Abstract
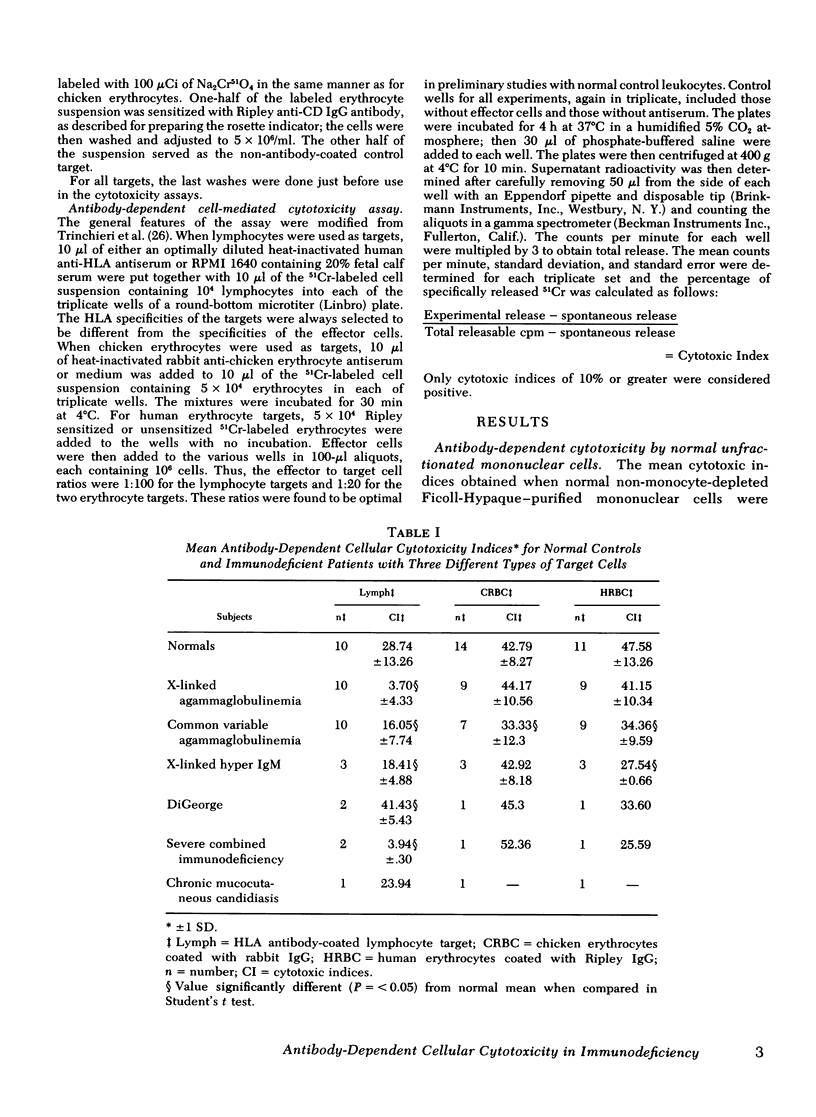
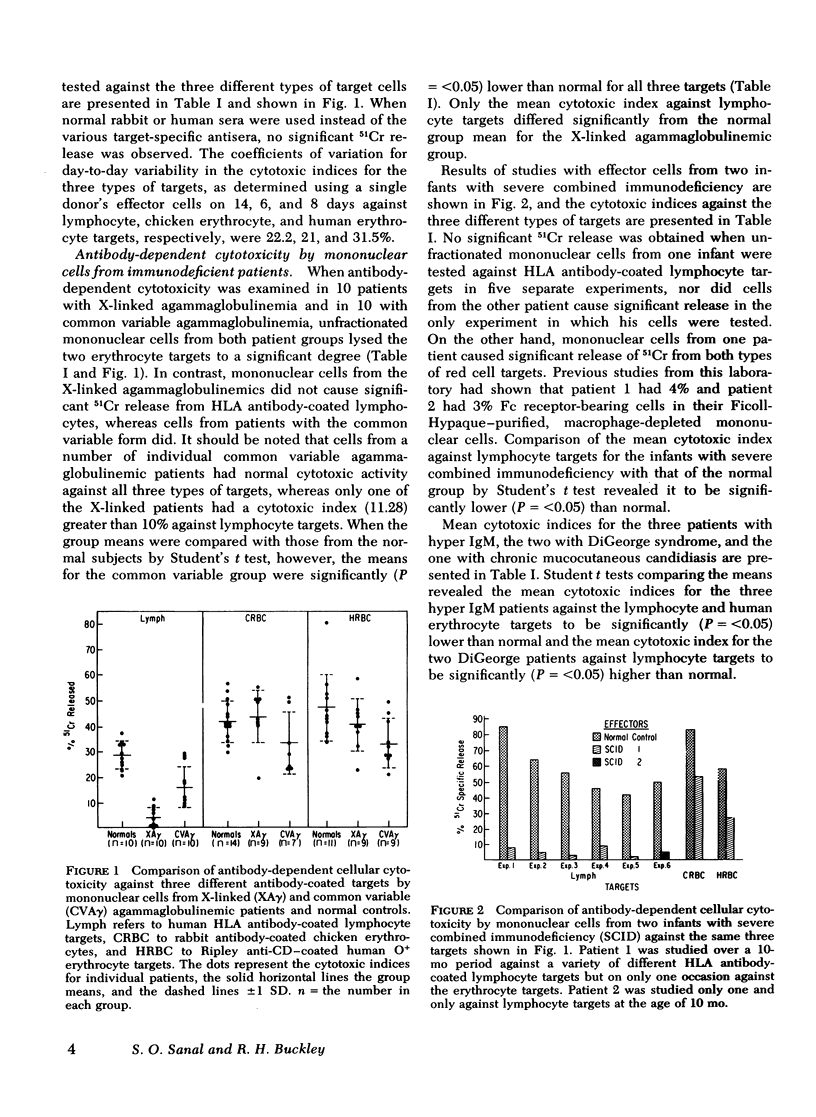
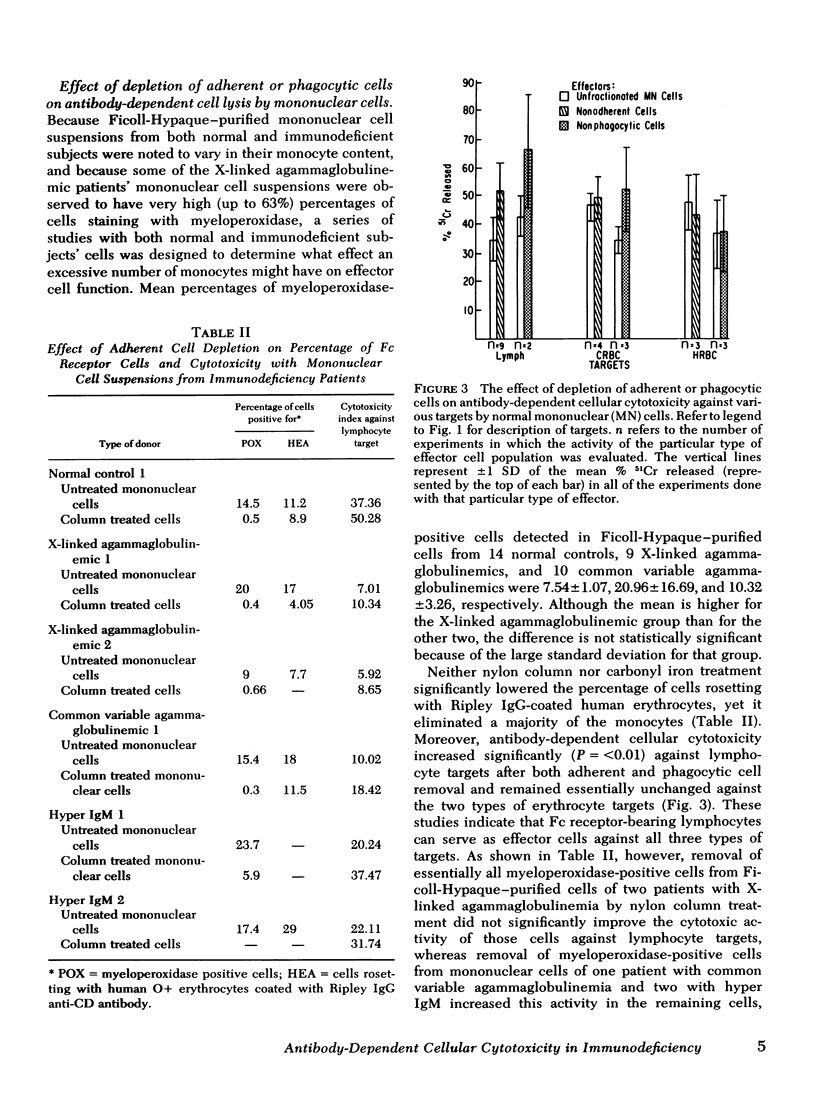
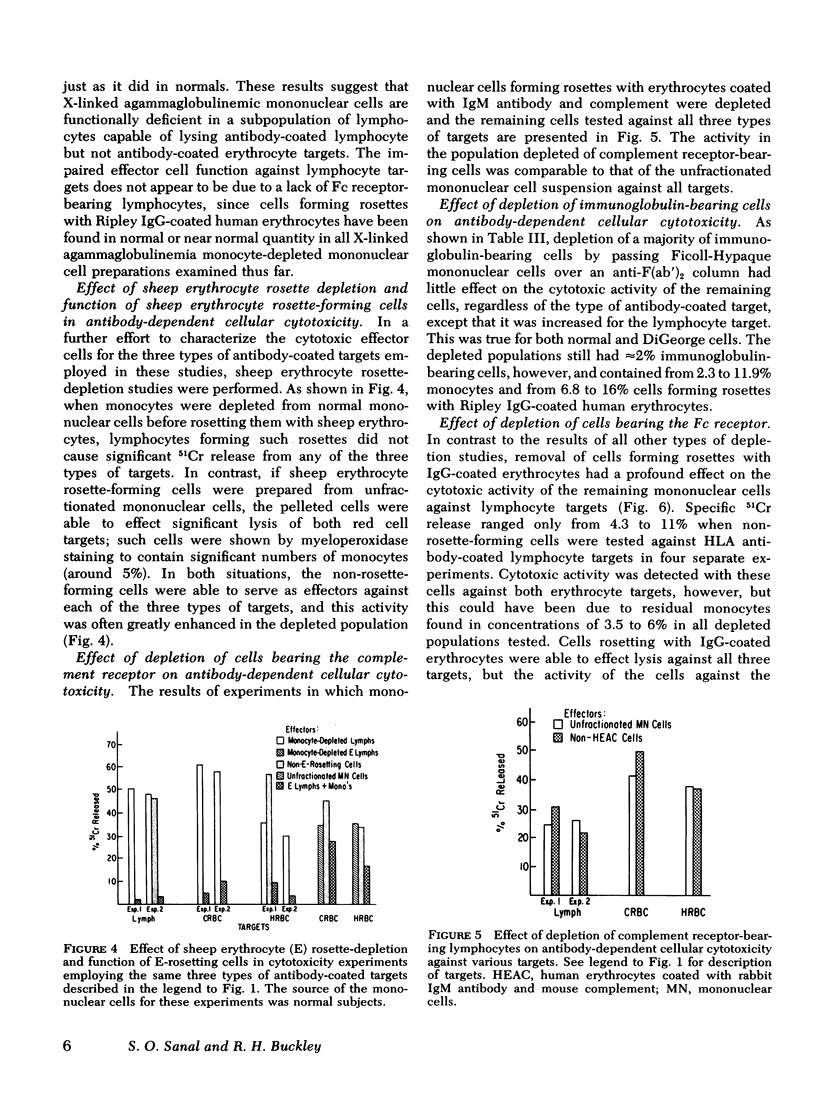
To gain insight into a possible role for antibody-dependent cell-mediated cytotoxicity in vivo, we examined the ability of leukocytes from 28 patients with primary immunodeficiency and from 20 normal controls to lyse three different types of antibody-coated targets in vitro. Mean cytotoxic indices ±1 SD elicited by unfractionated mononuclear cells from normal controls were 28.74±13.26 for human HLA antibody-coated lymphocyte targets, 42.79±8.27 for rabbit IgG antibody-coated chicken erythrocyte targets, and 47.58±10.34 for human anti-CD (Ripley)-coated O+ erythrocyte targets. Significantly (P=<0.05) lower than normal mean cytotoxic indices against lymphocyte targets were seen with effector cells from 10 patients with X-linked agammaglobulinemia (3.7±4.33), in 10 with common variable agammaglobulinemia (16.05±7.74), in 3 with immunodeficiency with hyper IgM (18.41±4.88), and in 2 with severe combined immunodeficiency (3.94±0.3). Antibody-dependent cytotoxicity against chicken erythrocytes was significantly (P=<0.05) lower than normal only in the common variable agammaglobulinemic group (33.33±12.3) and against human erythrocytes only in the common variable (34.36±9.59) and hyper IgM (27.54±0.66) groups. Rosette and anti-F(ab′)2 depletion studies with normal leukocytes indicated that a nonadherent, nonphagocytic, non-Ig-bearing, non-C receptor-bearing, Fc