Abstract
The circadian clock gene Period2 (PER2) has been suggested to be a tumor suppressor. However, detailed mechanistic evidence has not been provided to support this hypothesis. We found that loss of PER2 enhanced invasion and activated expression of epithelial-mesenchymal transition (EMT) genes including TWIST1, SLUG, and SNAIL. This finding was corroborated by clinical observation that PER2 down-regulation was associated with poor prognosis in breast cancer patients. We further demonstrated that PER2 served as a transcriptional corepressor, which recruited polycomb proteins EZH2 and SUZ12 as well as HDAC2 to octamer transcription factor 1 (OCT1) (POU2F1) binding sites of the TWIST1 and SLUG promoters to repress expression of these EMT genes. Hypoxia, a condition commonly observed in tumors, caused PER2 degradation and disrupted the PER2 repressor complex, leading to activation of EMT gene expression. This result was further supported by clinical data showing a significant negative correlation between hypoxia and PER2. Thus, our findings clearly demonstrate the tumor suppression function of PER2 and elucidate a pathway by which hypoxia promotes EMT via degradation of PER2.
Keywords: HIF1alpha, breast cancer stem cell
Circadian oscillation is a fundamental process that influences many physiological and biological processes. The master mammalian circadian clock, located in the suprachiasmatic nuclei (SCN) (1), coordinates peripheral circadian clocks in most tissues (2–5). These circadian rhythms are driven by a core set of clock genes. In human, there are at least nine clock genes including Period 1 (PER1), Period 2 (PER2), Period 3 (PER3), Cryptochrome 1 (CRY1), Cryptochrome 2 (CRY2), CLOCK, BMAL1, Casein kinase 1ε (CK1ε), and Timeless (TIM), which form a complex network of transcription-translation feedback loops and posttranslational modifications. In peripheral tissues, these circadian genes play important roles in tissue-specific responses to the circadian environment (6–8).
Recent studies in animal models and epidemiological analyses have suggested that disruption of circadian rhythms is associated with increased incidence of various epithelial cancers (9–12). This association can be explained by roles of circadian genes in the cell cycle, cell proliferation, DNA damage response, tumorigenesis, and angiogenesis. For example, in human prostate cancer cells, PER1 overexpression caused significant growth inhibition and apoptosis (13). Conversely, PER3 gene deletion in breast cancer was found to be associated with estrogen receptor-positive cancer recurrence (14). PER2 is functionally distinct from PER1 and PER3 despite their structural similarity (15), and each PER family member regulates target gene signaling pathways by distinct mechanisms (16–19). Similar to PER1 and PER3, cancer associations have also been reported for PER2 (20–30).
Additional lines of evidence suggest a role for PER2 in tumor suppression. Per2-deficient mice had low tumor incidence. However, on γ-irradiation, these mice became cancer prone (20). In humans, PER2 expression was found to be significantly reduced in both sporadic and familial primary breast cancers (21). A few breast cancers had PER2 mutations (22). In cases where PER2 was not mutated, altered PER2 promoter methylation was observed (23). Consistent with this, PER2 expression was significantly reduced in breast cancer stem cells (BCSCs) (24). Several studies have described a correlation between PER2 and cell cycle regulation or DNA damage response gene expression (20, 25–30). However, the underlying mechanism by which PER2 serves as a tumor suppressor remains to be elucidated.
We found that PER2 was essential for assembly of a repressor complex at OCT1 (POU2F1) sites on the promoters of the epithelial-mesenchymal transition (EMT) genes TWIST and SLUG. In hypoxia, PER2 protein was degraded, and the PER2 suppressor complex dissociated from the EMT promoters. The importance of this mechanism was supported by clinical correlation of decreased PER2 with hypoxia and poor prognosis in breast cancer patients. Our data demonstrate a mechanism by which PER2 suppresses breast tumor malignancy and elucidate a unique pathway by which hypoxia can release PER2 repression of EMT.
Results
Loss of PER2 Is Associated with Breast Tumorigenesis.
