Abstract
The aryl hydrocarbon receptor (AhR) has become increasingly recognized for its role in the differentiation and activity of immune cell subsets; however, its role in regulating the activity of natural killer (NK) cells has not been described. Here, we show that AhR expression is induced in murine NK cells upon cytokine stimulation. We show that in the absence of AhR, NK cells have reduced cytolytic activity and reduced capacity to control RMA-S tumor formation in vivo, despite having normal development and maturation markers. Although AhR was first identified to bind the xenobiotic compound dioxin, AhR is now known to bind a variety of natural exogenous (e.g., dietary) and endogenous ligands. We show that activation of AhR with an endogenous tryptophan derivative, 6-formylindolo[3,2-b]carbazole, potentiates NK cell IFN-γ production and cytolytic activity. Further, administration of 6-formylindolo[3,2-b]carbazole in vivo enhances NK cell control of tumors in an NK cell- and AhR-dependent manner. Finally, similar effects on NK cell potency occur with AhR dietary ligands, potentially explaining the numerous associations that have been observed in the past between diet and NK cell function. Our studies introduce AhR as another regulator of NK cell activity in vivo.
Keywords: FICZ; kynurenine, 3,3′-diindolylmethane (DIM); indole-3-carbinol (I3C)
Variation in natural killer (NK) cell cytotoxicity among the population is well known (1), but mechanisms underlying this variation are poorly understood. Although this variation is likely to result, in part, from genetic factors, there is increasing evidence that exogenous factors may influence NK cell activity significantly. Here, we describe the critical role of the aryl hydrocarbon receptor (AhR) in modulating NK cell antitumor activity. Importantly, this receptor can bind a variety of environmental factors, which can have both agonistic and antagonistic activity (2).
AhR, a member of the Per (period circadian protein)-Arnt (aryl hydrocarbon receptor nuclear translocator protein)-Sim (single-minded protein) (PAS) superfamily of proteins, is a cytoplasmic receptor that, upon ligand binding, translocates to the nucleus and binds the aryl hydrocarbon receptor nuclear translocator (ARNT). This AhR:ARNT heterodimer binds DNA and directs transcription of genes from specific DNA sequences called xenobiotic-response elements (XREs), also known as dioxin-response elements (DREs). As implied by this name for the response elements, AhR was initially studied for its role as a receptor for environmental contaminants and toxins, the most studied of which is 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD). Over the past 30 y, it has become increasingly clear that AhR binds a large number of natural compounds found in the diet and metabolites found endogenously (2, 3).
A role for AhR in the immune system was recently brought to the forefront when it was shown that AhR has an important function in the development and differentiation of regulatory T (Treg) cells and T helper 17 (Th17) cells. AhR ligands, such as TCDD and 6-formylindolo[3,2-b]carbazole (FICZ), can promote CD4 T-cell differentiation into these different subsets in an AhR-dependent manner (4–6). The fact that AhR is expressed in a number of hematopoietic populations implies that this receptor may play an important role in the biology of other immune subsets, as well. AhR expression has most recently been reported in specialized innate lymphoid cells (ILCs) (7, 8) and has been shown to have a critical role in the development of ILCs in the intestine (9). To date, however, a functional role for AhR in circulating NK cells has never been described.
In this study, we demonstrate that AhR expression is induced in conventional splenic NK cells upon NK cell cytokine stimulation and that determinants of AhR activity, including AhR ligands found in the diet, can modulate the antitumor effector functions of this immune subset. These data may help explain the variation in NK cell cytotoxicity that is found in the population and also present a unique therapeutic strategy for enhancing NK cell antitumor function.
Results
AhR Is Required for Proper Cytolytic Activity by Conventional NK Cells upon Cytokine Stimulation.
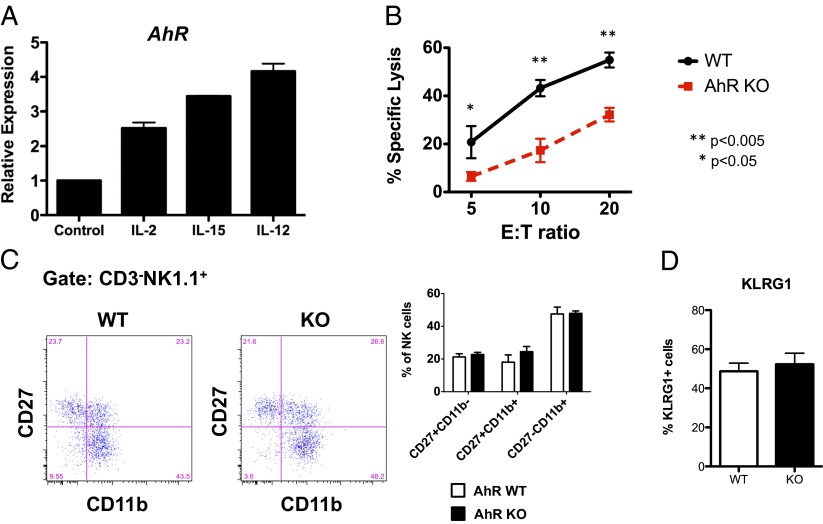
As with the IL-22 producing NK-like ILCs, NK cells also express AhR, albeit at low levels. Analysis of NK cells, purified by fluorescence-activated cell sorting (FACS) from the spleen and bone marrow (BM) of C57BL/6 (B6) mice, demonstrated that AhR is expressed in splenic NK cells more highly compared with BM NK cells (Fig. S1). Although AhR is preferentially expressed in the more immature splenic CD27+CD11b− population (Fig. S1) (10–12), surprisingly, the expression of AhR in splenic NK cells was also inducible upon stimulation ex vivo with interleukin (IL)-2, IL-15, or IL-12 (Fig. 1). Activation-induced expression of AhR in lymphocytes has been described in B cells activated by CD40 cross-linking (13), indicating that AhR activity may be able to modulate lymphocyte function at the effector level.
