus Apher Sci. 2004;30:111–117. doi: 10.1016/j.transci.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Minton J, Iqbal A, Eskiturk A, Irving W, Davies J. Hepatitis G virus infection in lymphoma and in blood donors. J Clin Pathol. 1998;51:676–678. doi: 10.1136/jcp.51.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitoh H, Moriyama M, Matsumura H, Goto I, Tanaka N, Aarakawa Y. The clinical significance of GBV-C/ HGV exposure in C-viral chronic liver disease and blood donors. Hepatolol Res. 2002;22:288–296. doi: 10.1016/s1386-6346(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 22.Roth WK, Waschk D, Marx S, Tschauder S, Zeuzem S, Bialleck H, Weber H, Seifried E. Prevalence of hepatitis G virus and its strain variant, the GB agent, in blood donations and their transmission to recipients. Transfusion. 1997;37:651–656. doi: 10.1046/j.1537-2995.1997.37697335162.x. [DOI] [PubMed] [Google Scholar]
- 23.Alter HJ, Nakatsuji Y, Melpolder J, Wages J, Wesley R, Shih JW, Kim JP. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N EnglJ Med. 1997;336:747–754. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- 24.Seifried C, Weber M, Bialleck H, Seifried E, Schrezen-meier H, Roth WK. High prevalence of GBV-C/HGV among relatives of GBV-C/HGV positive blood donors in blood recipients and in patients with aplas-tic anemia. Transfusion. 2004;44:268–274. doi: 10.1111/j.1537-2995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanci SY, Cevahir N, Kaleli I, Hanci V. Investigation of hepatitis G virus prevalence in hemodialysis patients and blood donors in Denizli, Turkey. Mikrobi-yol Bui. 2008;42:617–625. [PubMed] [Google Scholar]
- 26.Blair CS, Davidson F, Lycett C, McDonald DM, Hay-don GH, Yap PL, Hayes PC, Simmonds P, Gillon J. Prevalence, incidence, and clinical characteristics of hepatitis G virus/GB virus C infection in Scottish blood donors. J Infect Dis. 1998;178:1779–1782. doi: 10.1086/314508. [DOI] [PubMed] [Google Scholar]
- 27.Nordb0 SA, Krokstad S, Winge P, Skjeldestad FE, Dalen AB. Prevalence of GB virus C (also called hepatitis G virus) markers in Norwegian blood donors. J Clin Microbiol. 2000;38:2584–2590. doi: 10.1128/jcm.38.7.2584-2590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handajani R, Soetjipto Lusida MI, Suryohudoyo P, Adi P, Setiawan PB, Nidom CA, Soemarto R, Katayama Y, Fujii M, Hotta H. Prevalence of GB virus C/ hepatitis G virus infection among various populations in Surabaya, Indonesia, and identification of novel groups of sequence variants. J Clin Microbiol. 2000;38:662–668. doi: 10.1128/jcm.38.2.662-668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercier B, Barclais A, Botte C, Cantalube J, Coste J, Defer C, Gautreau C, Giannoli C, Halfon P, Lepot I, Loiseau P, Martial J, Montcharmont P, Merel P, Ouzan D, Ravera N, Follana J, Césaire R, Janot C, Lemaire J, De Micco P, Vezon G, Férec C. Prevalence of GBV C/HGV RNA and GBV C/HGV antibodies in French volunteer blood donors: results of a collaborative study. Vox Sang. 1999;76:166–169. doi: 10.1159/000031043. [DOI] [PubMed] [Google Scholar]
- 30.Yu ML, Chuang WL, Wang LY, Dai CY, Chiou SS, Sung MH, Chang CS, Chen SC, Wang CS, Chang TT, Chang WY. Status and natural course of GB virus C/hepatitis G virus infection among high-risk groups and volunteer blood donors in Taiwan. J Gastroenterol Hepatol. 2000;15:1404–1410. doi: 10.1046/j.1440-1746.2000.02359.x. [DOI] [PubMed] [Google Scholar]