PER2 expression in breast ductal carcinoma (DC) was significantly suppressed compared with normal mammary tissues (N) (SI Appendix, Fig. S1A; www.oncomine.org). Consistent with these clinical data, lower PER2 expression was observed in breast cancer cell lines SKBR-3 and MDA-MB-231 (MB-231) compared with normal mammary epithelial cells MCF-10A and H184B5F5/M10 (M10), both at the protein and mRNA levels (Fig. 1A; SI Appendix, Fig. S1B). Similar to PER2, PER1 expression was low in ductal carcinoma; however, its protein level did not differ between cancer and normal cell lines (Fig. 1B; SI Appendix, Fig. S1C). Neither clinical samples nor cell lines showed significant difference in PER3 gene or protein expression between cancer and normal mammary epithelial cells (Fig. 1C; SI Appendix, Fig. S1D). These data suggested a specific role of PER2 in breast tumorigenesis.
Fig. 1.
PER2 suppression promoted tumorigenic ability. (A–C) Immunoblot analysis of PER2 (A), PER1 (B), and PER3 (C) in breast cancer cells (MB-231 and SKBR-3) and normal mammary epithelial cells (MCF-10A and M10). Tubulin was used as a loading control. Relative expression (RE) level of PER protein in SKBR-3, MCF-10A, and M10 relative to MB-231 is indicated. (D and E) Soft agar colony formation (SACF) assay using MCF-10A (D) and SKBR-3 (E) cells infected with lentiviral-control shRNA (sh-Ctrl) or sh-PER2. (F) SACF assay using SKBR-3 cells transduced with lentiviral control (Ctrl) or PER2. PER2 expression in these cells was examined with immunoblot. Tubulin was used as a loading control. Data show means ± SD. *P < 0.05 (Student t test). (G) Tumorigenesis assay of NOD/SCID mice injected with SKBR-3 lentiviral Ctrl or PER2 overexpressing cells. Cell dose: 3 × 106 cells per fat pad. Five mice were used for each group. Data show means ± SD. *P < 0.05 (Student t test) (SI Appendix, Fig. S1).
Depletion of PER2 using shRNA increased colony-forming ability in both normal mammary epithelial (Fig. 1D) and breast cancer cells (Fig. 1E). In contrast, overexpression of PER2 reduced colony-forming ability of cancer cells (Fig. 1F), and tumors derived from PER2 overexpressing cells grew less in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (Fig. 1G). These data indicated a tumor suppressor role of PER2 in tumorigenesis.
Down-Regulation of PER2 Promotes Tumor Malignancy by Enhancing Invasion and Activating EMT Gene Expression.
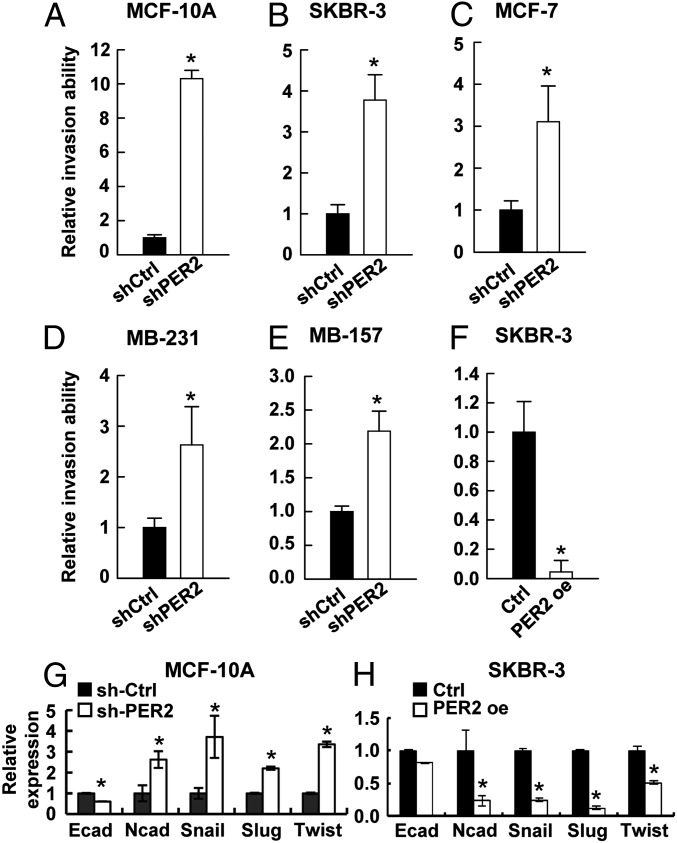
To test whether loss of PER2 contributes to tumor malignancy, we assayed invasion ability and found that PER2 depletion increased the invasion ability of normal epithelial MCF-10A cells and breast cancer cell lines SKBR-3, MCF-7, MB-231, and MDA-MB-157 (MB-157) (Fig. 2 A–E). In MCF-10A cells, down-regulation of PER2 resulted in a 10-fold induction of invasion ability (Fig. 2A). In less invasive SKBR-3 and MCF-7 cells, PER2 suppression promoted invasion ability by nearly fourfold (Fig. 2 B and C). Even in highly invasive MB-231 and MB-157 cells, down-regulation of PER2 could further enhance invasion ability by twofold (Fig. 2 D and E). In contrast, overexpression of PER2 inhibited invasion ability of SKBR-3 cells (Fig. 2F). However, PER2 overexpression did not suppress the invasion ability of MB-231 cells (SI Appendix, Fig. S2 A and B), possibly due to multiple highly activated EMT pathways in these cells (31).