Fig. 1.
AhR is required for proper cytolytic activity by conventional NK cells upon cytokine stimulation. (A) Splenic murine NK cells, enriched by magnetic bead negative selection, were cultured for 24 h in the presence of the indicated cytokines. Expression of AhR was assessed by qRT-PCR and normalized to expression of Hprt. (B) Enriched splenic murine NK cells from AhR−/− and AhR+/+ mice were cultured in IL-2 (1,000 U/mL) for 7 d and then assessed for cytolytic activity against RMA-S target cells in a 4-h calcein-release assay. (C) Fresh splenic murine NK cells (NK1.1+CD3−) from AhR−/− and AhR+/+ mice were assessed for CD27 and CD11b expression by FACS (n = 3 experimental replicates). (D) Fresh splenic murine NK cells (NK1.1+CD3−) from AhR−/− and AhR+/+ mice were assessed for KLRG1 expression by FACS (n = 3–5 mice per group). Results in this figure were reproduced at least once.
To evaluate the importance of AhR in conventional NK cells, we studied two mouse lines that have deficiencies in AhR. The first is a B6 strain congenic for the dilute brown non-agouti (DBA) allele of AhR (AhRd). This allele product has a 100-fold lower affinity for ligand than the native B6 allele product (AhRb); hence, it is effectively a B6 line with a ligand-insensitive AhR. The other line is a knockout of AhR on the B6 background. Both the AhRd and the AhR−/− lines (herein, collectively called “AhR-deficient”) are fully viable.
No appreciable differences in NK cell numbers among conventional splenic NKp46+NK1.1+CD3−CD19− NK cells in the AhR-deficient mice compared with wild-type (WT) littermates were seen. Further, with the exception of a subtle increase in the percentage of NKG2A+ NK cells in AhR−/− mice, we did not observe consistent baseline differences in the expression of CD27, CD11b, CD117, Ly49, NKG2D, TRAIL, Granzyme A, Granzyme B, or the activation marker KLRG1 (Fig. 1 and Figs. S1 and S2). However, there was a significant reduction in cytotoxic activity by IL-2–activated AhR−/− NK cells (Fig. 1B). Although there was a slight skewing of the CD27 and CD11b profile of the AhR−/− NK cells after IL-2 culture (Fig. S2D), it was the CD27+CD11b+ subset that increased. This subset has been been shown to have the highest cytotoxic activity in WT NK cells (10) and, therefore, the reduced cytotoxic activity we observed in the AhR−/− NK cells does not appear to be the result of a developmental defect. These findings led us to hypothesize that AhR−/− NK cells may have reduced antitumor effector functions in vivo.
NK Cells from AhR-Deficient Mice Have an Intrinsic Defect in Tumoricidal Activity.
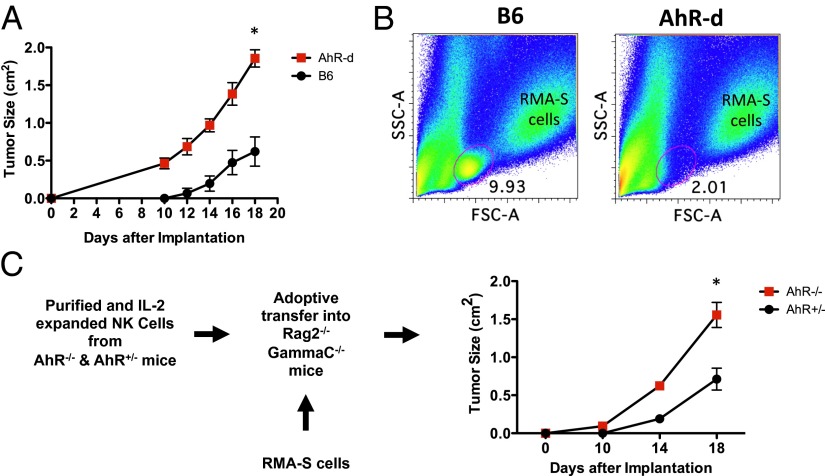
To assess the possibility that AhR is required for proper antitumor effector functions, we evaluated the ability of AhR-deficient mice to protect against RMA-S tumor growth in vivo. RMA-S is a murine lymphoma cell line that has reduced levels of MHC class I, and NK cells are both necessary and sufficient to control RMA-S tumor formation in WT mice (14). Consistent with our finding that AhR−/− NK cells have decreased cytolytic activity (Fig. 1B), we observed a defect in the ability of AhR-deficient mice to control RMA-S tumor formation (Fig. 2A).
Fig. 2.