receptor-bearing lymphocyte was the only effector capable of lysing HLA antiboyd-coated lymphocyte targets. Patients with infantile X-linked agammaglobulinemia and severe combined immunodeficiency appear to have a marked deficiency in this type of effector cell function.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brier A. M., Chess L., Schlossman S. F. Human antibody-dependent cellular cytotoxicity. Isolation and identification of a subpopulation of peripheral blood lymphocytes which kill antibody-coated autologous target cells. J Clin Invest. 1975 Dec;56(6):1580–1586. doi: 10.1172/JCI108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. H., Gilbertsen R. B., Schiff R. I., Ferreira E., Sanal S. O., Waldmann T. A. Heterogeneity of lymphocyte subpopulations in severe combined immunodeficiency. Evidence against a stem cell defect. J Clin Invest. 1976 Jul;58(1):130–136. doi: 10.1172/JCI108441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Calder E. A., Penhale W. J., McLeman D., Barnes E. W., Irvine W. J. Lymphocyte-dependent antibody-mediated cytotoxicity in Hashimoto thyroiditis. Clin Exp Immunol. 1973 Jun;14(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- Chess L., Evans R., Humphreys R. E., Strominger J. L., Schlossman S. F. Inhibition of antibody-dependent cellular cytotoxicity and immunoglobulin synthesis by an antiserum prepared against a human B-cell Ia-like molecule. J Exp Med. 1976 Jul 1;144(1):113–122. doi: 10.1084/jem.144.