Abstract
tRNA modifications are crucial to ensure translation efficiency and fidelity. In eukaryotes, the URM1 and ELP pathways increase cellular resistance to various stress conditions, such as nutrient starvation and oxidative agents, by promoting thiolation and methoxycarbonylmethylation, respectively, of the wobble uridine of cytoplasmic 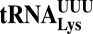 (tKUUU),
(tKUUU), 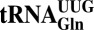 (tQUUG), and
(tQUUG), and 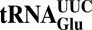 (tEUUC). Although in vitro experiments have implicated these tRNA modifications in modulating wobbling capacity and translation efficiency, their exact in vivo biological roles remain largely unexplored. Using a combination of quantitative proteomics and codon-specific translation reporters, we find that translation of a specific gene subset enriched for AAA, CAA, and GAA codons is impaired in the absence of URM1- and ELP-dependent tRNA modifications. Moreover, in vitro experiments using native tRNAs demonstrate that both modifications enhance binding of tKUUU to the ribosomal A-site. Taken together, our data suggest that tRNA thiolation and methoxycarbonylmethylation regulate translation of genes with specific codon content.
(tEUUC). Although in vitro experiments have implicated these tRNA modifications in modulating wobbling capacity and translation efficiency, their exact in vivo biological roles remain largely unexplored. Using a combination of quantitative proteomics and codon-specific translation reporters, we find that translation of a specific gene subset enriched for AAA, CAA, and GAA codons is impaired in the absence of URM1- and ELP-dependent tRNA modifications. Moreover, in vitro experiments using native tRNAs demonstrate that both modifications enhance binding of tKUUU to the ribosomal A-site. Taken together, our data suggest that tRNA thiolation and methoxycarbonylmethylation regulate translation of genes with specific codon content.
Keywords: translation regulation, SILAC, systems biology
To date, over 100 modifications of the four canonical RNA nucleotides have been described (1, 2). However, their biological relevance remains poorly understood. In yeast, over 25 distinct nucleotide modifications have been identified in tRNA molecules. Modifications at or near the anticodon are thought to be functionally important for translational efficiency and fidelity (3). In particular, the wobble position, residue 34, has emerged as a hot spot for modifications that restrict or increase wobbling (4, 5).
Uridine-34 (U34) is universally modified to 5-methyl-2-thio derivatives in the tRNAs encoding for lysine, glutamine, and glutamic acid, namely tKUUU, tQUUG, and tEUUC, respectively (6). Specifically, in yeast and higher eukaryotes, these tRNAs contain a 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U34). These modified tRNAs belong to split codon box families in which the synonymous A- and G-ending codons code for a different amino acid than U- and C-ending ones. In vitro, tRNAs containing either the s2 or the mcm5 modification on U34 are able to bind and translocate in ribosomes primed with A- and G-ending codons, but not U- and C-ending ones (7, 8). However, accurate protein translation in vivo also relies on correct charging by the aminoacyl-tRNA synthetases (9), tRNA gene copy numbers, and tRNA posttranscriptional modifications (10). Therefore, approaches to measure the in vivo relevance of RNA modifications and compare it to in vitro results are necessary to integrate these multifactorial contributions.
In yeast, the mcm5s2U34 modification relies on the URM1 pathway for the s2 (11–15) and on the ELP pathway for the mcm5 addition (16). The URM1 pathway contains the ubiquitin related modifier 1 (Urm1), its activating enzyme (Uba4), as well as the two proteins Needs Cla4 to Survive 2 and 6 (Ncs2 and Ncs6, respectively), and ThioUridine Modification protein 1 (Tum1), (12). The ELP pathway includes the six subunits of the Elp-complex (Elp1–6) and the TRna Methyltransferase complex containing Trm9 and Trm112 (16). Although thiolation is found exclusively on tKUUU, tQUUG, and tEUUC, Elp-dependent modifications are found on eight additional tRNAs.
Yeast cells lacking a functional URM1 or ELP pathway are sensitive to a wide range of drugs, suggesting a regulatory role of these modifications in translation under stress conditions. Moreover, U34 hypomodification of cytoplasmic tRNAs has been linked with defects in neural function and development in Caenorhabditis elegans (17, 18), with familial dysautonomia (19), and nonthiolated mitochondrial tRNAs are associated with the human disease myoclonic epilepsy with ragged red fibers (20). However, the underlying cellular and molecular mechanisms affected by lack of thiolation and methoxycarbonylmethylation are poorly understood.
Here, we present a proteome-wide analysis of changes in protein abundance induced in cells lacking tRNA thiolation and methoxycarbonylmethylation. We show that in vivo these tRNA modifications are not required for general translation, but specifically modulate translation of mainly AAA-, CAA-, and GAA-rich genes. Importantly, biochemical analysis using purified components revealed that these modifications of U34 specifically increase ribosomal A-site binding and peptide bond formation in vitro.
Results
Lack of URM1 Affects Only a Small Subset of the Proteome.
Relative protein abundance in urm1∆ yeast cells was compared with wild-type cells by stable isotope-labeling by amino acids in cell culture (SILAC) -based quantitative mass spectrometry. The density distribution of protein abundance ratios from a single biological replicate shows that most of the proteome is unaffected by lack of thiolation and that only a small fraction of proteins are differentially expressed (Fig. 1A). Similarly, quantification of [35S]Met and [35S]Cys incorporation into newly synthesized proteins by pulse-chase labeling showed no significant difference between wild-type and urm1∆ cells (Fig. 1B). Likewise, polysome profile analysis revealed that the percentage of polysomes and the ratio of 40S, 60S, and 80S particles in urm1∆ and uba4∆ cells were comparable to wild-type controls (Fig. 1C and Fig. S1A). Taken together, these experiments suggest that thiolation of U34 does not differentially impair general protein translation under standard growth conditions.