AhR-deficient NK cells have an intrinsic defect in tumoricidal activity. (A) RMA-S cells (1 × 105) were injected into the flanks of AhRd and WT mice (n = 8 per cohort), and tumor growth was monitored (*P < 0.05). These results were reproduced at least once. (B) Tumors from A were analyzed for tumor-infiiltrating lymphocytes by FACS. (C) RMA-S cells (2 × 105) were injected into flanks of Rag2−/−GammaC−/− mice (n = 4 per cohort), then 3 × 106 IL-2 (800 U/mL) expanded and FACS-sorted NK cells were injected by tail vein 24 h later. Tumor growth is indicated (*P < 0.01).
The increased tumor growth in the AhR-deficient mice was also correlated with a decrease in the percentage of tumor-infiltrating lymphocytes (TILs) (Fig. 2B and Fig. S3D). This decrease in TILs appeared to be generalized to all subsets, because there was a decrease in T cells (CD3+NKp46−), NK cells (CD3−NKp46+), and macrophages (CD3−CD11b+F4/80+) (Fig. S3A). The decreased NK cell infiltration was not due to a consistent difference in CXCR3 expression by the NK cells; however, we observed a decrease in the CXCR3 ligands being expressed within the microenvironment of tumors grown in the AhR−/− mice (Fig. S3 B and C). Further work is needed to understand which host cells are responsible for this difference.
To assess whether this defect in RMA-S tumor control was intrinsic to NK cells, we adoptively transferred FACS-purified, IL-2 expanded NK cells, derived from the spleens of either AhR−/− or AhR+/− mice, into RMA-S tumor-bearing Rag2−/−γc−/− mice, which lack T, B, and NK cells. Similar to our earlier findings, the mice receiving AhR−/− NK cells were not able to control RMA-S tumors as well as the mice receiving AhR+/− NK cells (Fig. 2C). Altogether, we conclude that the deficiency in antitumor activity in AhR mice is due in part to an intrinsic defect in NK cell activity that includes defective infiltration into tumors and decreased cytotoxic activity.
Activation of AhR Enhances NK Cell-Dependent Inhibition of Tumor Growth.
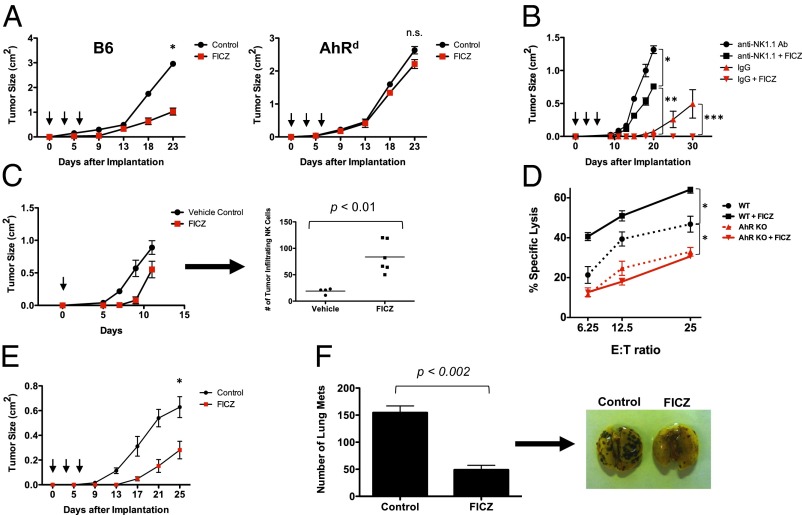
Because AhR activity can be modulated by agonistic and antagonistic ligands, we hypothesized that, in a wild-type physiologic situation, activation of AhR in vivo would enhance NK cell-mediated tumor rejection. To test this hypothesis, we used a well-characterized AhR agonist, FICZ, to activate AhR in vivo and in vitro. FICZ is a photo byproduct of tryptophan, which can be found naturally in vivo and has high, but reversible, affinity for AhR. When RMA-S tumor-bearing WT mice were administered FICZ, a significant inhibition of tumor growth was observed (Fig. 3A). The effect of FICZ on tumor growth was AhR dependent because the same inhibition was not observed in AhR-deficient mice. Importantly, the inhibition of tumor growth in the WT mice was not the result of FICZ acting directly on the RMA-S tumor cells, because RMA-S cells do not express AhR and the proliferation of RMA-S cells is not affected by FICZ when measured in vitro (Fig. S4). To determine whether the FICZ was acting through NK cells to mediate the tumor growth inhibition, we implanted RMA-S tumors into Rag1−/− mice (lacking T and B cells) that were injected with either anti-NK1.1 antibody to deplete NK cells or control IgG antibody. Cohorts of these mice were treated either with FICZ or control. Similar to our experience with WT mice, we observed significant tumor growth inhibition by FICZ in the control IgG-injected Rag1−/− mice (Fig. 3B). However, this tumor growth inhibition was largely abrogated when NK cells were depleted by anti-NK1.1 antibody injections, indicating that the effects seen with FICZ were primarily NK cell dependent. There was still a slight difference seen in the growth of tumors between the FICZ-treated and control groups among the NK cell-depleted Rag1−/− mice, suggesting that FICZ may also have effects on the myeloid compartment. This possibility is supported by evidence of AhR expression and function in macrophages and other myeloid lineages (15, 16). Nevertheless, the majority of FICZ-mediated tumor control in the Rag1−/− mice could be attributed to NK cells. Importantly, we observed an increase in the number of tumor-infiltrating NK cells within the tumors harvested from mice receiving FICZ (Fig. 3C). Further, NK cell cytotoxicity against RMA-S cells was significantly enhanced in the presence of FICZ (Fig. 3D). This effect by the compound depended on AhR, because no enhancement in cytotoxicity was observed when AhR−/− NK cells were exposed to FICZ. Thus, FICZ inhibits RMA-S tumor growth in vivo in both an AhR-dependent and NK cell-dependent manner.