Fig. 2.
PER2 suppression promoted invasion and EMT gene expression. (A–E) Invasion assay for MCF-10A (A), SKBR-3 (B), MCF-7 (C), MB-231 (D), and MB-157 (E) cells transduced with lentiviral sh-Ctrl or sh-PER2. (F) Invasion assay for SKBR-3 cells transduced with lentiviral control (Ctrl) or PER2. (G and H) mRNA expression of EMT markers (E-cadherin and N-cadherin) and regulators (SLUG, SNAIL, TWIST1) determined by qRT-PCR in MCF-10A infected with sh-Ctrl or sh-Per2 (G) and in Ctrl or PER2 overexpressing SKBR-3 (H). Data are means ± SD. *P < 0.05 (Student t test). (SI Appendix, Figs. S2 and S3).
It has been previously reported that EMT is the driving force of invasion and enhances stemness in epithelial cells (32, 33). Thus, we examined the expression of EMT markers (E-cadherin and N-cadherin), as well as key EMT regulators (SNAIL, SLUG, and TWIST1). The elevated invasion ability of PER2-depleted MCF-10A cells was associated with up-regulation of N-cadherin, SNAIL, SLUG, and TWIST1 and down-regulation of E-cadherin, as well as a morphological change from epithelial phenotype to an elongated and spindle-like morphology (Fig. 2G; SI Appendix, Fig. S3). Conversely, decreased invasion ability of PER2-overexpressing SKBR-3 cells was associated with repression of these EMT genes, with the exception of E-cadherin (Fig. 2H). In other breast cancer cell lines, different EMT regulators were up-regulated by PER2 knockdown (SI Appendix, Fig. S2 C–F). This observation indicated that, although invasion mediated by PER2 suppression was observed in various breast cancer cells, the targets of PER2-mediated EMT gene regulation were more cell type specific. Consistent with these results in culture, Twist1 and Snail were up-regulated in mammary glands of Per2-deficient (Per2−/−) mice compared with the WT (SI Appendix, Fig. S2G). Slug expression may also have been slightly higher, although the difference was not statistically significant. Together these data indicated a new role of PER2 in EMT regulation.
PER2 Is Recruited to EMT Promoters via OCT1 (POU2F1).