- 31.Björkman P, Nauclér A, Winqvist N, Mushahwar I, Widell A. A case-control study of transmission routes for GB virus C/ hepatitis G virus in Swedish blood donors lacking markers for hepatitis C virus infection. Vox Sang. 2001;81:148–153. doi: 10.1046/j.1423-0410.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- 32.Kar P, Bedi P, Berry N, Chakravorty A, Gupta RK, Saha R, Das BC. Hepatitis G virus (HGV) infection in voluntary and commercial blood donors in India. Diagn Microbiol Infect Dis. 2000;38:7–10. doi: 10.1016/s0732-8893(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 33.Loiseau P, Mariotti M, Corbi C, Ravera N, Girot R, Thauvin M, Portelette E, Manette X, Roudot-Tho-raval F, Benbunan M, Lefrère JJ. Prevalence of hepatitis G virus RNA in French blood donors and recipients. Transfusion. 1997;37:645–650. doi: 10.1046/j.1537-2995.1997.37697335161.x. [DOI] [PubMed] [Google Scholar]
- 34.Mastouri M, Safer IL, Pozzetto B, Bourlet T, Khedher M. Prevalence of hepatitis G virus among Tunisian blood donors. East Mediterr Health J. 2005;11:1053–1060. [PubMed] [Google Scholar]
- 35.Oliveira LA, Martins RM, Carneiro MA, Teles SA, Silva SA, Cardoso DD, Lampe E, Yoshida CF. Prevalence and genotypes of GB virus C/hepatitis G virus among blood donors in central Brazil. Mem Inst Oswaldo Cruz. 2002;97:953–957. doi: 10.1590/s0074-02762002000700005. [DOI] [PubMed] [Google Scholar]
- 36.Yan J, Chen LL, Luo YH, Mao YF, He M. High frequencies of HGV and TTV infections in blood donors in Hangzhou. World J Gastroenterol. 2001;7:637–641. doi: 10.3748/wjg.v7.i5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Bio-chem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 38.Biagini P, Todd D, Bendinelli M, Hino S, Mankertz A, Mishiro S, Niel C, Okamoto H, Radial S. Genus Anello-virus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy. Eightth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2005. pp. 335–341. [Google Scholar]
- 39.Okamoto H. TT viruses in animals. Curr Top Microbiol Immunol. 2009;331:35–52. doi: 10.1007/978-3-540-70972-5_3. [DOI] [PubMed] [Google Scholar]
- 40.Inami T, Konomi N, Arakawa Y, Abe K. High prevalence of TT virus DNA in human saliva and semen. J Clin Microbiol. 2000;38:2407–2408. doi: 10.1128/jcm.38.6.2407-2408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krekulova L, Rehak V, Killoran P, Madrigal N, Riley LW. Genotypic distribution of TT virus (TTV) in a Czech population: evidence for sexual transmission of the virus., din Viro! 2001;23:31–41. doi: 10.1016/s1386-6532(01)00185-8. [DOI] [PubMed] [Google Scholar]
- 42.Iso K, Suzuki Y, Takayama M. Mother-to-infant transmission of TT virus in Japan. Int J Gynaecol Obstet. 2001;75:11–19. doi: 10.1016/s0020-7292(01)00450-7. [DOI] [PubMed] [Google Scholar]
- 43.Itoh K, Takahashi M, Ukita M, Nishizawa T, Okamoto H. Influence of primers on the detection of TT virus DNA by polymerase chain reaction. J Infect Dis. 1999;180:1750–1751. doi: 10.1086/315107. [DOI] [PubMed] [Google Scholar]
- 44.Khoshbaten M, Kheradpezhouh M, Taremi M, Gachkar L, Naghili B, Aghabozorgi S, Shahnazi T. Prevalence of TTV DNA in blood donors in Tabriz -Iran. Pajoohandeh. 2005;6:367–371. [Google Scholar]
- 45.Pourshams A, Azimi K, Kiani L, Sarrafi M, Farhadi Langroody M, Malekzadeh R. TT virus infection among Iranian blood donors: its prevalence and relationship to serum alanine aminotransferase (ALT) level. Govaresh. 2004;9:106–109. [Google Scholar]
- 46.Jalali Far MA, Zandieh T, Imam SJ, Galehdari H, Pour-fathollah AA, Babà Ahmadi M. TTV prevalence in Ahwaz blood donors by using semi-nested PCR. Sci J Blood Transfus Organ. 2007;3:389–395. [Google Scholar]
- 47.Zandie T, Pourfathollah AA, Aminikafiabad S, Samiei SH, Ranjbar Kermani F, Ghafari K, Sobhani M, Kovari M, Ataei Z. Measurement of TTV prevalence rate by PCR method in plasma of blood donors and recipients in Tehran. Sci J Blood Transfus Organ. 2006;3:153–160. [Google Scholar]
- 48.Bouzari M, Mokhtarzadeh A. Frequency of TTV and its role in HBV and HCV infected blood donors in East Azarbayejan province of Iran. Sci J Blood Transfus Organ. 2009;5:247–255. [Google Scholar]
