Abstract
Host species have evolved mechanisms that can inhibit pathogen replication even after a cell has been successfully invaded. Here we show that tripartite-motif protein 21 (TRIM21), a ubiquitously expressed E3 ubiquitin ligase that targets viruses inside the cytosol, protects mice against fatal viral infection. Upon infection with mouse adenovirus-1, naive mice lacking TRIM21 succumb to encephalomyelitis within 7 d. In contrast, wild-type mice rapidly up-regulate TRIM21 and control viremia. Trim21 heterozygous mice have a haploinsufficiency phenotype in which reduced TRIM21 expression leads to a viral load that is higher than wild types but lower than knockouts. TRIM21 is a high-affinity antibody receptor that allows antibodies to operate inside an infected cell. In passive transfer experiments at high viral dose, antisera that fully protects wild-type mice fails to protect most Trim21 knockout animals. These results demonstrate that TRIM21 provides potent antiviral protection and forms an important part of the humoral immune response.
Despite surveillance by professional leukocytes, many viruses successfully infect their target cells. There is therefore a need for antiviral mechanisms that operate inside infected cells to inhibit pathogen replication. A key problem facing any such intracellular mechanism is how to detect and target viruses, given their antigenic diversity and capacity for change. The only known proteins that can target viruses and keep pace with their constant evolution are antibodies, yet these are considered to be purely extracellular in their location. Recently, we showed that antibodies that attach to viruses before infection are brought inside the cell during viral entry and that these antibody-virus complexes are detected by TRIM21, a high-affinity mammalian antibody receptor (1–3). TRIM21 interaction with IgG is highly conserved both within and between different mammalian species. For instance, mouse TRIM21 is capable of binding human IgG and vice versa (2). Upon binding to antibody–virus complexes, TRIM21 flags them for rapid degradation in a process that is dependent upon the proteasome and the AAA ATPase VCP (4), thereby preventing viral replication. This combination of humoral immune targeting and innate immune activity has the capacity to provide important protection against viral infection (5). However, these responses are classically considered to work at different stages during the infection cycle. Furthermore, although we have observed potent antiviral activity in vitro, these experiments are carried out in the absence of cell-mediated immunity. Here we investigate the importance of TRIM21 in protecting against viral infection in the context of whole-animal immunity.
Mouse adenovirus 1 (MAV-1) causes dose-dependent fatal encephalomyelitis in C57BL/6 mice (6). Survival from acute infection is highly dependent on the humoral immune response. Antibody-deficient mouse strains such as btk−/−, Jh, and µMT are particularly susceptible to disease, having increased viral loads in the brain and other organs and increased mortality (7). Passive transfer of neutralizing antibodies is sufficient to protect btk−/− mice and reduce viremia in a RAG−/− strain (7). In contrast, depletion of natural killer cells has a limited affect on both viremia and survival (8), whereas mouse strains lacking natural killer cells, CD4+ T cells, CD8+ T cells, macrophages, perforin, or MHC class I or II all-clear MAV-1 infection (9, 10). Given the importance of antibody immunity in MAV-1 infection, we chose MAV-1 as a model pathogen to investigate the role of TRIM21 in protecting animals against viral infection.