Fig. 1.
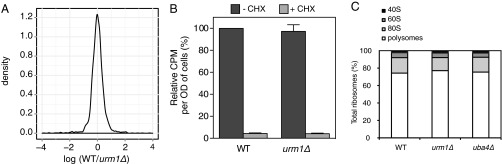
General translation is unaffected by lack of URM1. (A) Density plot of log2(WT/urm1∆) protein abundance ratios from a single SILAC-based proteomics experiment. (B) Wild-type and urm1∆ cells were pulsed with [35S]Met and [35S]Cys in the presence (+) or absence (−) of cycloheximide (CHX). [35S] incorporation into proteins was quantified by liquid scintillation counting. Counts per minute were normalized to the wild-type value. Data show mean ± SEM of three independent experiments. (C) Quantification of the polysome profiles from wild-type, urm1∆, and uba4∆ cells separated on a 6–45% sucrose gradient showing the distribution of 40S, 60S, and 80S particles and polysomes as percentage of total ribosomes from the average of three independent experiments. See also Fig. S1.
Lack of URM1 or ELP3 Impairs Protein Expression of AAA-, CAA-, AAG-, and GAA-rich mRNAs.
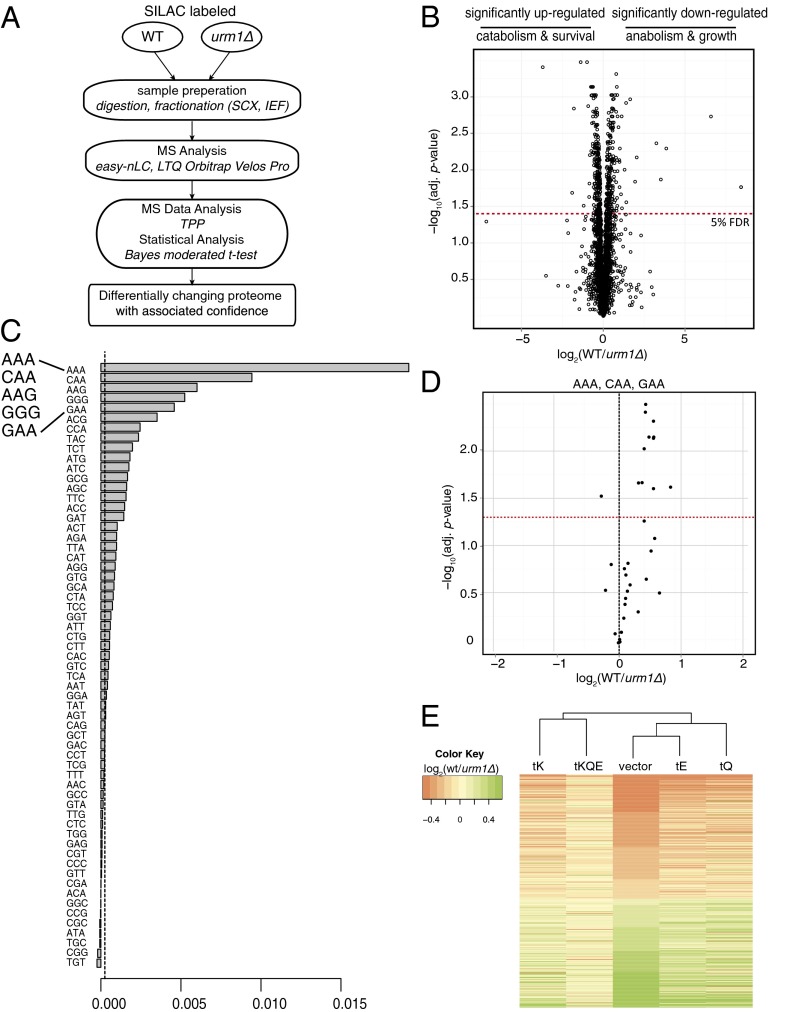
To identify differentially expressed proteins in the absence of a functional URM1 pathway, we designed a SILAC-based experimental workflow aimed at maximizing proteome coverage and sensitivity (Fig. 2A and Fig. S1B). Statistical significance of protein ratios from six biological replicates was calculated using an empirical Bayes moderated t test (21). To maximize proteome coverage, the samples were extensively fractionated at the protein and peptide levels and analyzed using a combination of multiple database search engines (22). Overall, we identified 3,818 proteins, corresponding to 57% of the predicted yeast proteome, at a 1% false-discovery rate (FDR) at the protein level. Small variations in the distribution of protein ratios across the biological replicates were normalized using median normalization (Fig. S1C). The data did not show any trend in protein ratios vs. number of peptides used for the computation either before or after normalization (Fig. S1D). Statistical analysis identified 267 proteins to be significantly (5% FDR) down-regulated and 286 proteins to be up-regulated in urm1∆ cells (Fig. 2B and Dataset S1, Table S1). Gene ontology analysis of the differentially expressed proteins showed that down-regulated proteins were enriched for anabolic processes, such as translation initiation and elongation, whereas up-regulated proteins were enriched for catabolic processes such as proteasomal degradation (Dataset S1, Tables S2 and S3). To assess whether the mcm5 modification similarly affects protein abundance in elp3Δ mutants, we used a second SILAC-based proteomics approach that compared protein abundances between urm1Δ and elp3Δ cells. This analysis quantified 243 (85%) from the significantly up-regulated and 199 (74.5%) from the significantly down-regulated proteins identified in the urm1Δ vs. wild-type experiment (Dataset S1, Table S4). Importantly, the levels of all these proteins were comparable in urm1∆ and elp3∆ cells, implying that the URM1- and ELP-pathways control an overlapping set of target proteins. In total, only 12 proteins, two of which were Elp3 and His3 (the histidine auxotrophic marker used to delete ELP3), were found to be significantly changing in elp3Δ compared with the urm1Δ mutants.