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Cooper M. D., Faulk W. P., Fudenberg H. H., Good R. A., Hitzig W., Kunkel H., Rosen F. S., Seligmann M., Soothill J., Wedgwood R. J. Classification of primary immunodeficiencies. N Engl J Med. 1973 May 3;288(18):966–967. doi: 10.1056/NEJM197305032881814. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J., Ossorio R. C., Fahey J. L. A comparison of human lymphoid cells in antibody-dependent cellular cytotoxicity (ADCC). Clin Immunol Immunopathol. 1975 Jan;3(3):377–384. doi: 10.1016/0090-1229(75)90025-2. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kovithavongs T., Rice G., Thong K. L., Dossetor J. B. Effector cell activity in antibody mediated cell dependent immune lysis. II. Evidence for different populations of effector cells for different targets. Cell Immunol. 1975 Jul;18(1):167–175. doi: 10.1016/0008-8749(75)90045-3. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Bundy B. M., Pitchon H. E., Blaese R. M., Strober W. The effector cells in human peripheral blood mediating mitogen-induced cellular cytotoxicity and antibody-dependent cellular cytotoxicity. J Immunol. 1976 Nov;117(5 Pt 1):1472–1481. [PubMed] [Google Scholar]
- Nelson D. L., Strober W., Abelson L. D., Bundy B. M., Mann D. L. Distribution of alloantigens on human Fc receptor-bearing lymphocytes: the presence of B cell alloantigens on sIg-positive but not sIg-negative lymphocytes. J Immunol. 1977 Mar;118(3):943–946. [PubMed] [Google Scholar]