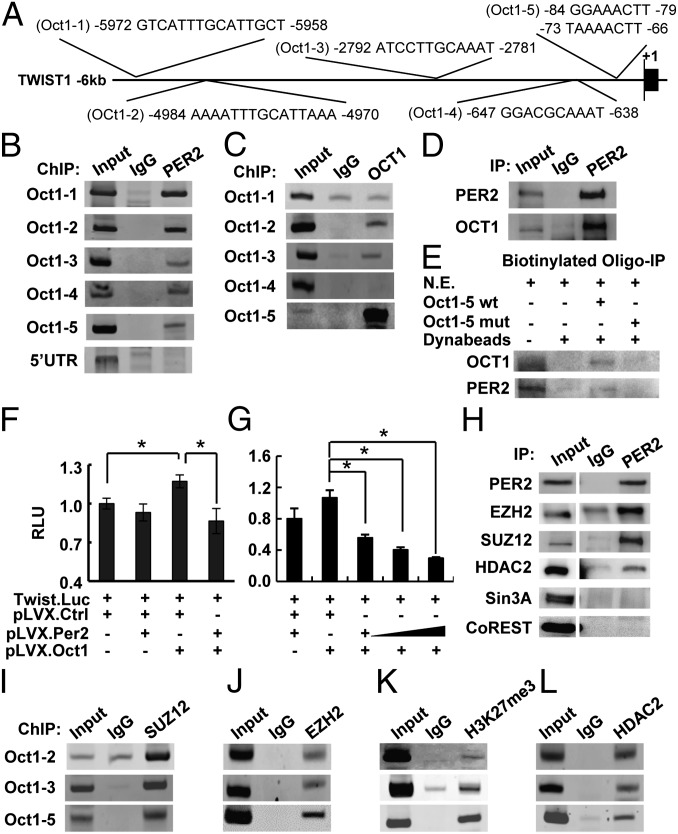
PER2 does not contain a DNA binding domain. Therefore, it must regulate gene expression as a cofactor, which interacts with other transcription regulators. Using several promoter analysis databases (34–37), we identified OCT1 as a transcription factor that may recruit PER2 to binding sites on the TWIST1, SLUG, and SNAIL promoters (Fig. 3A; SI Appendix, Fig. S4A). To test this hypothesis, we performed ChIP assays in MCF-10A cells using an anti-PER2 antibody and primer sets amplifying 150- to 250-bp fragments spanning the predicted OCT1 binding regions. PER2 was associated with all predicted OCT1 sites of the TWIST1, SLUG, and SNAIL promoters but not the 5′-UTR or the far site control region (Fig. 3B; SI Appendix, Fig. S4B). ChIP assays using an anti-OCT1 antibody further confirmed strong binding of OCT1 to the proximal OCT1 sites on the TWIST1 (Oct1-5) and SLUG (Oct1-2) promoters (Fig. 3C; SI Appendix, Fig. S4C). A weaker OCT1 binding to the TWIST1 Oct1-2 and Oct1-3 sites and the SLUG Oct1-1 site was also observed (Fig. 3C; SI Appendix, Fig. S4C). However, ChIP assay failed to detect any binding to the OCT1 site of the SNAIL promoter (SI Appendix, Fig. S4C) or to the TWIST1 Oct1-1 and Oct1-4 sites (Fig. 3C).
Fig. 3.
PER2 recruited corepressors to suppress OCT1-mediated TWIST1 promoter activity. (A) Diagram showing the five predicted OCT1 sites (Oct1-1–Oct1-5) on the TWIST1 promoter. (B and C) ChIP of PER2 (B) or OCT1 (C) on the TWIST1 promoter in MCF-10A cells. Normal IgG (IgG) and a site in 5′-UTR were used as controls for PER2-promoter association. (D) Coimmunoprecipitation (Co-IP) of PER2 and OCT1 in MCF-10A cells. Normal IgG was used as a control. (E) Immunoprecipitation of OCT1 or PER2 using MCF-10A nuclear extract (N.E.) and biotin-labeled oligonucleotides containing WT or mutated (mut) Oct1-5 site. (F and G) Relative fold change in luciferase activity of the TWIST1 reporter construct in HEK-293T cells transiently cotransfected with OCT1 or/and PER2 expression plasmids. Data show means ± SD. *P < 0.05 (Student t test). (H) Co-IP of PER2 and EZH2, SUZ12, HDAC2, Sin3A, or CoREST in MCF-10A cells. (Left) EZH2 and HDAC2 immunoblots were input images from the same blots with different exposure time. (I–L) ChIP of SUZ12 (I), EZH2 (J), trimethylated Histone 3 lysine 27 (H3K27me3) (K), and HDAC2 (L) in MCF-10A cells (SI Appendix, Figs. S4 and S5).