Fig. 3.
Activation of AhR enhances NK cell-mediated control of tumor growth. (A) RMA-S cells (3 × 105) were implanted on WT and AhRd mice (n = 6 per cohort), then treated with FICZ (3 μg per mouse, i.p.) or vehicle control on days 0, 2, and 4 (arrows) (*P < 0.001; n.s., not significant). These results were reproduced at least once. (B) Rag1−/− mice (n = 4 per cohort) were implanted with RMA-S cells (1 × 105) and treated with anti-NK1.1 antibody (PK136) i.p. to deplete NK cells or IgG control antibody. The mice were then treated with FICZ (3 μg per mouse, i.p.) or vehicle control on days 0, 2, and 4. Tumor growth is plotted (*P < 0.002; **P < 0.0001; ***P < 0.05). (C) RMA-S tumor cells (1 × 106) were implanted into the s.c. compartment in Rag1−/− mice. FICZ was administered i.p. once at day 0. Tumors were harvested at day 12, dissociated, and analyzed by FACS for tumor-infiltrating NKp46+ cells. These results were reproduced at least once. (D) Magnetic bead-enriched NK cells from AhR−/− and AhR+/+ mice were cultured in IL-2 (1,000 U/mL) for 7 d, either with FICZ or vehicle control. The NK cells were then incubated for 4 h with RMA-S target cells in a calcein-release cytotoxicity assay (*P < 0.05). There were no statistically significant differences in cytotoxicity between the AhR KO and AhR KO + FICZ groups. Results were reproduced at least once. (E) Rag1−/− mice (n = 8 per cohort) were implanted with B16 melanoma cells (1 × 105) and treated with FICZ or vehicle control, as above (*P < 0.05). These results were reproduced at least once. (F) Rag1−/− mice (n = 3 per cohort) were injected with B16 cells (5 × 105) via tail vein and then treated with FICZ or vehicle control, as above. Lungs were harvested and fixed in Bouin’s solution, and metastatic implants were counted. Representative images of lungs are shown.
This inhibition of tumor growth by FICZ is likely to apply to other tumor types because FICZ treatment of Rag1−/− mice bearing B16 melanoma tumors led to delayed tumor growth (Fig. 3E). In addition, FICZ treatment inhibited B16 melanoma implantation into the lungs of Rag1−/− mice injected with tumor cells via the tail vein (Fig. 3F). This metastasis model has been used in the past to demonstrate the importance of NK cells in controlling blood-borne melanoma cells (17–21). Specifically, depletion of NK cells before tail vein injection of B16 cells has been shown to result in a significantly greater number of tumor implantation sites in the lungs. As seen with RMA-S tumor targets, an enhanced killing of B16 tumor cells in vitro was seen with IL-2–stimulated NK cells cultured in FICZ. Thus, the enhanced inhibition of tumor growth, and possibly metastases, by FICZ is likely to apply to epithelial type cancers in addition to hematopoietic neoplasms.
Activation of AhR Enhances NK Cell Production of IFN-γ.
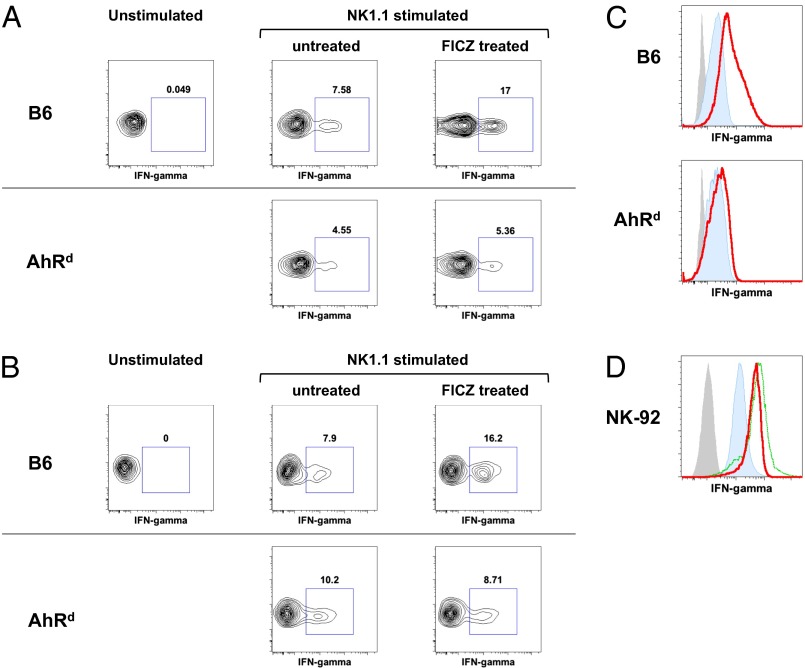
In addition to the effect of AhR activation on NK cell cytotoxicity, IFN-gamma (IFN-γ) production by NK cells was also enhanced by FICZ in an AhR-dependent manner. Splenocytes from WT mice injected with FICZ intraperitoneally (i.p.) had a greater than twofold increase in the percentage of NK cells producing IFN-γ in response to plate-bound anti-NK1.1 versus splenocytes from untreated WT mice (Fig. 4A). This effect of FICZ on IFN-γ production was not observed in the NK cells from AhRd mice, indicating FICZ’s effects are specifically mediated through AhR. Further, the enhancement of NK cell effector functions by AhR was not limited to NK1.1 stimulation. We also observed enhanced IFN-γ production and CD107 expression when NK cells were stimulated through NKG2D in the presence of FICZ. Thus, the ability of AhR activation to enhance NK cell effector functions appears to work with multiple modes of NK cell stimulation (Fig. S5).