- 49.Bouzari M, Shaykh Baygloo N. Primers designed for detection of TT virus also detect SEN virus. Res J Biol Sci. 2008;3:1063–1066. [Google Scholar]
- 50.Charlton M, Adjei P, Poterucha J, Zein N, Moore B, Therneau T, Krom R, Wiesner R. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology. 1998;28:839–842. doi: 10.1002/hep.510280335. [DOI] [PubMed] [Google Scholar]
- 51.Werno AM, Wang Z, Schroeder BA, Woodfield G, Croxson MC. Prevalence and phylogenetic characterisation of TT-virus in the blood donor population of Auckland, New Zealand. J Med Virol. 2000;62:109–114. doi: 10.1002/1096-9071(200009)62:1<109::aid-jmv17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 52.Handa A, Dickstein B, Young N, Brown K. Prevalence of the newly described human circovirus, TTV, in United States blood donors. Transfusion. 2000;40:245–251. doi: 10.1046/j.1537-2995.2000.40020245.x. [DOI] [PubMed] [Google Scholar]
- 53.Ho TF, Yang SC, Huang YT, Hsieh MH. TT Virus infection in screened Taiwanese blood donors. Vox Sang. 2000;79:198–200. doi: 10.1159/000056730. [DOI] [PubMed] [Google Scholar]
- 54.de Castro Amarante MF, Kashima S, Covas DT. TT virus (TTV) genotyping in blood donors and multiple transfused patients in Brazil. Virus Genes. 2007;35:503–509. doi: 10.1007/s11262-007-0124-x. [DOI] [PubMed] [Google Scholar]
- 55.Nakano T, Park YM, Mizokami M, Choi JY, Orito E, Ohno T, Kato T, Kondo Y, Tanaka Y, Kato H, Kato T, Kim BS. TT virus infection among blood donors and patients with non-B, non-C liver diseases in Korea. ] Hepatol. 1999;30:389–393. doi: 10.1016/s0168-8278(99)80095-6. [DOI] [PubMed] [Google Scholar]
- 56.Kalkan A, Ozdarendeli A, Bulut Y, Sarai Y, Ozden M, Kelestimur N, Toraman ZA. Prevalence and geno-typic distribution of hepatitis GB-C/HG and TT viruses in blood donors, mentally retarded children and four groups of patients in Eastern Anatolia, Turkey. Jpn J Infect Dis. 2005;58:222–227. [PubMed] [Google Scholar]
- 57.Urwijitaroon Y, Barusrux S, Chunlertlith K, Mairiang P, Yoshimura H. Torquetenovirus infection among northeastern Thai blood donors. Southeast Asian J Trop Med Public Health. 2007;38:686–689. [PubMed] [Google Scholar]
- 58.Hashish MH, El-Barrawy MA, Mahmoud OA, Abdel Rahman NW. TT virus among blood donors in Alexandria. J Egypt Public Health Assoc. 2005;80:651–664. [PubMed] [Google Scholar]
- 59.Alfaresi MS, Elnazer AM, Alzaabi AS, Elkoush AA, Islam AA. Transfusion transmitted virus in screened United Arab Emirates blood donors. Saudi Med J. 2006;27:58–62. [PubMed] [Google Scholar]
- 60.Matsumoto A, Yeo AE, Shih JW, Tanaka E, Kiyosawa K, Alter HJ. Alter transfusion-associated TT virus infection and its relationship to liver disease. Hepatol-ogy. 1999;30:283–288. doi: 10.1002/hep.510300118. [DOI] [PubMed] [Google Scholar]
- 61.Bendinelli M, Pistello M, Maggi F, Fornai C, Freer G, Vatteroni ML. Molecular properties, biology, and clinical implications of TT virus, a recently identified widespread infectious agent of humans. Clin Microbil Rev. 2001;14:98–113. doi: 10.1128/CMR.14.1.98-113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24:5926–5930. doi: 10.1038/sj.onc.1208980. [DOI] [PubMed] [Google Scholar]
- 63.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 64.Farid R, Etemadil M, Baradaranl H, Nikbir BP. Seroepidemiology and virology of HTLV-1 in the city of Mashhad, northeastern Iran. Serodiagn Immunother Infect Dis. 1993;5:251–252. [Google Scholar]
- 65.Ghafouri M, Ameli MR. Comparing prevalence of transfusion transmitted viral infections in various population groups of South Khorasan. Sci J Blood Transfus Organ. 2011;7:242–248. [Google Scholar]
- 66.Rezvan H, Ahmad J, Farhadi M. A cluster of HTLV-1 infection in northeastern of Iran. Transfus Today. 1996;27:8–11. [Google Scholar]