Fig. 2.
URM1 is important for efficient expression of a subset of AAA-, CAA-, AAG-, and GAA-rich genes. (A) Schematic representation of the quantitative proteomics workflow used to assess differential translation in urm1∆ cells. (B) Volcano plot of protein abundance ratios vs. their bayes normalized t test calculated confidence. Results shown are of six biological replicates. Red dotted line: 5% FDR chosen as statistical significance. (C) Barplot representation of the variable importance learned by a random forest algorithm used to predict the ability of different codon abundances in classifying proteins in significantly up- and down-regulated sets. Dotted line: absolute value of the lowest predictor. (D) Proteins with the corresponding highest frequency (1% of the genome) of AAA, CAA, and GAA codons represented in the volcano plot from Fig. 2B. Red dotted line: 5% FDR. (E) Heatmap of log2(WT/urm1∆) protein abundance ratios of the significantly up- and down-regulated proteins from Fig. 2B in cells overexpressing tKUUU, tQUUG, and tEUUC individually or in combination compared with cells without plasmid. Columns are clustered based on Euclidean distance. Figs. S1–S3.
To identify the codons that best separated the significantly up- and down-regulated datasets, we performed an unsupervised machine learning analysis using a random forest algorithm of the urm1Δ vs. wild-type data. A plot of variable importance (Fig. 2C) illustrates that the codons AAA, CAA, AAG, GGG, and GAA, in decreasing order of their importance, were the best at classifying the data in up- or down-regulated sets. Further analysis suggested that codons AAA, CAA, and GAA, and to a lesser extent AAG, were significantly enriched in the down-regulated dataset, whereas GGG was enriched in the up-regulated dataset and was associated with up-regulated mRNAs (Fig. 2D, Figs. S2 and S3, and Dataset S1, Tables S5 and S6). The results of the analysis were first tested for stability in silico by varying the random seed. We then tested the predictions in vivo by assessing the ability of individual tRNAs to rescue the differential protein abundance using SILAC-based proteomics of wild-type vs. urm1∆ cells overexpressing tKUUU, tQUUG, and tEUUC, either individually or in combination. The heatmap in Fig. 2E shows that overexpression of all three tRNA species reverts most of the differentially expressed proteins back to wild-type levels. This finding indicates that URM1 activity in tRNA thiolation is mainly responsible for the observed differential protein abundance. Complete clustering using Euclidean distances recapitulates and validates the relative importance of different codons predicted by the random forest (dendrogram in Fig. 2E).
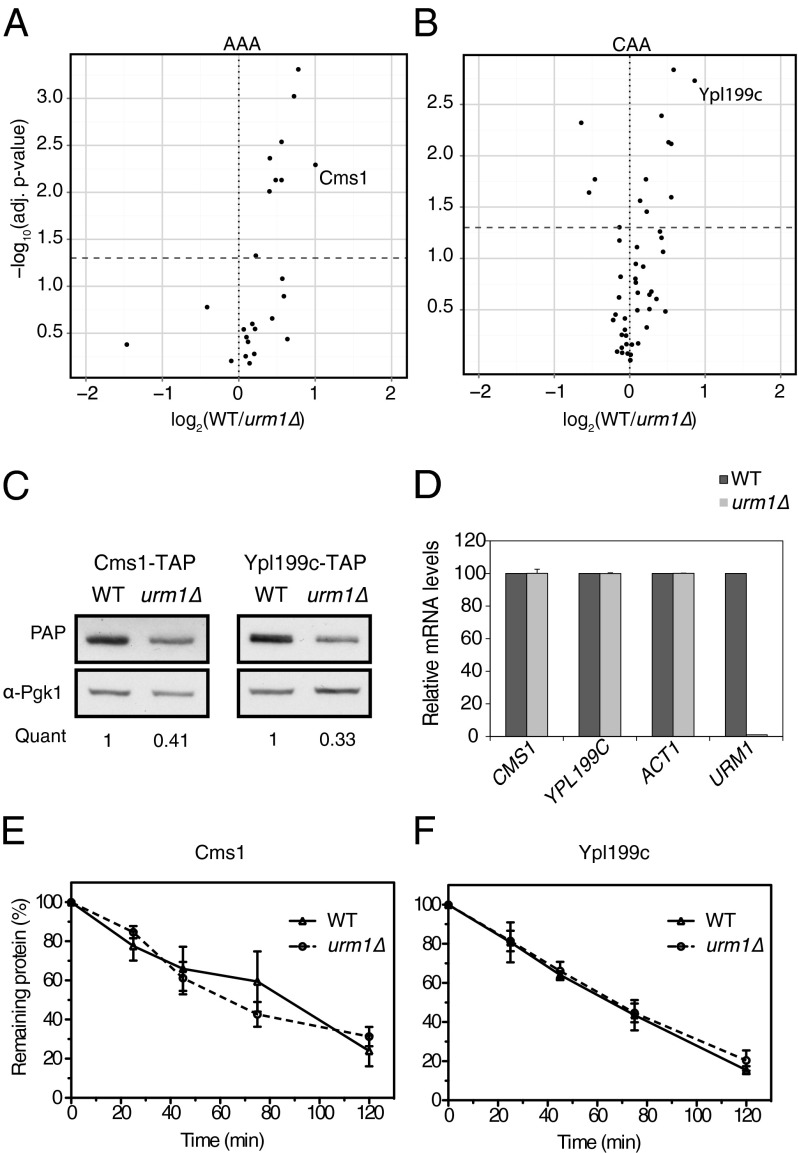