Results
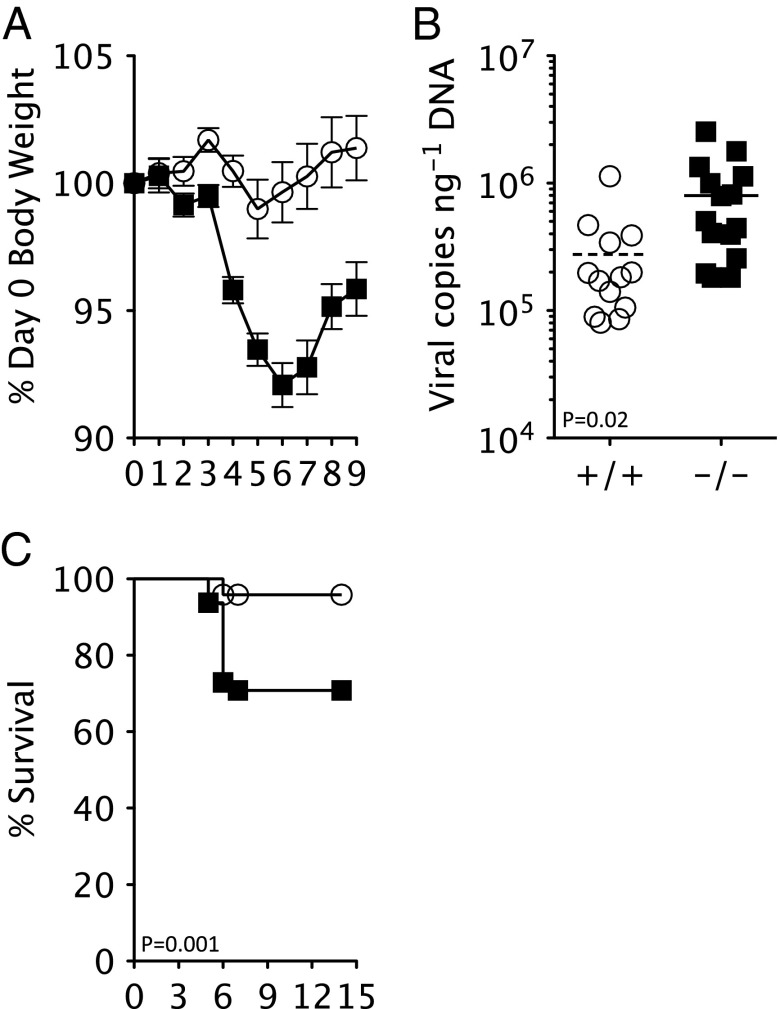
The importance of TRIM21 in virus pathophysiology was investigated by comparing in vivo infection of C57BL/6 (T21+/+) and knockout Trim21 C57BL/6 mice (11) (T21−/−) with MAV-1, a virus that causes dose-dependent hemorrhagic encephalomyelitis (6). Upon infection with 360 tissue culture infectious dose units (TCID50) of MAV-1, T21−/− mice rapidly lost body weight (Fig. 1A) and began to display clinical signs associated with MAV-induced encephalomyelitis, including hunching, ataxia, and spastic paralysis (12). This was in contrast to relatively stable body weights and fewer clinical signs in T21+/+ animals. MAV-induced encephalomyelitis is the result of viral replication in the brain, leading to damage of the endothelium and dysfunction of the blood–brain barrier (12). Weight loss in T21−/− mice was accompanied by significantly higher viremia in the brain and good correlation was observed between these two measures of infection (Fig. 1B and Fig. S1). Terminal infection occurred in ∼25% of T21−/− mice after 4–6 d, whereas end points were reached in only 2 of 48 T21+/+ animals tested (Fig. 1C). Thus, genetic ablation of Trim21 results in a highly statistically significant (P < 0.001) survival defect in mice upon naive infection with MAV-1.
Fig. 1.
MAV-1 causes fatal infection in naive TRIM21−/− mice. (A) Change in body weight upon infection of T21+/+ (white circles) and T21−/− (black squares) mice with 360 TCID50 MAV-1. (B) Brain viral copies at day 7 postinfection in T21+/+ (n = 13) and T21−/− (n = 14). (C) Survival of T21+/+ (n = 48) and T21−/− (n = 47) mice.
To understand how TRIM21 is able to provide such rapid antiviral protection, we examined viral load, IFN-γ induction, and TRIM21 production in the brain during the first week of infection. We observed rapid up-regulation of IFN-γ transcripts and this was closely matched by a corresponding increase in TRIM21 (Fig. 2A). This result correlates with the observed IFN induction of TRIM21 in vitro (3). Viremia was found to increase until day 5, then rapidly decline, decreasing by ∼50-fold by day 7. Peak viremia therefore closely matches onset of mortality, whereas its decline follows strong induction of IFN-γ and TRIM21. The relationship between viremia and cytokine induction prevents meaningful comparison of inflammatory responses between genotypes because T21−/− mice have a higher viral load than T21+/+. However, both genotypes induce a broadly similar pattern of inflammatory cytokines upon infection (Fig. 2B), in agreement with published studies on LPS treatment (11).
Fig. 2.