Fig. 4.
Activation of AhR enhances NK cell IFN-γ expression. (A) Splenocytes from WT and AhRd mice, injected with FICZ (3 μg per mouse) or vehicle control 48 h earlier, were stimulated ex vivo with plate-bound anti-NK1.1 antibody, stained for intracellular IFN-γ, and analyzed by FACS. Gate, DAPI−CD3−NKP46+. (B) Splenocytes (10 × 106) from WT and AhRd mice (Ly5.2) were adoptively transferred into B6 Ly5.1 mice. The mice were then treated with FICZ (3 μg per mouse) or vehicle control. The recipient spleens were harvested 72 h later and stimulated and analyzed as in A. Gate, Ly5.2 donor cells. (C) IL-2 (400 U/mL) cultured splenic NK cells from WT and AhRd mice were treated with FICZ (200 nM) for 7 h and stained for IFN-γ. Blue, IFN-γ staining; gray, unstained; red, IFN-γ staining in presence of FICZ. (D) Human NK-92MI NK cell line was treated with FICZ (200 nM) for 7 h and stained for IFN-γ. Blue, IFN-γ staining; gray, unstained; green, IFN-γ staining in presence of PMA (200 nM); red, IFN-γ staining in presence of FICZ. Results in this figure were reproduced at least once.
The mechanism by which FICZ enhances NK cell IFN-γ production and cytotoxicity is not clear. Although there was an increase in NKG2D expression seen on AhR-activated NK cells (Fig. S2B), this increase alone cannot explain the enhanced cytotoxicity of RMA-S or B16 tumor cells, because these cells do not express NKG2D ligands. Further, we also did not observe alterations in NK cell maturation subsets that could account for the differences in effector functions (Fig. S6). We did not observe direct stimulation of NK cells by exposure to FICZ; specifically, FICZ was not able to stimulate IFN-γ production by NK cells without another source of stimulation (Fig. S6). Therefore, we hypothesize that activation of AhR by FICZ lowers the threshold for NK cell stimulation but is not able to stimulate NK cells by itself.
To determine whether FICZ is acting directly on NK cells, in a cell-intrinsic fashion, to enhance the IFN-γ production or indirectly through another cell population (which may also express AhR), we performed adoptive transfer experiments in which splenocytes from WT or AhRd mice were injected into B6 Ly5.1-congenic mice. These recipients were treated with either FICZ or vehicle control, injected i.p., and after 72 h, splenocytes were isolated and stimulated ex vivo with plate-bound anti-NK1.1 antibody. We observed that FICZ enhanced IFN-γ production upon NK1.1 cross-linking in the adoptively transferred WT NK cells but not in the AhRd NK cells. Because the recipient mice have intact immune cell compartments with the WT allele of AhR, an indirect effect of AhR stimulation by FICZ (through another cell type) would have resulted in equal enhancement of IFN-γ production by the AhRd donor-derived NK cells. The failure to enhance IFN-γ production by the adoptively transferred AhRd donor-derived NK cells indicates that FICZ is acting directly on NK cells and not indirectly through another cell population.
In support of FICZ acting directly on NK cells, we also observed that FICZ is able to enhance IFN-γ production by IL-2 cultured/activated NK cells, in which AhR expression has been induced (Figs. 1 and 4C). In addition, FICZ treatment of the human NK cell line, NK-92MI, which expresses human AhR, was also able to enhance production of IFN-γ (Fig. 4D) in the presence of IL-2. These cells already have a high baseline production of IFN-γ, probably resulting from the IL-2 necessary to maintain them in culture. However, the addition of FICZ to the culture increased the expression of IFN-γ, measured by intracellular staining and FACS, almost to a level achieved by PMA stimulation. Thus, activation of AhR in human NK cells appears to enhance IFN-γ production, similar to what we observed with mouse NK cells. This observation suggests that agonistic ligands of AhR may have a profound NK cell-mediated antitumor effect in humans.
AhR Activation by Compounds Found in the Diet.