Down-regulated candidates exhibiting a significant codon-bias were selected for further analysis (Fig. 3 A and B). Protein abundance was measured by Western blotting and confirmed reduced expression levels in urm1∆ cells (Fig. 3C and Fig. S4A). Quantitative real-time PCR revealed that mRNA abundance of all but one candidate were comparable in wild-type and urm1∆ cells (Fig. 3D and Fig. S4B), implying that changes at the protein level are responsible for their differential protein expression. To discriminate between differential degradation and translation, we quantified the stability of Cms1 and Ypl199c in wild-type and urm1∆ cells after blocking translation by cycloheximide (Fig. 3 E and F and Fig. S4C). Both proteins were degraded with half-lives of ∼60 and 70 min, respectively, in wild-type and urm1∆ cells, implying that the observed changes in protein levels are caused by reduced translation rates in urm1∆ cells. Taken together, these data suggest that efficient translation of mRNA molecules rich in AAA, CAA, GAA, and AAG codons requires functional URM1 and ELP pathways in vivo.
Fig. 3.
The URM1-pathway specifically regulates translation of Cms1 and Ypl199c. (A and B) Volcano plots of protein abundance ratios with statistical significance measured by quantitative proteomics of the top 1.5% yeast genes with the highest (A) AAA or (B) CAA codon frequency. (C) Western blot of TAP-tagged Cms1 or Ypl199c from wild-type and urm1∆ cells using PAP antibodies or anti-Pgk1 as loading control. The quantification indicates the urm1∆/WT protein abundance ratio averaged from three independent experiments. (D) The mRNA levels of CMS1 and YPL199C and the ACT1 or URM1 controls were measured by quantitative PCR in wild-type and urm1∆ cells. Data show the mean ± SEM of three independent experiments. (E and F) The protein stability of TAP-tagged (E) Cms1 and (F) Ypl199c in wild-type and urm1∆ cells was determined by Western blot of extracts prepared at the indicated time points (min) after CHX addition. Protein amount over time were compared with amount at time 0. Data show the mean ± SEM of three independent experiments. See also Figs. S2 and S4.
s2 and mcm5 Modifications of U34 in tKUUU, tQUUG, and tEUUC Are Required for Codon-Specific Translation in Vivo.
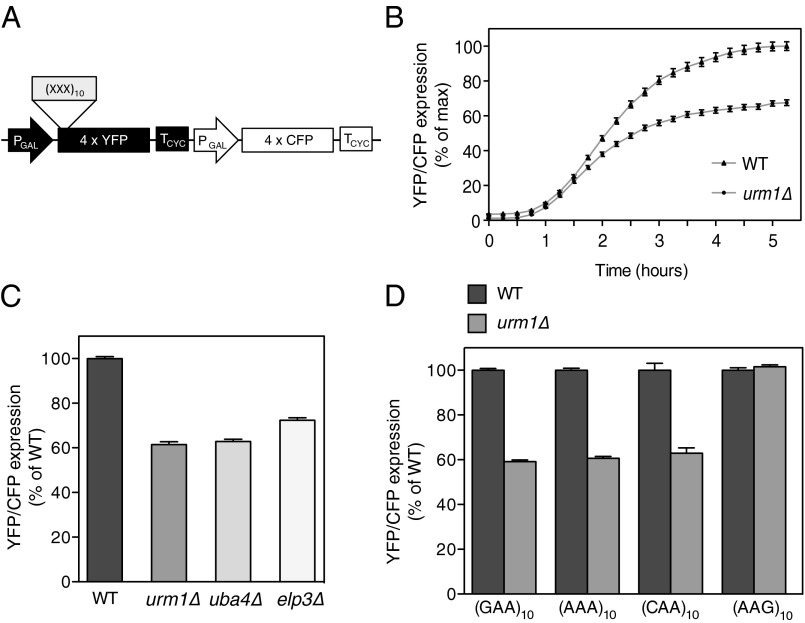
To further assess codon use and translation regulation, we constructed an in vivo codon-specific translation reporter (Fig. 4A) consisting of quadruple-cyan fluorescent protein (CFP) (4× CFP) and quadruple-venus (4× YFP, yellow fluorescent protein), both inducible by the addition of β-estradiol (23). CFP and YFP are very similar and stable molecules, thus ruling out codon composition and degradation effects. Additionally, YFP and CFP can be readily quantified in vivo by fluorescence microscopy in single cells (Fig. S5A), thereby accounting for cell-to-cell variations and extrinsic effects, such as cell-cycle stage (24). Although YFP fluorescence reports on codon-specific translation caused by a “codon trap” of N-terminally inserted codon-enriched sequences, CFP expression controls for the general expression capacity of the same cell (Fig. S5B).
Fig. 4.