Coimmunoprecipitation (Co-IP) showed a strong interaction between endogenous PER2 and OCT1 in MCF-10A cells (Fig. 3D). Also, in vitro biotinylated oligonucleotide immunoprecipitation assays confirmed that binding of OCT1 and PER2 on both the TWIST1 (Oct1-5) and SLUG (Oct1-2) promoters (Fig. 3E; SI Appendix, Fig. S4D) involved the PER2–OCT1 interaction. OCT1 and PER2 binding was disrupted when the OCT1 binding sites were mutated (Fig. 3E; SI Appendix, Fig. S4D). These results indicated that, although PER2 lacks a DNA binding domain, it can be recruited to the TWIST1 and SLUG promoters through OCT1.
PER2 Suppresses OCT1-Mediated TWIST1 and SLUG Promoter Activity.
Transient reporter assays using reporter vectors containing −139 to +48 (Oct1-5 site) of the TWIST1 proximal promoter (38) or −908 to −2 (including the Oct1-2 binding site) of the SLUG promoter region (39) demonstrated that PER2 acted as a transcriptional corepressor. OCT1 alone acted as a transactivator and induced both TWIST1 and SLUG promoter activities. PER2 alone had no significant effect, consistent with its lack of DNA binding domain (Fig. 3F; SI Appendix, Fig. S4E). However, when PER2 and OCT1 were coexpressed, both promoter activities were suppressed in a dose-dependent manner (Fig. 3 F and G; SI Appendix, Fig. S4E). These results indicated that PER2 could be recruited and suppress TWIST1 or SLUG expression via association with OCT1. To confirm these results, the EMT gene levels in SKBR-3 cells with perturbed OCT1 or PER2 expression was evaluated. OCT1 depletion reduced EMT gene expression, confirming its transactivator role (SI Appendix, Fig. S5A). PER2 overexpression repressed these EMT genes, but OCT1 depletion in the PER2-overexpressing cells abolished PER2-mediated EMT suppression (SI Appendix, Fig. S5B). These data suggested that OCT1 is required for PER2-mediated EMT gene suppression.
PER2 Suppresses TWIST1 and SLUG Transcription by Recruiting EZH2, SUZ12, and HDAC2.
Corepressor complexes can be large multiprotein complexes that include polycomb repressive complex 2 (PRC2), which catalyzes trimethylation of histone H3 lysine 27 (H3K27me3), as well as histone deacetylases (HDACs), which deacetylate lysine residues on the core histones. Both of these histone modifications are associated with repression of transcription. Using Co-IP assays, we found a PER2 interaction with EZH2, SUZ12, and HDAC2, but not with other corepressors such as Sin3A and CoREST, which are known to complex with HDACs (40, 41) (Fig. 3H). ChIP assays indicated colocalization of PER2 and EZH2/SUZ12 on the OCT1-binding regions of the TWIST1 and SLUG promoters (Fig. 3 I and J; SI Appendix, Fig. S4 F and G). ChIP assays also detected the repressive trimethylated histone 3 lysine 27 (H3K27me3) mark on all of the OCT1/PER2/EZH2/SUZ12 binding regions (Fig. 3K; SI Appendix, Fig. S4H). HDAC2 was also found associated with all of these promoter regions (Fig. 3L; SI Appendix, Fig. S4I). Taken together, these data suggested a mechanism by which PER2 suppressed TWIST1 and SLUG transcription by recruitment of a repressor complex containing EZH2, SUZ12, and HDAC2.
Hypoxia Down-Regulates PER2 Posttranslationally to De-Repress EMT Gene Expression.
Hypoxia promotes EMT, invasion, and metastasis (38, 42), whereas our data indicated that PER2 represses EMT genes. To test whether hypoxia promotion of EMT involves a change in PER2, we examined PER2 protein levels of MCF-10A cells at various times under hypoxia (1% O2) (Fig. 4A). A significant decrease of PER2 protein was observed within 6 h after transfer to 1% O2 along with induction of the hypoxia marker HIF1α (Fig. 4A). Cycloheximide treatment and the pulse-chase assay showed that the half-life of PER2 was reduced under hypoxia (Fig. 4B; SI Appendix, Fig. S6). Such a rapid decrease in PER2 half-life suggested that a hypoxia-dependent posttranslational modification such as phosphorylation may promote PER2 degradation (43). It has been reported that phosphorylation of PER2 S662 facilitates phosphorylation of other PER2 serines by casein kinase-1 δ/ε (CK1δ/ε) and subsequently triggers proteasomal degradation of the hyperphosphorylated PER2 (44, 45). Consistent with this, an increase in hyperphosphorylated PER2 on hypoxia was detected by a PER2-S662 phospho-specific antibody (Fig. 4C), and PER2 translocation from nucleus to cytoplasm was observed after 6 h of hypoxia (Fig. 4D; SI Appendix, Fig. S7A). A phospho-null PER2 mutant (S662A) could not be degraded in hypoxia (SI Appendix, Fig. S8). Also, treatment with proteasome inhibitors MG132 and lactacystin prevented PER2 degradation (Fig. 4E) and increased ubiquitinated PER2 (SI Appendix, Fig. S9). These data indicated that hypoxia induced PER2 posttranslational modification, leading to proteasome degradation.