Early immune response to MAV-1 infection. (A) Fold induction of TRIM21 (black squares) and IFN-γ (black circles) transcripts and viral copies (black triangles) in the brains of T21+/+ mice infected with MAV-1 during the first week of infection. (B) Fold induction of inflammatory cytokines in T21+/+ (white circles) and T21−/− (black squares) mice by day 9.
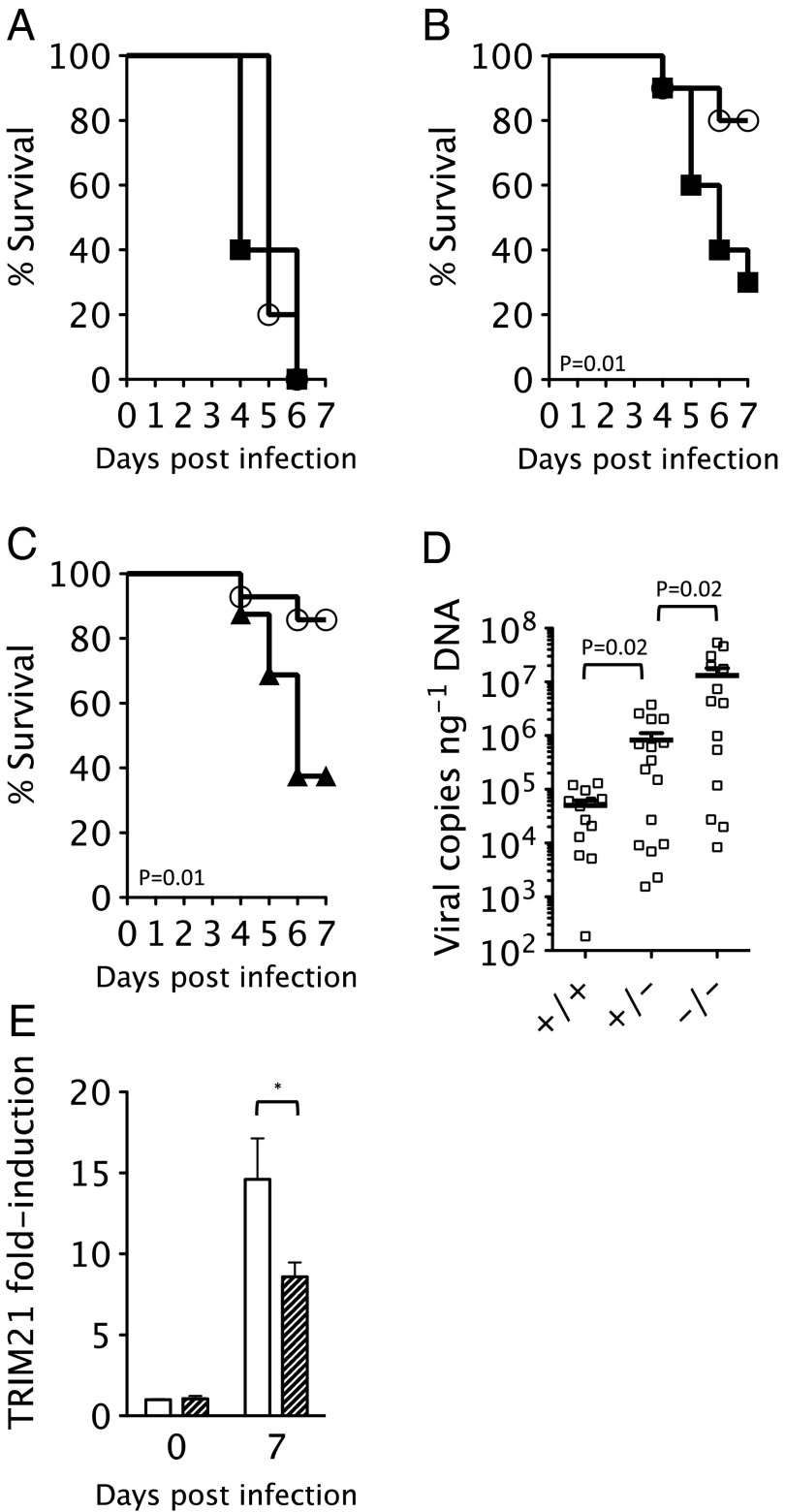
The antiviral activity of TRIM21 in vitro is antibody-mediated (3). To confirm that TRIM21 immunity in vivo is similarly antibody-dependent, we challenged mice with a lethal viral dose and tested the ability of either early (9 d) or late antiserum (72 d) to protect against infection. At a viral dose of 3.6 × 103 TCID50, all mice of both genotypes reached clinical endpoints by 6 d postinfection (Fig. 3A). Passive transfer of early antiserum at days −1, 1, and 3 was sufficient to protect most T21+/+ animals; however, 70% of T21−/− mice still succumbed to fatal infection by day 7 (Fig. 3B). These results are consistent with previous studies demonstrating a critical role for antibodies and early B-cell function in preventing MAV-1 infection (7). However, they also suggest that although TRIM21 and antibody together provide effective immunity, neither component alone is sufficient for complete protection.