URM1 is required for efficient translation of AAA-, GAA-, and CAA-enriched reporters. (A) Schematic representation of the dual-fluorescent codon-specific translation reporter. β-Estradiol–inducible quadruple-venus (4× YFP) or quadruple-CFP (4× CFP) proteins serve as codon-specific translation reporters and internal translation control, respectively. Codon-traps composed of a run of 10 identical codons, (XXX)10, are inserted at the N-terminus of YFP. (B) Time course of (CAA)10 translation reporter expression induced at time 0 in wild-type and urm1∆ cells. Data show the mean YFP/CFP ratio ± SEM from at least 100 cells plotted as percentage of maximum expression. (C and D) Expression of the translation reporter after 3-h induction with (C) (CAA)10 codon-trap in different mutants or (D) different codon-traps in wild-type and urm1∆ cells. Data show the mean YFP/CFP ratio ± SEM from at least 1,000 cells plotted as percent of wild-type control. See also Fig. S5.
We first analyzed a codon-trap of 10 consecutive CAA codons, (CAA)10, in a time-course experiment. After a delay of 45 min, corresponding to the maturation time of the fluorophores, both wild-type and urm1∆ cells showed reporter expression, but the expression in urm1∆ cells was significantly slower and only reached ∼60% of wild-type expression after 5 h (Fig. 4 B and C). Reexpression of Urm1 from a centromeric plasmid restored efficient translation (Fig. S5C). A similar defect was also observed in uba4∆ and elp3∆ cells (Fig. 4C), implying that s2 and the mcm5 modifications are both required for efficient translation. Similarly, tRNA thiolation also influenced translation of (AAA)10 or (GAA)10 codon-traps (Fig. 4D). Interestingly, inclusion of the synonymous G-ending codon, (AAG)10, recognized by the nonthiolated tKUUC, did not affect translation of YFP in urm1∆ cells, demonstrating that the URM1-dependent decrease in reporter expression is specific to the codon use and not the amino acid composition. Taken together, these data suggest that efficient translation of AAA-, CAA-, and GAA-rich reporters requires functional URM1 and ELP pathways in vivo.
U34 Modifications Enhance Ribosomal A-Site Binding and Dipeptide Formation Rates in Vitro.
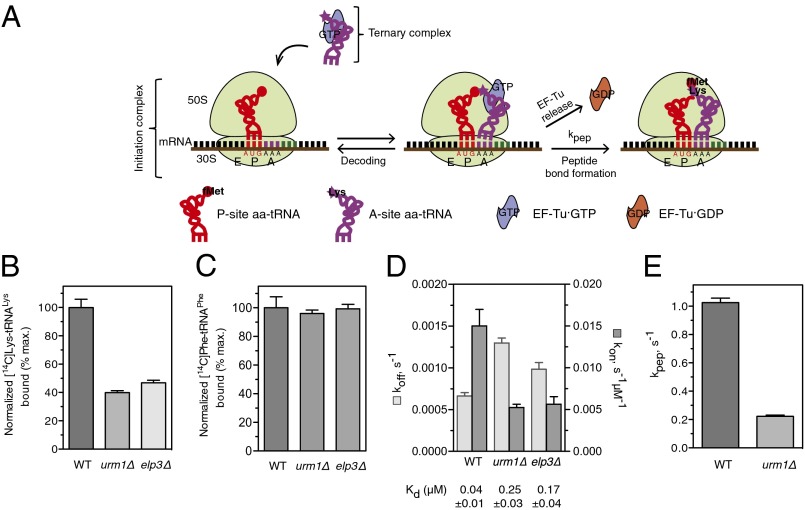
To investigate how thiolation and methoxycarbonylmethylation promote efficient translation of cognate codons, we compared the effects of native and hypomodified yeast tRNAs on binding to the A-site of the ribosome and dipeptide formation in vitro (Fig. 5A). tRNALys isolated from wild-type, urm1∆, or elp3∆ yeast cells were aminoacylated (aa) with [14C]-labeled lysine and incubated with EF-Tu·GTP to form ternary complex. The [14C]Lys-tRNALys ternary complex was mixed with 70S initiation complex from Escherichia coli loaded with f[3H]Met-tRNAfMet and mRNA containing an AAA codon following the AUG initiation codon, and subsequently the A-site binding was measured. Interestingly, A-site binding of aa-tRNALys from urm1Δ was decreased by 60% compared with wild-type controls (Fig. 5B), demonstrating an important role of tRNA thiolation to enhance cognate codon binding. A similar effect was observed with tRNAs extracted from elp3∆ cells that lack the mcm5 modification (Fig. 5B), suggesting that both modifications enhance A-site binding. As a control, [14C]Phe-tRNAPhe ternary complex was mixed with 70S initiation complex from E. coli loaded with f[3H]Met-tRNAfMet and mRNA containing an UUC codon following the AUG initiation codon. As expected, the A-site binding of [14C]Phe-tRNAPhe from wild-type, urm1∆, or elp3∆ cells was not significantly different (Fig. 5C), demonstrating that the observed binding defects of [14C]Lys-tRNALys are indeed caused by the missing U34 modifications.
Fig. 5.
tRNA thiolation of U34 promotes A-site binding and dipeptide formation in vitro. (A) Schematic illustration of the decoding and peptide bond formation processes. (B and C) A-site binding of [14C]Lys-tRNALys (B) or for control [14C]Phe-tRNAPhe (C) isolated from wild-type, urm1∆, or elp3∆ cells containing tKUUU (mcm5s2U34, mcm5U34, or s2U34 modifications) and tFGAA, were measured after incubation of initiation complex with ternary complex. Data show the mean [14C] signal ± SEM of three independent experiments plotted as percentage of wild-type. (D) The rate constants of tRNA dissociation (koff) and tRNA association (kon) of peptidyl-tRNALys prepared from wild-type, urm1∆, or elp3∆ cells. The equilibrium dissociation constant (Kd) ± SEM is shown below the graph. (E) The rate of dipeptide formation (kpep) using tRNALys isolated from wild-type and urm1∆ cells.