- Pape G. R., Troye M., Perlmann P. Characterization of cytolytic effector cells in peripheral blood of healthy individuals and cancer patients. I. Surface markers and K cell activity after separation of B cells and lymphocytes and Fc-receptors by column fractionation. J Immunol. 1977 Jun;118(6):1919–1924. [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Müller-Eberhard H. J. Cytolytic lymphocytic cells with complement receptor in human blood. Induction of cytolysis by IgG antibody but not by target cell-bound C3. J Exp Med. 1975 Feb 1;141(2):287–296. doi: 10.1084/jem.141.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S., Heppner G., Brawn R. J., Nelson K. Specific killing of tumor cells in vitro in the presence of normal lymphoid cells and sera from hosts immune to the tumor antigens. Int J Cancer. 1972 Mar 15;9(2):316–323. doi: 10.1002/ijc.2910090209. [DOI] [PubMed] [Google Scholar]
- Rachelefsky G. S., McConnachie P. R., Ammann A. J., Terasaki P. I., Stiehm E. R. Antibody-dependent lymphocyte killer function in human immunodeficiency diseases. Clin Exp Immunol. 1975 Jan;19(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Schiff R. I., Buckley R. H., Gilbertsen R. B., Metzgar R. S. Membrane receptors and in vitro responsiveness of lymphocytes in human immunodeficiency. J Immunol. 1974 Jan;112(1):376–386. [PubMed] [Google Scholar]
- Ting A., Terasaki P. I. Influence of lymphocyte-dependent antibodies on human kidney transplants. Transplantation. 1974 Oct;18(4):371–373. [PubMed] [Google Scholar]
- Trinchieri G., De Marchi M., Mayr W., Savi M., Ceppellini R. Lymphocyte antibody lymphocytolytic interaction (LALI) with special emphasis on HL-A. Transplant Proc. 1973 Dec;5(4):1631–1649. [PubMed] [Google Scholar]