Fig. 3.
Protection by early MAV-1 antisera is dependent upon TRIM21 expression. (A) Survival of T21+/+ (white circles) and T21−/− mice (black squares) after infection with 3.6 × 103 TCID50 of MAV-1. (B) Survival of T21+/+ (n = 14) and T21−/− (n = 15) mice upon infection with 3.6 × 103 TCID50 MAV-1 in the presence of passively transferred early antisera. (C) Survival of T21+/+ (n = 14) and T21+/− (n = 16) mice (black triangles) upon infection with 3.6 × 103 TCID50 MAV-1 in the presence of passively transferred early antisera. (D) Brain viral copies in T21+/+, T21+/−, and T21−/− mice after infection with 3.6 × 103 TCID50 MAV-1 in the presence of early antisera. (E) Induction of TRIM21 in T21+/+ (white bar) or T21+/− (hatched bar) after challenge with 3.6 × 103 TCID50 MAV-1 and early antisera 7 d postinfection.
Repeating the passive transfer experiments in Trim21 heterozygous animals (T21+/−) resulted in a similar survival profile to the homozygous knockouts (Fig. 3C). To investigate this more closely, we measured viremia and TRIM21 levels in the different genotypes 7 d postinfection. Viremia was >250-fold higher in knockout animals than in T21+/+, with heterozygotes almost exactly in between (16-fold more virus than T21+/+ but 16-fold lower than T21−/−) (Fig. 3D). The intermediate viremia phenotype of the T21+/− mice closely correlates with TRIM21 induction, because heterozygous animals produce approximately half as much TRIM21 transcript as T21+/+ animals (Fig. 3E). These data suggest that heterozygous mice are haploinsufficient, producing sufficient TRIM21 to reduce viremia but not enough for animal survival. This finding agrees with in vitro data in which efficiency of TRIM21-mediated virus neutralization was shown to be proportional to the level of TRIM21 expression in target cells (13).
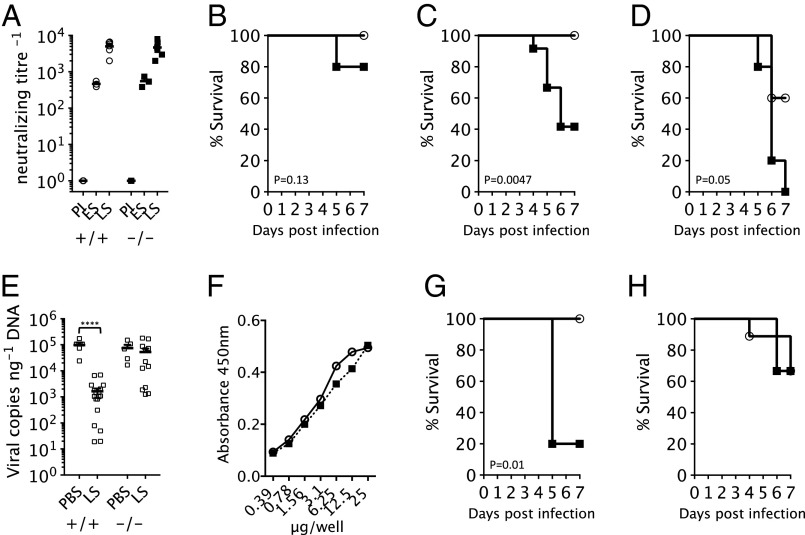
Vaccination can provide protective immunity through the induction of potently neutralizing antibodies (14–16). Conversely, antibody deficiencies result in increased acute and persistent infection (17). To investigate the role of TRIM21 in protective immunity, we repeated the high-dose viral experiments using late antisera collected from mice 72 d postinfection. As expected, the neutralizing titer of late antisera was significantly higher than early antisera; therefore, a range of late serum dilutions were tested for their ability to protect against MAV-1 infection in vivo. Of note, we observed no significant difference in the titer of antibodies produced in T21+/+ or T21−/− mice, indicating that although TRIM21 uses antibodies, it is not essential for their production (Fig. 4A). Passive transfer on days −1 and 3 of late antisera diluted 