To determine the kinetic parameters of A-site binding, ribosomal pretranslocation complexes bearing fMet[14C]Lys-tRNALys were prepared and peptidyl-tRNA dissociation was followed by nitrocellulose binding assay. The equilibrium dissociation constant, Kd, and the rate constant of tRNA dissociation (koff) from and association (kon) with the A-site were calculated from the apparent dissociation rates and the final level of the reaction. Strikingly, the kon for peptidyl-tRNALys from urm1Δ cells (5 × 10−3 s−1·μM−1) and elp3∆ cells (5.6 × 10−3 s−1·μM−1), were about three-times lower than for tRNAs from wild-type control cells (1.5 × 10−2 s−1·μM−1), whereas koff was higher with tRNALys from urm1∆ cells (1.3 × 10−3 s−1) and elp3∆ cells (1 × 10−3 s−1) compared with tRNALys from wild-type controls (6.6 × 10−4 s−1) (Fig. 5D).
Because the final step in decoding results in peptide bond formation, we measured kpep, the rate of ribosome-catalyzed formation of f[3H]Met[14C]Lys-tRNALys dipeptides, using quench-flow analysis with rapid mixing of an excess of initiation complex with ternary complex (EF-Tu·GTP·[14C]Lys-tRNALys). Dipeptide formation was five-times slower with aa-tRNALys tRNA from urm1∆ cells compared with wild-type with apparent rate constants, kpep, of 0.2 s−1 and 1 s−1, respectively (Fig. 5E). Together, these in vitro experiments demonstrate that wobble position modifications stabilize cognate codon-anticodon interactions at the ribosome, and thereby enhances the efficiency of translation.
Discussion
U34 Modifications Stabilize Binding of Cognate tRNAs to the A-Site and Promote Peptide Bond Formation.
Our in vitro and in vivo experiments show that the s2 and the mcm5 modifications at the wobble position stabilize binding of cognate tRNAs, raising the possibility that tRNA modifications at the anticodon loop may generally enhance efficiency and fidelity of translation. Consistent with this notion, Trm9, involved in the mcm5 modification, was recently shown to contribute to translation fidelity upon stress conditions (25). In Saccharomyces cerevisae, loss of thiolation does not abolish the presence of the mcm5 modification, and cells lacking either mcm5 or s2 are viable. However, simultaneous loss of mcm5 and s2 is lethal (26), suggesting that these U34 modifications cooperatively promote translation. Indeed, previous in vitro studies showed that a synthesized anticodon stem loop fragment of human LYS3 tRNA hASLLysUUU lacking the mcm5s2U34 and t6A37 modifications was unable to bind to AAA and AAG codons at the ribosomal A-site (27, 28). In this study, we used native tRNAs isolated from mutant yeast cells to examine the effect of the loss of a single tRNA modification on ribosomal binding. These data suggest that URM1- and ELP-mediated U34 modifications cooperate to increase binding of cognate tRNAs to the ribosomal A-site and subsequent peptide bond formation, thereby promoting efficient protein translation.
U34 Thiolation and Methoxycarbonylmethylation Control Efficient Translation of Specific mRNAs in Vivo.
To better understand the in vivo biological relevance of tRNA wobble uridine thiolation, we used an unbiased data-driven approach to analyze the differential proteome of urm1∆ and wild-type cells. Bioinformatic analysis revealed that thiolation is required for the efficient expression of mRNAs rich in the cognate AAA, CAA, and GAA codons. Previous in vitro studies implicated thiolation in the efficient recognition of G-ending codons (7, 29). Interestingly, we also found differential translation of mRNAs rich in AAG but not CAG or GAG codons in urm1∆ cells. In vivo, G-ending codons are recognized by cognate, nonthiolated tRNAs. Yeast cells lacking tQCUG or tECUC are nonviable, suggesting that the thiolated tQUUG and tEUUC cannot efficiently recognize CAG and GAG codons (30). However, AAG codons are preferentially used in highly expressed proteins, and tKUUU might be required to supplement the translational capacity for these AAG-rich proteins. Remarkably, the synthetic translation reporters did not detect a defect in the translation of an AAG codon trap in urm1∆ cells, suggesting that thiolated tKUUU only plays a marginal role in the recognition of highly AAG-biased genes. Similarly, although AAA, CAA, and GAA constitute 11.4% of the coding genome, our results indicate that overall translation is not differentially affected in exponentially growing cells lacking a functional URM1 pathway. Only mRNAs strongly biased in Urm1-dependent codons, such as CMS1 and YPL199C, both of which belong to the upper quartile for AAA and CAA codon abundance, respectively, were translated 50% less efficiently in urm1∆ cells. Importantly, we established that the same set of mRNAs was also differentially translated in an elp3∆ mutant. These findings are consistent with the dynamic regulation of translation in vivo, where initiation constitutes the rate-limiting step under nonlimiting conditions, but elongation is very fast (31, 32). Interestingly, despite the fact that GAA is more abundant than AAA, our data suggest that the latter is the major cause of differential translation. However, some AAA-, CAA-, and GAA-rich mRNAs did not show the predicted decrease in protein expression levels, suggesting that the position of thiolation-dependent codons in the mRNA may also be important or that compensatory mechanisms may operate in these cases.