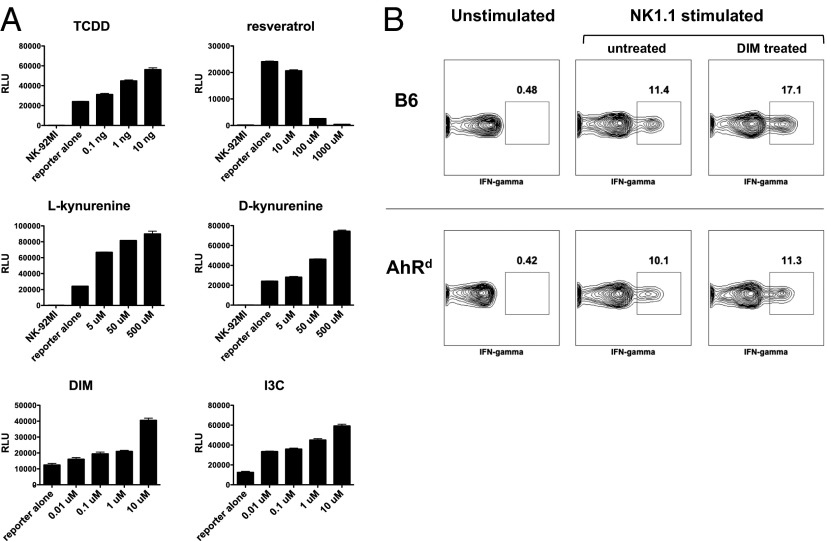
The fact that a number of dietary compounds can bind and activate AhR suggests that NK cell effector functions may be modulated by exogenous dietary compounds in this manner. To begin to investigate this possibility, we constructed an AhR luciferase-reporter cell line, using NK-92MI human NK cells (which express AhR) that were stably transfected with the luciferase gene under the control of a promoter containing multiple AhR binding XRE sites. Incubation of this cell line with various known AhR agonists—e.g., TCDD, kynurenine, 3,3′-diindolylmethane (DIM), and indole-3-carbinol (I3C)—induced luciferase expression in a dose-dependent manner (Fig. 5). Likewise, the known AhR antagonist, resveratrol, inhibited luciferase expression. Interestingly, there was a baseline expression of luciferase that was inhibited by the resveratrol, suggesting the presence of AhR agonistic ligands in the culture medium, an observation that has been made (22). Kynurenine, a tryptophan metabolite produced by indoleamine 2,3-dioxygenase (IDO) within tumors, has been shown to be an AhR agonist (23) and, thus, may serve a role in activating AhR in NK cells within the tumor microenvironment.
Fig. 5.
Dietary AhR agonists can modulate NK cell activity. (A) NK92-MI NK cell line was stably transfected with a luciferase reporter under the control of a promoter containing multiple AhR binding XRE sites. Candidate AhR ligands were incubated with the reporter cell lines, and luciferase activity was measured by colorimetric assay. (B) Splenocytes from WT and AhRd mice, injected with DIM (50 μg) i.p. or vehicle control 48 h earlier, were stimulated ex vivo with plate-bound anti-NK1.1 antibody, stained for intracellular IFN-γ, and analyzed by FACS.
To evaluate the possibility that compounds found in the diet can modulate NK cell function through AhR, we administered DIM to mice, and splenocytes were then harvested and stimulated ex vivo by plate-bound anti-NK1.1 antibody. The DIM treatment of WT mice resulted in an increase in the percent of NK cells producing IFN-γ (Fig. 5B), similar to the effects we observed with FICZ. The effect of DIM on IFN-γ production was not observed in the NK cells from AhRd mice, indicating that the DIM effects were specifically mediated through AhR.
Discussion
In this study, we have demonstrated that AhR expression is induced in conventional NK cells upon stimulation and that activation of this receptor is a critical modulator of NK cell antitumor effector functions. Importantly, our loss-of-function studies demonstrate a cell-intrinsic defect in NK cell cytolytic and antitumor activity in vitro and in vivo. Conversely, the activation of AhR significantly potentiates NK cell cytotoxicity, IFN-γ production, and in vivo control of tumor formation.
Because AhR binds a number of exogenous ligands, it is intriguing to consider the possibility that an individual’s exposure to such ligands in the environment (both agonistic and antagonistic) may modulate NK cell activity in vivo. In fact, the connection between dietary compounds, protection from tumor formation, and NK cell activity has been widely observed and studied; however, no mechanism has been proposed. Our findings that AhR can modulate NK cell effector functions are thus potentially revealing when one considers the vast number of AhR ligands found in the diet and the environment that could modulate NK cell activity. Soy products, for example, contain high levels of isoflavones, which have been shown to enhance IL-2–stimulated NK cell activity against B16 melanoma cells and have been associated with decreased incidences of prostate and breast cancer (24, 25). Another example of a dietary product that influences NK cell activity are the isothiocyanates that result from the metabolism of compounds in the family of cruciferous vegetables (26, 27), and epidemiologic data have indicated a correlation between intake of cruciferous vegetables and decreased risk of cancer development (28, 29). Importantly, both isoflavones and isothiocyanates are known ligands of AhR (2). The influence of exogenous AhR ligands on NK cell function may help explain the heterogeneity in NK cell potency found among the population and may lead to new approaches in immunotherapy.
It remains to be determined whether endogenous ligands, such as FICZ, play a physiologic role in NK cell homeostasis. Interestingly, kynurenine, the tryptophan metabolite produced by IDO in tumors, has been shown to be a potent AhR agonist (23). Although kynurenine can inhibit T cells through AhR (30), it will be important to determine whether kynurenine plays a role in activating NK cells within the tumor microenvironment.