1/1,280 was sufficient to mediate complete protection of T21+/+ animals, whereas in 20% of T21−/− mice, MAV-1 was still fatal (Fig. 4B). A more dramatic difference was observed at 1/2,560 dilution, which resulted in complete survival of T21+/+ but 60% mortality of T21−/− mice (Fig. 4C). At a further dilution of 1/10,240, antiserum began to lose efficacy in T21+/+ animals (60% survival) and was completely ineffective in T21−/− mice (0% survival) (Fig. 4D). These results indicate that TRIM21 has an important role in protective immunity. Furthermore, the data support the hypothesis that TRIM21 is synergistic with, rather than independent of, antibodies because the difference in survival between genotypes is dependent upon antibody dose. In particular, a dose–response is observed wherein the protection mediated by less concentrated antiserum is more dependent upon TRIM21. This correlates with our observations in vitro that TRIM21 is able to use very few antibody molecules to prevent infection (13).
Fig. 4.
Late MAV-1 antisera reduces viremia and protects T21+/+ but not T21−/− mice. (A) Neutralizing titer of preimmune (PI), early antisera (ES), and late antisera (LS) produced by T21+/+ (circles) and T21−/− (squares) mice. (B–D) Survival upon infection with 3.6 × 103 TCID50 MAV-1 in the presence of passively transferred LS at dilutions of 1/1,280 (B), 1/2,560 (C), or 1/10,240 (D). n = 5 for each genotype except in C, where n = 12. (E) Brain viral copies in T21+/+ and T21−/− mice after infection with 3.6 × 103 TCID50 MAV-1 in the presence of PBS or LS. (F) Binding of pooled serum antibody (squares) or F(ab′)2 fragments (circles) to MAV-1 virus as detected by ELISA. (G and H) Survival upon infection with 3.6 × 103 TCID50 MAV-1 in the presence of passively transferred pooled serum antibody (G) or F(ab′)2 fragments (H) at 1 mg/mL or 0.1 mg/mL, respectively.
TRIM21 localizes to antibody-coated viruses that have invaded the cytosol and targets them for degradation in a process that is dependent upon the proteasome and the AAA ATPase VCP (3, 4). Consistent with this mechanism, we observe higher viremia in T21−/− than T21+/+ mice in all our in vivo experiments. Controlling viremia is crucial to survival, and animals exceeding a critical threshold succumb to fatal infection. In high-dose experiments, without addition of antisera, viremia reaches a MAV-1 copy number of ∼105 per nanogram of genomic DNA in both genotypes and no mice survive. Addition of late antisera reduces viremia in T21+/+ animals ∼100-fold, allowing them to recover from infection (Fig. 4E). However, the same antisera has little effect on viral load in T21−/− animals, explaining their much higher mortality rate.