Although mutations in the URM1 and ELP pathways increase sensitivity to a number of stresses (12, 33), we found that under rich conditions changes in protein-expression levels are small, and indeed they do not induce a discernable phenotype other than slow growth. Nevertheless, under certain stress conditions small differences in the translation of specific proteins might become relevant. For example, cells lacking components of the URM1/ELP pathway as well as cells lacking CMS1, the translation of which is significantly reduced in urm1∆ cells, are associated with quinine sensitivity (34).
Finally, because the proportion of U34 modified tRNAs is tissue-dependent and altered by stress (17, 35), both the frequency of URM1/ELP-dependent codons and the regulation of tRNA posttranscriptional modification might contribute to the fine-tuning of protein expression.
Materials and Methods
Further details on protocols are described in SI Materials and Methods.
SILAC Experiments.
Cells were labeled with SILAC as described previously (36), and processed and analyzed as described in Fig. S1C.
Quantitative RT-PCR.
Quantitative RT-PCR was performed using the SYBR Green I kit (Roche).
[35S] Metabolic Labeling and Polysome Profiles.
Equal amounts of cells were pulsed for 15 min with [35S]Met and [35S]Cys (Hartmann Analytics; SCIS-103) and chased for 5 min with cold amino acids. Protein extracts for polysome analysis were separated on a 6–45% sucrose gradient, and ribosome content recorded with a teledyne ISCO UA-6 detector.
Expression of Fluorescent Reporter and Cell Imaging.
Expression of the translation reporters was induced with 50 nM β-estradiol and images were taken with an inverted epi-fluorescence microscope (Ti-Eclipse, Nikon). YFP/CFP fluorescence was measured in single cells, and analyzed with YeastQuant software (24).
Cycloheximide Chase.
Samples of equal volume were taken at different times after translation block with 100 μg/mL cycloheximide and cell extracts probed with PAP (Sigma; P1291), anti-Pgk1 (Invitrogen; 459250), anti-Act1.
Biochemical and Kinetic Assays.
In vitro experiments with purified ribosomes and EF-Tu·GTP·[14C]Lys-tRNALys or EF-Tu·GTP·[14C]Phe-tRNAPhe ternary complexes were performed in buffer A (50 mM Tris⋅HCl pH 7.5, 70 mM NH4Cl, 30 mM KCl, 7 mM MgCl2). Ribosome complex preparation and experiments testing stability of peptidyl-tRNA binding to the A site were performed as in (37). Quench-flow assays were performed at 24 °C in a KinTek RQF-3 apparatus with 2 µM initiation complex and 0.6 µM ternary complex. Dipeptides were analyzed by RP-HPLC (38).
Supplementary Material
Acknowledgments
We thank S. Pelet, S. Leidel, and M. Stark for reagents and advice; members of the M.P. laboratory for discussions; A. Smith and R. Dechant for critical reading of the manuscript; the University of Washington and J. Eng for granting us access to UW SEQUEST v2012.01.2; and David A. Tirrell (California Institute of Technology) for providing the plasmid pQE32-FRS-sc encoding for the His6-tagged phenylalanyl-tRNA synthetase from Saccharomyces cerevisiae. V.A.N.R. is a member of the Molecular Life Science doctorate program, and M.P. of the Competence Center for Systems Physiology and Metabolic Diseases. This work is funded in part by the Scottish Institute for Cell Signalling (P.G.A.P.); the College of Life Sciences, University of Dundee, Innovation Pipeline for Translational Science LUPS/ERDF/2008/2/1/0429 (to P.G.A.P.); an Ambizione grant of the Swiss National Science Foundation (to P.G.A.P.); the Scottish Institute for Cell Signalling (K.T.); the College of Life Sciences Bursary at the University of Dundee and by the Discovery Scholarship (to K.T.); the Max Planck Society (M.V.R., A.L.K. and J.M.); and the Deutsche Forschungsgemeinschaft (M.V.R.). This project was supported by the Promedica Foundation, and work in the M.P. laboratory by the European Research Council, the Swiss National Science Foundation, and the Eidgenössiche Technische Hochschule Zurich.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300781110/-/DCSupplemental.