- 67.Khameneh ZR, Baradaran M, Sepehrvand N. Survey of the seroprovalence of HTLV I/II in hemodialysis patients and blood donors in Urmia. Saudi J Kidney Dis Transpl. 2008;19:838–841. [PubMed] [Google Scholar]
- 68.Tarhini M, Kchour G, Zanjani DS, Rafatpanah H, Otrock ZK, Bazarbachi A, Farid R. Declining tendency of human T-cell leukaemia virus type I carrier rates among blood donors in Mashhad, Iran. Pathology. 2009;41:498–499. doi: 10.1080/00313020903041010. [DOI] [PubMed] [Google Scholar]
- 69.Karimi A, Nafici MR, Imani R. Comparison of human T-cell leukemia virus type-1 (HTLV-1) seroprevalence in high risk patients (thalassemia and hemodialysis] and healthy individuals from Charmahal - Bakhtiari province, Iran. Kuwait Med J. 2007;39:259–261. [Google Scholar]
- 70.Abbaszadegan MR, Gholamin M, Tabatabaee A, Farid R, Houshmand M, Abbaszadegan M. Prevalence of human T-lymphotropic virus type 1 among blood donors from Mashhad, Iran. J Clin Microbiol. 2003;41:2593–2595. doi: 10.1128/JCM.41.6.2593-2595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sertöz R, Turhan A, Bozkurt H, Samhoglu P, Degirmenci A, Aydmok Y, Erensoy S. Investigation of anti-HTLV I/II seroprevalence in healthy blood donors in Izmir region, Turkey. Mikrobiyol Bui. 2010;44:579–584. [PubMed] [Google Scholar]
- 72.Naman R, Klayme S, Naboulsi M, Mokhbat J, Jradi O, Ramia S. HTLV-I and HTLV-II infections in volunteer blood donors and high-risk groups in Lebanon. ] Infect. 2002;45:29–31. doi: 10.1053/jinf.2002.1006. [DOI] [PubMed] [Google Scholar]
- 73.Brennan M, Runganga J, Barbara JA, Contreras M, Tedder RS, Garson JA, Tuke PW, Mortimer PP, McAlpine L, Tosswill JH. Prevalence of antibodies to human T-cell leukaemia/lymphoma virus in blood donors in north London. BMJ. 1993;307:1235–1239. doi: 10.1136/bmj.307.6914.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tynell E, Andersson S, Lithander E, Arneborn M, Blomberg J, Hansson HB, Krook A, Nomberg M, Ramstedt K, Shanwell A, Bjorkman A. Screening for human T cell leukaemia/lymphoma virus among blood donors in Sweden: cost effectiveness analysis. BMJ. 1998;316:1417–1422. doi: 10.1136/bmj.316.7142.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6058. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 76.Chaudhari CN, Shah T, Misra RN. Prevalence of human T cell leukaemia virus amongst blood donors. MJAFI. 2009;65:38–40. doi: 10.1016/S0377-1237(09)80052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maeda Y, Furukawa M, Takehara Y, Yoshimura K, Miyamoto K, Matsuura T, Morishima Y, Tajima K, Okochi K, Hinuma Y. Prevalence of possible adult T-cell leukemia virus-carriers among volunteer blood donors in Japan: a nation-wide study. Int J Cancer. 1984;33:717–720. doi: 10.1002/ijc.2910330602. [DOI] [PubMed] [Google Scholar]
- 78.Rezvan H, Abolghassemi H, Amini Kafiabad S. Transfusion-transmitted infections among multi-transfused patients in Iran: a review. Transfus Med. 2007;17:425–433. doi: 10.1111/j.1365-3148.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 79.Petersen LR, Roehrig JT. West Nile virus: a reemerging global pathogen. Emerg Infect Dis. 2001;7:611–614. doi: 10.3201/eid0704.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Komar O, Robbins MB, Klenk K, Blitvich BJ, Marle-nee NL, Burkhalter KL, Gubler DJ, Gonzàlvez G, Pena CJ, Peterson AT, Komar N. West Nile virus transmission in resident birds, Dominican Republic. Emerg Infect Dis. 2003;9:1299–1302. doi: 10.3201/eid0910.030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 82.Fereidouni SR, Ziegler U, Linke S, Niedrig M, Modir-rousta H, Hoffmann B, Groschup MH. West Nile virus monitoring in migrating and resident water birds in Iran: are common coots the main reservoirs of the virus in wetlands? Vector Borne Zoonotic Dis. 2001;1:1377–1381. doi: 10.1089/vbz.2010.0244. [DOI] [PubMed] [Google Scholar]