To substantiate that TRIM21 antiviral activity is antibody-dependent, we compared the protective affects of F(ab′)2 fragments in T21+/+ and T21−/− mice. F(ab′)2 fragments can interact with antigen in a similar manner to intact IgG but they lack part of the constant fragment (Fc). TRIM21 does not bind to F(ab′)2 fragments because of the missing Fc, and we have previously shown that TRIM21 cannot use F(ab)′2 to neutralize infection in vitro (3). Therefore, we would predict that F(ab′)2 fragments should give the same protection in both genotypes, unless TRIM21 possesses antiviral activity independent of antibody. To test this, we repeated high-dose MAV-1 infections and passively transferred F(ab′)2 fragments prepared from pooled human serum antibody because of the limited quantity of immune sera available. An ELISA against whole virus was used to show that pooled serum antibody contains antibodies capable of binding to virus and that F(ab′)2 fragments had no diminution of binding capability. Passive transfer of F(ab′)2 fragments resulted in the same MAV-1–induced mortality in both genotypes, whereas transfer of intact serum antibody resulted in complete survival of T21+/+ mice but 80% mortality among T21−/− mice. Although differences in serum half-life, tissue penetration, and bioavailability between IgG and F(ab′)2 prevent direct comparison of the protection offered by these different antibody variants, comparison of the genotype responses to each variant indicates that TRIM21 does not influence survival independently of antibody.
Discussion
These data show that TRIM21 is a physiologically relevant antiviral gene that prevents fatal infection by reducing viremia. The protection provided by TRIM21 is antibody-dependent, consistent with its role as an Fc receptor. This is supported by the observation that although intact IgG is more protective in wild-type than TRIM21 knockout mice, F(ab′)2 fragments, which TRIM21 cannot bind, are equally protective in both genotypes. Furthermore, the degree of difference in survival between genotypes observed in the presence of intact IgG is dependent upon antibody dose. TRIM21 expression is induced by IFN, which increases neutralization of infection in vitro (3). During in vivo MAV-1 infection, TRIM21 is rapidly up-regulated in parallel with IFN and this is followed by a decline in viremia. The degree of TRIM21 expression is crucial both to the efficiency of neutralization and the level of persistent fraction in vitro (13). In cells expressing insufficient TRIM21, there is a high level of infectivity even in the presence of saturating concentrations of neutralizing antibody. Our in vivo data correlate with these findings and the importance of robust TRIM21 expression. Heterozygous animals produce significantly less TRIM21 than wild types and in the presence of antibody have higher viremia. The TRIM21 produced in heterozygous mice appears to be functional, as these animals have lower viremia than knockouts, but it is not sufficient to prevent fatal infection in our model. This suggests that the reduced neutralization efficiency that occurs as a result of decreased TRIM21 expression can have a direct effect on animal survival.
The system of protection mediated by TRIM21 is difficult to classify using traditional definitions because it is mediated neither by professional cells nor is it cell-autonomous. Similarly, the immunity it provides is neither wholly innate nor adaptive and appears to be important both in a naive and protective context. It is a system that uses antibodies to provide viral targeting and an IFN-induced receptor to mediate an effector response. TRIM21 is distinct from other antibody effectors in that it functions intracellularly rather than extracellularly and is expressed in most histogenetic cell lineages rather than solely in professional leukocytes. One consequence of this difference may be that each infection event, independent of cell type, could present an opportunity for TRIM21-mediated neutralization, in contrast to the surveillance-based protection afforded by professional immunity. It is important to note, however, that TRIM21 is likely to be effective only against nonenveloped viruses because enveloped viruses may shed their antibodies alongside their envelope proteins during membrane fusion. TRIM21 may also vary in its effectiveness against nonenveloped viruses because of differences in viral entry and cellular tropism. Although TRIM21 is widely expressed, some areas of the body have restricted antibody access. For instance, there is a lower concentration of antibodies in the brain than in serum. In this regard, the breakdown of the blood–brain barrier mediated by MAV-1 may be significant because it might contribute to the susceptibility of the virus to antibodies (18). It is also noteworthy that the deletion of Trim21 does not impair the survival of C57BL/6 mice to the same degree as ablating antibodies all together, as in the Btk−/− mouse (7). This suggests that other antibody receptors or antibody-dependent processes provide a significant contribution to survival. It is possible that there are other intracellular antibody receptors involved, in addition to TRIM21. Finally, it is tempting to speculate that other immune proteins, such as complement and acute-phase proteins, could act in a similar manner to antibodies and mediate intracellular immune responses.
Experimental Procedures
Cell Lines and Viruses.
MAV-1 (ATCC) was prepared by two rounds of CsCl centrifugation as previously described (3), and infectious titer was determined by endpoint dilution assay in 3T3 cells. TCID50 was calculated by the Reed–Muench method (19).