The role of AhR in modulating the maturation and function of various immune subsets is becoming increasingly more apparent with the recently described functions of AhR in Th17 and Treg cells (4–6), dendritic cells (31), gammadelta T cells (32), and IL-22–producing innate lymphoid cells (9). Our study shows how AhR can significantly influence the effector functions of mature conventional NK cells and introduces AhR as another mechanism of NK cell regulation that should be considered in future studies of NK cell activation.
Materials and Methods
Mice.
C57BL/6, AhRd, Ahr+/−, and B6 Rag1−/− mice were obtained from Jackson Laboratory. B10;B6 Rag2−/−γc−/− mice were obtained from Taconic. AhR−/− mice were established by breeding AhR+/− mice and confirmed by the genotyping strategy outlined by the vendor. Mice were kept under specific pathogen-free conditions, and 6- to 8-wk-old mice were used for the experiments. All studies were performed in accordance with protocols approved by the Stanford University Institutional Animal Care and Use Committee.
Cell Culture.
RMA (murine T-cell lymphoma), RMA-S (RMA variant with decreased cell surface expression of major histocompatibility complex class I molecules), and p815 (lymphoma-like mastocytoma) cells were cultured in RPMI medium 1640 with 10% (vol/vol) FBS, 2 mM l-glutamine, and 2 mM nonessential amino acid (Gibco). B16 (murine melanoma) and Yac-1 cells were grown in DMEM nutrient mixture F-12 HAM (DMEM/F12; Invitrogen) supplemented with 10% (vol/vol) FBS (Gibco-BRL) at 37 °C in 5% CO2. The NK-92MI human NK cell line was cultured in alpha medium supplemented with 2 mM l-glutamine, 0.2 mM i-inositol, 20 mM folic acid, 10−4 M 2-mercaptoethanol, 12.5% (vol/vol) FBS, and 12.5% (vol/vol) horse serum (Stem Cell Technologies). Culture media were renewed every 2–3 d depending on cell density, and subculture was conducted when confluence was reached.
Establishment of s.c. and Lung Metastatic Tumors.
Mice were anesthetized with inhalational isoflurane for all tumor injections. To establish tumors in the flanks of C57BL/6 and AhRd mice, tumor cells were resuspended in 200 μL of PBS and injected into the s.c. compartment with a 25 gauge needle. Tumor formation and growth was measured every 2–3 d. To establish tumor lung metastatic implants, mice were injected with B16 melanoma cells in PBS via the tail vein. Mice were monitored up to 3–4 wk depending on symptoms and treatments received.
In Vitro Cytotoxicity Assay.
One microliter of 10 mM calcein AM solution (Invitrogen/Life Technologies) was added to 106 target cells in 2 mL of HBSS/5% FBS and incubated at 37 °C in 5% CO2 for 60 min. The loaded target cells were then washed twice with RPMI medium 1640/10% FBS and resuspended in the same medium. NK cells purified by negative selection (Stem Cell Technologies) were used as effector cells. Effector cells were mixed with 105 target cells at variable effector:target (E:T) ratios in a final volume of 100 μL by using 96-well round-bottom plates. Each E:T ratio was tested in triplicate. After cell mixing, cell suspensions were centrifuged at 300 × g for 5 min and then incubated at 37 °C in 5% CO2 for 4 h. The spontaneous release of calcein was determined by incubating loaded target cells in medium alone, and maximal release was determined by adding 0.1% Triton X (Sigma) to lyse all of the target cells. After completion of incubation, plates were centrifuged at 300 × g for 5 min, and 100 μL of supernatant from each sample was transferred to a 96-well plate (Optiplate 96F; Perkin-Elmer) and fluorescence was measured on a fluorometer (SpectraMax M3 Microplate Reader; Molecular Devices) at an excitation wavelength of 480 nm and emission wavelength of 538 nm. The median value for each triplicate was used in the calculation of cytotoxicity. Cytotoxicity, measured as percent specific release of calcein, was calculated by using the following formula: Percent specific release = (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100.
Flow Cytometry.
Antibodies to CD3 (2C11), NK1.1 (PK136), IFN-γ (XMG1.2), NKp46 (29A1.4), CD27 (LG.3A10), CD11b (M1/70), Ly5.2 (1O4), and isotype control antibodies were obtained from BD Pharmingen. Antibodies to NKG2A (16A11), TRAIL (N2B2), and NKG2D (CX5) were obtained from eBioscience. An antibody to Granzyme A (3G8.5) was obtained from Santa Cruz Biotechnology, and Granzyme B (GB11) was obtained from Invitrogen/Life Technologies. Antibodies to human IFN-γ were obtained from R&D Systems. For lymphocyte staining, cells were incubated with antibodies in FACS solution [1× PBS (Invitrogen), 2% FCS, and 2 mM EDTA] on ice for 30 min. They were then washed three times with FACS solution, followed by flow cytometry analysis (FACSAriaII; BD Biosciences). For intracellular cytokine staining, cells first stained with antibodies to surface markers and were then permeabilized and fixed by using Cytofix/Cytoperm (BD Pharmingen) according to the manufacturer's instructions. Cells were washed and incubated with antibodies to intracellular targets for 30 min on ice and then washed and analyzed by flow cytometry.
Cytokine Stimulation Assay.