- 83.Saidi S. Viral antibodies in preschool children from the Caspian area, Iran. Iranian J Pubi Health. 1974;3:83–91. [Google Scholar]
- 84.Saidi S, Tesh R, Javadian E, Nadim A. The prevalence of human infection of West Nile virus in Iran. Iranian J Pubi Health. 1976;5:8–14. [Google Scholar]
- 85.Hollinger FB, Kleinman S. Transfusion transmission of West Nile virus: a merging of historical and contemporary perspectives. Transfusion. 2003;43:992–997. doi: 10.1046/j.1537-2995.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 86.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–459. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 87.Pezzotti P, Piovesan C, Barzon L, Cusinato R, Cattai M, Pacenti M, Piazza A, Franchin E, Pagni S, Bressan S, Martello T, Potenza R, Scipioni C, Ammendola R, Breda A, Palu G, Russo F, Rezza G. Prevalence of IgM and IgG antibodies to West Nile virus among blood donors in an affected area of north-eastern Italy, summer 2009. Euro Surveill. 2011;16 doi: 10.2807/ese.16.10.19814-en. pii:19814. [DOI] [PubMed] [Google Scholar]
- 88.Ergünay K, Saygan MB, Aydoğan S, Menemenlioglu D, Turan HM, Özkul A, Us D. West Nile virus seroprevalence in blood donors from central Anatolia, Turkey. Vector Borne Zoonotic Dis. 2010;10:771–775. doi: 10.1089/vbz.2009.0130. [DOI] [PubMed] [Google Scholar]
- 89.Alfaresi M, Elkoush A. West Nile virus in the blood donors in UAE. Indian J Med Microbiol. 2008;26:92–93. doi: 10.4103/0255-0857.38875. [DOI] [PubMed] [Google Scholar]
- 90.Pealer LN, Martin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, Goodman JL, Chamberland ME. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 91.Sharifi Z, Mahmoodian Shooshtari M, Talebian A. A study of West Nile virus infection in Iranian blood donors. Arch Iran Med. 2010;13:1–4. [PubMed] [Google Scholar]
- 92.Kojima H, Kaita KD, Zhang M, Giulivi A, Minuk GY. Genomic analysis of a recently identified virus (SEN virus) and genotypes D and H by polymerase chain reaction. Antiviral Res. 2003;60:27–33. doi: 10.1016/s0166-3542(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 93.Akiba J, Umemura T, Alter HJ, Kojiro M, Tabor E. SEN virus: epidemiology and characteristics of a transfusion-transmitted virus. Transfusion. 2005;45:1084–1088. doi: 10.1111/j.1537-2995.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 94.Sagir A, Kirschberg O, Heintges T, Erhardt A, Häussinger D. SEN virus infection. Rev Med Virol. 2004;14:141–148. doi: 10.1002/rmv.421. [DOI] [PubMed] [Google Scholar]
- 95.Sharifi Z, Mahmoodian-Shooshtari M, Talebian A. The prevalence of SEN virus infection in blood donors in Iran. Arch Iranian Med. 2008;11:423–426. [PubMed] [Google Scholar]
- 96.Ghasemi Dehkordi P, Doosti A. The prevalence of SEN virus infection in blood donors and chronic hepatitis B and C patients in Chaharmahal Va Bakhtiari Province. J Cell Anim Biol. 2011;5:182–186. [Google Scholar]
- 97.Karimi-Rastehkenari A, Bouzari B. High frequency of SEN virus infection in thalassemic patients and healthy blood donors in Iran. Virol J. 2010;7:1–7. doi: 10.1186/1743-422X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Umemura T, Yeo AE, Sottini A, Moratto D, Tanaka Y, Wang RY, Shih JW, Donahue P, Primi D, Alter HJ. SEN virus infection and its relationship to transfusion-associated hepatitis. Hepatology. 2001;33:1303–1311. doi: 10.1053/jhep.2001.24268. [DOI] [PubMed] [Google Scholar]
- 99.Shibata M, Wang RY, Yoshiba M, Shih JW, Alter HJ, Mitamura K. The presence of a newly identified infectious agent (SEN virus) in patients with liver diseases and in blood donors in Japan. J Infect Dis. 2001;184:400–404. doi: 10.1086/322050. [DOI] [PubMed] [Google Scholar]