- Wilfert C. M., Buckley R. H., Mohanakumar T., Griffith J. F., Katz S. L., Whisnant J. K., Eggleston P. A., Moore M., Treadwell E., Oxman M. N. Persistent and fatal central-nervous-system ECHOvirus infections in patients with agammaglobulinemia. N Engl J Med. 1977 Jun 30;296(26):1485–1489. doi: 10.1056/NEJM197706302962601. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]
- Wisloff F., Froland S. S. Antibody-dependent lymphocyte-mediated cytotoxicity in man: no requirement for lymphocytes with membrane-bound immunoglobulin. Scand J Immunol. 1973;2(2):151–157. doi: 10.1111/j.1365-3083.1973.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Wisloff F., Froland S. S., Michaelsen T. E. Antibody-dependent cytotoxicity mediated by human Fc-receptor-bearing cells lacking markers for B- and T-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):139–154. doi: 10.1159/000231208. [DOI] [PubMed] [Google Scholar]
- Wong L., Wilson J. D. The identification of Fc and C3 receptors on human neutrophils. J Immunol Methods. 1975 Apr;7(1):69–76. doi: 10.1016/0022-1759(75)90131-3. [DOI] [PubMed] [Google Scholar]
- Zawodnik S. A., Bonnard G. D., Gautier A. E., Macdonald H. R. Antibody-dependent cell-mediated destruction of human erythrocytes sensitized in ABO and rhesus fetal-maternal incompatibilities. Pediatr Res. 1976 Sep;10(9):791–796. doi: 10.1203/00006450-197609000-00006. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]