Anti-NK1.1 antibody (PK136; 1 μg/mL) were bound on plastic (six-well plates) overnight in PBS at 4 °C. Spleen lymphocytes (1 × 106 cells per well) isolated from mice were stimulated for 7 h in the presence of 1 μL/mL Golgi-stop (BD Pharmingen). Cells were subsequently stained with antibodies surface markers, then fixed, permeabilized, and incubated with anti–IFN-γ antibody with the Cytofix/Cytoperm kit (BD Pharmingen) according to the manufacturer’s instructions before flow cytometric analysis.
Quantitative RT-PCR.
Total RNA was extracted from cells by using the Qiagen RNeasy mini kit (Qiagen). First-strand cDNA (cDNA) was synthesized from 50 to 500 ng of RNA by using SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative real-time RT-PCR (qRT-PCR) was performed by using a validated TaqMan Gene Expression Assay (Applied Biosystems) in accordance with the manufacturer's instructions. Assays were performed in triplicate on an Applied Biosystems 7900HT system. All human and mouse primers were purchased from Applied Biosystems. The expression of genes was normalized to the housekeeping Hprt gene. Change in expression (fold) for each gene was calculated as 2−Δ(ΔCT) where ΔCT = CT (target) − CT (housekeeping), and Δ(ΔCT) = ΔCT (treated) − ΔCT (control). Reaction product purity was confirmed by examination of melting curves for a single peak.
Luciferase Assay.
To make a stable reporter cell line, NK92MI cells were infected with lentivirus containing Cignal XRE Reporter (SABiosciences) and selected with 1 μg/mL puromycin (Invitrogen) according to manufacturer’s protocol. For reporter gene analysis, NK92MI reporter cells (1 × 105) were plated onto 96-well plates and stimulated with variable concentrations of ligands. FICZ was purchased from Biomol-Enzo Life Sciences and all other ligands (TCDD, Resveratrol, l-kynurenine, d-kynurenine, DIM, and I3C) were purchased from Sigma-Aldrich. Cells were harvested at 7 h or 18 h after treatment and firefly luciferase activity was measured by using a Dual-Luciferase Assay System (Promega). The fluorescence intensity was measured by using FLUOstar OPTIMA (BMG Labtech).
Statistics.
For statistical comparison between groups, the paired two-tailed Student t test was used. Analyses were performed by using the statistics tools of Microsoft Excel. Mean values are shown unless otherwise indicated. Errors and error bars represent SD unless otherwise stated. Differences that have P values <0.05 is considered significant.
Supplementary Material
Acknowledgments
We thank all members of the J.B.S. laboratory and Sungjin Kim and Ravi Uppaluri for their invaluable advice throughout the project. J.B.S. is supported by National Institutes of Health Grant R01CA158516, a Stanford Cancer Center 2010 Developmental Cancer Research Award Grant, and by gifts from Kathy Knudsen, the John and Jill Freidenrich Foundation, and the Harold Simmons Foundation. L.Z. is supported by the National Science Foundation Graduate Research Fellowship. O.M,-S. is supported by the Tobacco Related Diseases Research Program. J.D.B. is supported by a grant from the Cancer Research Institute, National Institutes of Health Grants K08CA128893 and R01CA157885, American Cancer Society Grant ACS-IRG 70-002, Cancer Research Coordinating Committee Grant 6-34384, the Concern Foundation, and The Hartwell Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302856110/-/DCSupplemental.
References
- 1.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 4.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 6.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 7.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes T, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32(6):803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 11.Watt SV, Andrews DM, Takeda K, Smyth MJ, Hayakawa Y. IFN-gamma-dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J Immunol. 2008;181(8):5323–5330. doi: 10.4049/jimmunol.181.8.5323. [DOI] [PubMed] [Google Scholar]
- 12.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68(20):8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 13.Allan LL, Sherr DH. Constitutive activation and environmental chemical induction of the aryl hydrocarbon receptor/transcription factor in activated human B lymphocytes. Mol Pharmacol. 2005;67(5):1740–1750. doi: 10.1124/mol.104.009100. [DOI] [PubMed] [Google Scholar]
- 14.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413(6852):165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013;25(6):335–343. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- 16.Platzer B, et al. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J Immunol. 2009;183(1):66–74. doi: 10.4049/jimmunol.0802997. [DOI] [PubMed] [Google Scholar]
- 17.Richie JP. Abrogation of hematogenous metastases in a murine model by natural killer cells. Surgery. 1984;96(2):133–138. [PubMed] [Google Scholar]
- 18.Gorelik E, Wiltrout RH, Okumura K, Habu S, Herberman RB. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J Cancer. 1982;30(1):107–112. doi: 10.1002/ijc.2910300118. [DOI] [PubMed] [Google Scholar]
- 19.Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980;65(5):929–935. [PubMed] [Google Scholar]
- 20.Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97(6):2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezrich JD, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo TL, et al. Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J Nutr. 2001;131(12):3251–3258. doi: 10.1093/jn/131.12.3251. [DOI] [PubMed] [Google Scholar]
- 25.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 26.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36(5):377–383. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(9):733–748. [PubMed] [Google Scholar]
- 29.Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr. 2008;47(Suppl 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 30.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 31.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182(11):6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 32.Kadow S, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J Immunol. 2011;187(6):3104–3110. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.