Fig. 4.
Hypoxia-induced PER2 degradation resulted in suppressor complex dissociation and de-repression of TWIST1 promoter transactivation. (A) Time course assay using MCF-10A cells cultured in normoxia or hypoxic conditions (1% oxygen). PER2 levels were determined by Immunoblotting. HIF1α accumulation was used to verify the activation of hypoxic response. (B) Time course assay using MCF-10A treated with cycloheximide (CHX) (100 μg/mL) in normoxia or hypoxia. PER2 expression was determined by immunoblotting. (C) Phosphorylated PER2 species in MCF-10A cells cultured in hypoxia were detected using immunoblotting with PER2-S662 phospho-specific antibody. (D) Immunofluorescence (IF) of PER2 in MCF-10A cells at 6 h in normoxia or hypoxia. Nuclear matrix protein p84 and DAPI were used as nuclear staining controls. Arrows indicate PER2 staining. (Scale bar, 10 μm.) (E) Time course assay using MCF-10A treated with proteasome inhibitors MG132 (20 μM) or lactacystin (20 μM) in hypoxia. PER2 expression was determined by immunoblotting. (F) Time course reporter assay using MCF-10A transfected with the TWIST1 promoter construct in hypoxia. Relative fold change in luciferase activity was shown. Data shown is means ± SD. *P < 0.05 (Student t test). (G) Time course ChIP of PER2, OCT1, EZH2, SUZ12, HeK27me3, HDAC2, HIF1α, and acetylated lysine (Ac-lysine) on Oct1-5 region of the TWIST1 promoter in MCF-10A cells cultured in hypoxia. For all immunoblot assays, tubulin was used as a loading control. All experiments were performed at least two times with similar results. One representative result is shown (SI Appendix, Figs. S6–S9).
Transient reporter assays showed that both the TWIST1 and SLUG promoter activities were induced on hypoxia treatment (Fig. 4F; SI Appendix, Fig. S7B). ChIP analysis of MCF-10A cells under 0–24 h of hypoxia demonstrated that promoter-bound PER2 could no longer be detected after the first 6 h of hypoxia (Fig. 4G; SI Appendix, Fig. S7 C and D). Correspondingly, strong binding of EZH2 and HDAC2 was only detected at the beginning of hypoxia treatment when PER2 was still associated with the promoter (Fig. 4G; SI Appendix, Fig. S7 C and D). Later in the hypoxia time course when PER2 binding was no longer detected, the repressive epigenetic mark of H3K27 trimethylation decreased, whereas the activating mark of lysine acetylation increased (Fig. 4G; SI Appendix, Fig. S7 C and D). Importantly, a strong HIF1α binding signal on the TWIST1 promoter was observed at 24 h on hypoxia treatment (Fig. 4G). These data suggested a progression by which hypoxia activates EMT by first removing PER2-mediated repression and then transactivating EMT gene expression by HIF1α.