Experimental Infections.
C57BL/6 wild-type (T21+/+) and TRIM21-deficient mice (T21−/−) were obtained from Jackson Laboratories. Seven- to 10-wk-old mice were used in infection experiments, which were conducted in accordance with the 19.b.7 moderate severity limit protocol and Home Office Animals (Scientific Procedures) Act (1986). All animal work was licensed under the UK Animals (Scientific Procedures) Act, 1986 and approved by the Medical Research Council Animal Welfare and Ethical Review Body. Throughout the protocol, animals were weighed and observed twice daily for clinical signs of infection, which included subdued behavior, pilo-erection, hunched posture, ataxia, and paresis. Animals that reached the end of the experiment, lost more than 20% of initial body weight, or showed clinical signs that failed to improve over a 6-h period were killed. Brains were collected and snap frozen in liquid nitrogen. Serum was prepared from intracardiac blood and frozen in liquid nitrogen.
For naive infection experiments, T21+/+ and T21−/− mice were infected by i.p. injection with 360 TCID50 MAV‐1 in 100 μL PBS. In passive transfer of mice sera experiments, T21+/+ and T21−/− mice were i.p. injected with either 100 μL of pooled early immune serum, collected 9 d post MAV-1 challenge, or 200 μL late immune serum, collected after three rounds of immunization with MAV-1, at the appropriate dilution or PBS (control group) on days −1, 1, and 3 (early serum transfer experiment) or days −1 and 3 (late serum transfer experiment) after infection with 3.6 × 103 TCID50 MAV‐1. In passive transfer of pooled human IgG or F(ab′)2 experiments (Athens R&T), T21+/+ and T21−/− mice were i.p. injected with either 1 mg of intact serum antibody or 0.1 mg F(ab′)2 fragment in 100 μL PBS on days −1, 0, 3, 5 (intact) or days −1, 0, 1, 3, and 5 F(ab′)2 postinfection with 3.6 × 103 TCID50 MAV‐1.
Genomic DNA Isolation and RNA Preparation.
Frozen brains were split along the sagittal fissure and homogenized in 200 μL PBS using a Tissue Ruptor (Qiagen). DNA was purified from 50 μL of the homogenate using QIAamp DNA mini kit (Qiagen). A total of 100 ng of total DNA was used per quantitative PCR (qPCR) reaction. To measure gene expression levels, 80-mg sections of forebrain were cut from frozen brains. Total RNA was isolated using the RNeasy Lipid Tissue mini kit (Qiagen) according to the manufacturer’s protocol. cDNA was prepared by reverse transcription using SuperScript III according to the manufacturer’s recommended protocol (Invitrogen). A total of 1 μL of the total 20 μL RT product was used per qPCR reaction.
Neutralizing Antibody Assay.
T21+/+ C57BL/6 mouse embryonic fibroblast (MEF) cells were plated at 2 × 104 per well in 96-well plates and allowed to adhere overnight. Heat-inactivated mouse sera were diluted twofold in 2,000 TCID50 of MAV-1 per milliliter and incubated for 1 h at 37 °C. Media were removed from the cells and 25 μL of the virus–serum mixtures was added to wells in quintuplicate. Virus was allowed to adsorb for 1 h at 37 °C. DMEM with 1% heat-inactivated calf serum was added and the wells were monitored over 14 d for cytopathic effect. Antibody neutralizing titer was calculated as described (19).
MAV-1 ELISA.
Streptavidin-coated plates (Roche) were incubated with biotinylated MAV-1 followed by blocking with 2% (wt/vol) Marvell in PBS (PBSM). Pooled human IgG or F(ab′)2 dilutions in PBSM were incubated on the plate for 1.5 h. MAV-1–specific antibodies were detected with goat anti-human IgG F(ab′)2-HRP (AbD serotec).
qPCR and RT-qPCR.
All qPCR and RT-qPCR reactions were done using a StepOne Plus Real Time PCR machine (ABI). qPCR and RT‐qPCR reactions were set up in 20 μL total volume, with 10 μL 2XTaqMan universal gene expression master mixes (ABI) and 1 μL of the following 20× gene expression assays (ABI) or primer and probe mixes (Eurofins).
MAV-1.