References
- 1.Czerwoniec A, et al. MODOMICS: A database of RNA modification pathways. 2008 update. Nucleic Acids Res. 2009;37(Database issue):D118–D121. doi: 10.1093/nar/gkn710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limbach PA, Crain PF, McCloskey JA. Summary: The modified nucleosides of RNA. Nucleic Acids Res. 1994;22(12):2183–2196. doi: 10.1093/nar/22.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agris PF, Vendeix FA, Graham WD. tRNA’s wobble decoding of the genome: 40 years of modification. J Mol Biol. 2007;366(1):1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Krüger MK, Pedersen S, Hagervall TG, Sørensen MA. The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J Mol Biol. 1998;284(3):621–631. doi: 10.1006/jmbi.1998.2196. [DOI] [PubMed] [Google Scholar]
- 5.Murphy FV, 4th, Ramakrishnan V, Malkiewicz A, Agris PF. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat Struct Mol Biol. 2004;11(12):1186–1191. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- 6.Takai K, Yokoyama S. Roles of 5-substituents of tRNA wobble uridines in the recognition of purine-ending codons. Nucleic Acids Res. 2003;31(22):6383–6391. doi: 10.1093/nar/gkg839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarian C, et al. Accurate translation of the genetic code depends on tRNA modified nucleosides. J Biol Chem. 2002;277(19):16391–16395. doi: 10.1074/jbc.M200253200. [DOI] [PubMed] [Google Scholar]
- 8.Phelps SS, Malkiewicz A, Agris PF, Joseph S. Modified nucleotides in tRNA(Lys) and tRNA(Val) are important for translocation. J Mol Biol. 2004;338(3):439–444. doi: 10.1016/j.jmb.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 9.Beuning PJ, Musier-Forsyth K. Transfer RNA recognition by aminoacyl-tRNA synthetases. Biopolymers. 1999;52(1):1–28. doi: 10.1002/(SICI)1097-0282(1999)52:1<1::AID-BIP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Novoa EM, Pavon-Eternod M, Pan T, Ribas de Pouplana L. A role for tRNA modifications in genome structure and codon usage. Cell. 2012;149(1):202–213. doi: 10.1016/j.cell.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Huang B, Lu J, Byström AS. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14(10):2183–2194. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leidel S, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458(7235):228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 13.Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem. 2008;283(41):27469–27476. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- 14.Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37(4):1335–1352. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci USA. 2008;105(47):18255–18260. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, Johansson MJO, Byström AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11(4):424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Tuck S, Byström AS. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 2009;5(7):e1000561. doi: 10.1371/journal.pgen.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewez M, et al. The conserved Wobble uridine tRNA thiolase Ctu1-Ctu2 is required to maintain genome integrity. Proc Natl Acad Sci USA. 2008;105(14):5459–5464. doi: 10.1073/pnas.0709404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson SL, et al. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet. 2001;68(3):753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umeda N, et al. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J Biol Chem. 2005;280(2):1613–1624. doi: 10.1074/jbc.M409306200. [DOI] [PubMed] [Google Scholar]
- 21.Ting L, et al. Normalization and statistical analysis of quantitative proteomics data generated by metabolic labeling. Mol Cell Proteomics. 2009;8(10):2227–2242. doi: 10.1074/mcp.M800462-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shteynberg D, et al. (2011) iProphet: Multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol Cell Proteomics 10(12): M111.007690. [DOI] [PMC free article] [PubMed]
- 23.Louvion JF, Havaux-Copf B, Picard D. Fusion of GAL4-VP16 to a steroid-binding domain provides a tool for gratuitous induction of galactose-responsive genes in yeast. Gene. 1993;131(1):129–134. doi: 10.1016/0378-1119(93)90681-r. [DOI] [PubMed] [Google Scholar]
- 24.Pelet S, Dechant R, Lee SS, van Drogen F, Peter M. An integrated image analysis platform to quantify signal transduction in single cells. Integr Biol (Camb) 2012;4(10):1274–1282. doi: 10.1039/c2ib20139a. [DOI] [PubMed] [Google Scholar]
- 25.Patil A, et al. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biol. 2012;9(7):990–1001. doi: 10.4161/rna.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björk GR, Huang B, Persson OP, Byström AS. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13(8):1245–1255. doi: 10.1261/rna.558707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarian C, et al. Modified nucleoside dependent Watson-Crick and wobble codon binding by tRNALysUUU species. Biochemistry. 2000;39(44):13390–13395. doi: 10.1021/bi001302g. [DOI] [PubMed] [Google Scholar]
- 28.Vendeix FA, et al. Human tRNA(Lys3)(UUU) is pre-structured by natural modifications for cognate and wobble codon binding through keto-enol tautomerism. J Mol Biol. 2012;416(4):467–485. doi: 10.1016/j.jmb.2011.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sen GC, Ghosh HP. Role of modified nucleosides in tRNA: Effect of modification of the 2-thiouridine derivative located at the 5′-end of the anticodon of yeast transfer RNA Lys2. Nucleic Acids Res. 1976;3(3):523–535. doi: 10.1093/nar/3.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson MJO, Esberg A, Huang B, Björk GR, Byström AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol. 2008;28(10):3301–3312. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli. Science. 2009;324(5924):255–258. doi: 10.1126/science.1170160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milon P, Konevega AL, Gualerzi CO, Rodnina MV. Kinetic checkpoint at a late step in translation initiation. Mol Cell. 2008;30(6):712–720. doi: 10.1016/j.molcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Bauer F, et al. Translational control of cell division by Elongator. Cell Rep. 2012;1(5):424–433. doi: 10.1016/j.celrep.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dos Santos SC, Sá-Correia I. A genome-wide screen identifies yeast genes required for protection against or enhanced cytotoxicity of the antimalarial drug quinine. Mol Genet Genomics. 2011;286(5–6):333–346. doi: 10.1007/s00438-011-0649-5. [DOI] [PubMed] [Google Scholar]
- 35.Chan CT, et al. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010;6(12):e1001247. doi: 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Godoy LMF, et al. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature. 2008;455(7217):1251–1254. doi: 10.1038/nature07341. [DOI] [PubMed] [Google Scholar]
- 37.Konevega AL, et al. Purine bases at position 37 of tRNA stabilize codon-anticodon interaction in the ribosomal A site by stacking and Mg2+-dependent interactions. RNA. 2004;10(1):90–101. doi: 10.1261/rna.5142404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katunin VI, Muth GW, Strobel SA, Wintermeyer W, Rodnina MV. Important contribution to catalysis of peptide bond formation by a single ionizing group within the ribosome. Mol Cell. 2002;10(2):339–346. doi: 10.1016/s1097-2765(02)00566-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.