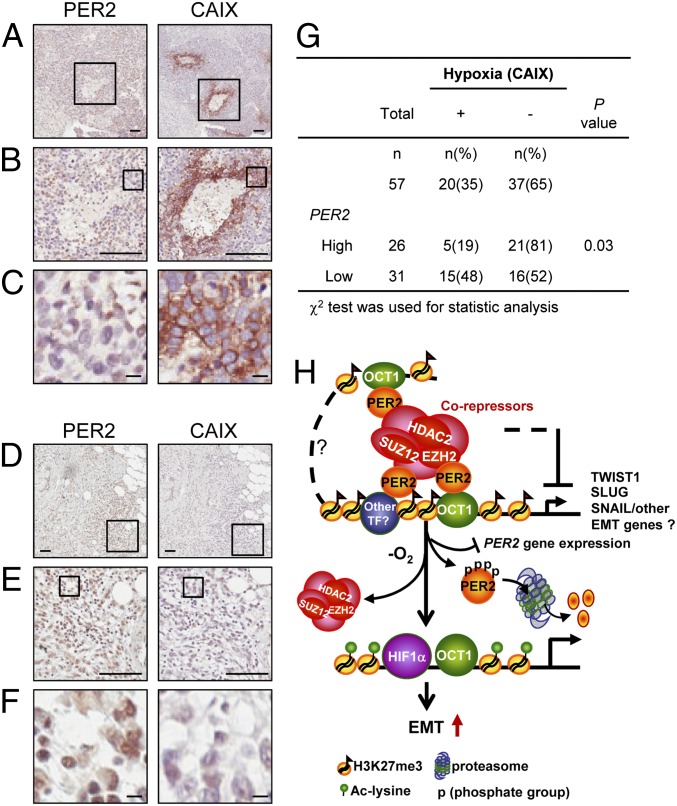
To confirm these in vitro findings, we obtained a cohort of 101 breast cancer specimens. The characteristics of these cases are given in SI Appendix, Table S1. Immunohistochemistry (IHC) of serial paraffin embedded sections of 57 breast cancer cases found a significant negative correlation between PER2 expression and hypoxia using CAIX (carbonic anhydrase IX), a HIF1α downstream target, as a hypoxia marker (46) (Fig. 5 A–G; P = 0.03). Severely hypoxic samples (high CAIX membrane staining) showed low or nondetectable PER2 nuclear staining (Fig. 5 A–C). In contrast, samples with no detectable CAIX membrane staining showed high PER2 nuclear staining (Fig. 5 D–F). Consistent with this result, PER2 gene expression in MCF-10A cells treated with hypoxia was down-regulated (SI Appendix, Fig. S10A). Data from human breast tumor gene expression profiling (31) also found a significant negative correlation between CAIX and PER2 expression (SI Appendix, Fig. S10B; r = −0.35, P = 0.03). These results indicated that hypoxia not only promoted PER2 protein degradation, but also triggered PER2 gene suppression by an as yet unknown mechanism. Our in vitro experiments and clinical data all support a model whereby PER2 down-regulation is a key step in induction of EMT gene expression under hypoxia (Fig. 5H).
Fig. 5.
Hypoxia induces PER2 suppression. (A–F) Representative pictures of the IHC serial sections of PER2 nuclear and CAIX membrane staining in a CAIX-positive/PER2-low case (A–C) and a CAIX-negative/PER2-high case (D–F). Boxes show the enlarged area. (A and D) Original magnification, 100×. (Scale bar, 100 μm.) B and E, original magnification, 400×. (Scale bar, 100 μm.) C and F, original magnification, 1,600×. (Scale bar, 10 μm.) (G) Correlation of CAIX with PER2 gene expression in 57 breast cancer cases. χ2 test was used. (H) Diagram of PER2 function as a repressor in EMT regulation. PER2 recruits a corepressor complex including EZH2, SUZ12, and HDAC2 to the promoters of EMT genes via interaction with OCT1. Hypoxia induces PER2 protein proteasomal degradation and PER2 gene suppression and causes dissociation of corepressors, which leads to reactivation of EMT gene expression (SI Appendix, Fig. S10).
Suppression of PER2 Is Associated with Tumor Malignancy and Poor Clinical Outcome in Breast Cancer Patients.