5′TTTTGTCCTGTGGCATTTGA (MAV-1 Hexon reverse), 5′GGCCAACACTACCGACACTT (MAV-1 Hexon forward), 5′CATTCCAGCCAACTTATGGCTCGGC (MAV-1 Hexon FAM/TAMRA probe) (TRIM21 exon 2 forward), 5′GAACTTCAGGGTCAGCTT.
Expression assays.
Mm00447364_m1 (TRIM21), Mm01336162_m1 (IL-1), Mm00445259_m1 (IL-4), Mm00446190_m1 (IL-6), Mm00443260_g1 (TNF-α), Mm00441242_m1 (CCL2), Mm03030145_gH (IFN-α), Mm00439552_s1 (IFN-β1), Mm01168134_m1 (IFN-γ), Mm00607939_s1 (Actin beta control).
Supplementary Material
Acknowledgments
We thank Michael Neuberger and Cristina Rada for technical assistance. This work was funded by UK Medical Research Council Grant U105181010 and European Research Council Grant IAI-281627.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301918110/-/DCSupplemental.
References
- 1.James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci USA. 2007;104(15):6200–6205. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci USA. 2008;105(16):6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallery DL, et al. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci USA. 2010;107(46):19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauler F, Mallery DL, McEwan WA, Bidgood SR, James LC. AAA ATPase p97/VCP is essential for TRIM21-mediated virus neutralization. Proc Natl Acad Sci U S A. 2012;109(48):19733–19738. doi: 10.1073/pnas.1210659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwan WA, Mallery DL, Rhodes DA, Trowsdale J, James LC. Intracellular antibody-mediated immunity and the role of TRIM21. Bioessays. 2011;33(11):803–809. doi: 10.1002/bies.201100093. [DOI] [PubMed] [Google Scholar]
- 6.Charles PC, Guida JD, Brosnan CF, Horwitz MS. Mouse adenovirus type-1 replication is restricted to vascular endothelium in the CNS of susceptible strains of mice. Virology. 1998;245(2):216–228. doi: 10.1006/viro.1998.9180. [DOI] [PubMed] [Google Scholar]
- 7.Moore ML, McKissic EL, Brown CC, Wilkinson JE, Spindler KR. Fatal disseminated mouse adenovirus type 1 infection in mice lacking B cells or Bruton’s tyrosine kinase. J Virol. 2004;78(11):5584–5590. doi: 10.1128/JVI.78.11.5584-5590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welton AR, Gralinski LE, Spindler KR. Mouse adenovirus type 1 infection of natural killer cell-deficient mice. Virology. 2008;373(1):163–170. doi: 10.1016/j.virol.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley SL, Welton AR, Harwood KM, Van Rooijen N, Spindler KR. Mouse adenovirus type 1 infection of macrophages. Virology. 2009;390(2):307–314. doi: 10.1016/j.virol.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore ML, Brown CC, Spindler KR. T cells cause acute immunopathology and are required for long-term survival in mouse adenovirus type 1-induced encephalomyelitis. J Virol. 2003;77(18):10060–10070. doi: 10.1128/JVI.77.18.10060-10070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimi R, et al. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J Immunol. 2009;182(12):7527–7538. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guida JD, Fejer G, Pirofski LA, Brosnan CF, Horwitz MS. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57BL/6 but not BALB/c mice. J Virol. 1995;69(12):7674–7681. doi: 10.1128/jvi.69.12.7674-7681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwan WA, et al. Regulation of virus neutralization and the persistent fraction by TRIM21. J Virol. 2012;86(16):8482–8491. doi: 10.1128/JVI.00728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edghill-Smith Y, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 15.Emini EA, et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355(6362):728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 16.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 17.Sanna PP, Burton DR. Role of antibodies in controlling viral disease: Lessons from experiments of nature and gene knockouts. J Virol. 2000;74(21):9813–9817. doi: 10.1128/jvi.74.21.9813-9817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralinski LE, Ashley SL, Dixon SD, Spindler KR. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol. 2009;83(18):9398–9410. doi: 10.1128/JVI.00954-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed LMH. A simple method for estimating fifty per cent endpoints. Am J Epidemiol. 1938;27(3):493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.