To explore whether PER2 can influence clinical outcomes, we analyzed PER2 expression in the 101 breast cancer tissue specimens. Consistent with our molecular mechanism, PER2 expression was inversely correlated with tumor size (P = 0.002; Fig. 6A), tumor stage using the TNM (tumor, node, metastasis) staging system (Pearson’s correlation, r = −0.22, P = 0.02), and histological grading (P < 0.001; Fig. 6B). This correlation indicated an association between reduction of PER2 expression and tumor malignancy. Moreover, a lower level of PER2 expression was associated with poor prognosis in breast cancer patients (P = 0.01; Fig. 6C). The hazard ratio of patients with high PER2 expression was 0.37-fold (95% CI: 0.14–0.99; P = 0.04) of those with low PER2 expression after adjusting for age, tumor size, lymph node status, and estrogen receptor and progesterone receptor expression (SI Appendix, Table S2). Further analysis showed that patients with a low level of PER2 and high level of TWIST1 (PER2low/TWIST1high) had worse prognosis compared with those with PER2high/TWIST1low (group 1 vs. group 2, P = 0.03; Fig. 6D). In high TWIST1 expression cases, low PER2 expression was still associated with worse survival rate (group 1 vs. group 4, P = 0.04; Fig. 6D), indicating that other PER2 target genes in addition to TWIST1 may contribute to this clinical outcome. These results indicated that down-regulation of PER2 enhances tumor malignancy in breast cancer patients.
Fig. 6.
PER2 suppression is associated with tumor malignancy and poor prognosis. (A) Regression analysis of PER2 mRNA expression vs. tumor size in 101 breast cancer patients. (B) Trend test of PER2 expression and histological grading in breast cancer patients. (C) Comparison of the overall survival periods of patients with different levels of PER2 gene expression using the Kaplan-Meier method. (D) Comparison of the overall survival periods of patients with different patterns of PER2 and TWIST1 gene expression using the Kaplan–Meier method. (E) Schematic diagram of the central role of PER2 in tumorigenesis promoted by hypoxic microenvironments.
Discussion
Our results provide a unique mechanism describing how PER2 serves as a tumor suppressor to restrict cancer development. First, we demonstrated that loss of PER2 in breast epithelial cells, either normal or cancerous cells, was able to modulate EMT, an important part of cancer stem cell formation as well as the first step in conversion of early stage tumors into invasive malignancies. Consistent with all of these mechanistic data, a positive relationship between loss of PER2 expression and breast cancer malignancy was observed. Second, we demonstrated that PER2 serves as a corepressor to assemble a repression complex at OCT1 binding sites for this tumor suppressor function. This PER2-OCT1 interaction effectively converted OCT1 sites, which normally activate expression, into repressor sites by recruitment of a polycomb repressor complex including EZH2 and SUZ12, as well as HDAC2. The repression was released in response to hypoxia, which caused PER2 protein degradation and thus destroyed the connection between OCT1 sites and the repressor complex (Fig. 5H). Importantly, this mechanism appears to be specific to PER2, because PER1 and PER3 were not degraded on hypoxia treatment (SI Appendix, Fig. S11). Also, hypoxia-induced PER2 protein degradation is HIF1α independent, because PER2 level did not change when a nondegradable HIF1α was overexpressed (SI Appendix, Fig. S12). These observations further indicate that PER2 has a distinct function in tumor progression and that PER2 degradation under hypoxia is a specific mechanism to reactivate EMT gene expression. Taken together, our results provide a mechanistic underpinning for a central role of PER2 in tumorigenesis promoted by hypoxic microenvironments (Fig. 6E).
The data indicated that PER2 regulates EMT genes in a cellular-specific manner, whereas PER2 down-regulation could promote tumorigenicity and invasion ability independently of cellular context. Thus, there could be a more complex role of PER2 in EMT involving additional PER2 target genes. PER2 was the core component of a repressor complex assembled at OCT1 sites, and lack of PER2 led to dissolution of the repressor complex and reversal of the repressive epigenetic marks (Fig. 4). We did observe differences between promoter sites that indicate that additional transcription factors may modify PER2 binding or that there may be heterogeneity in the repressor complex anchored by PER2 (Figs. 2 and 3; SI Appendix, Figs. S2 and S13). Study of these cell type– and promoter-specific differences will further elucidate the role of PER2.
The connection between EMT and cancer stemness is also of interest. For example, BMI1, an important factor for maintaining cancer stem cell self-renewal ability, was directly up-regulated by TWIST1 and played an essential role for hypoxia-mediated EMT (33). Also, SLUG expression, along with SOX9, could convert differentiated luminal cells into mammary stem cells and promote BCSC phenotypes (47). These observations are consistent with our findings that PER2 lies upstream of TWIST1 and SLUG (Fig. 2) and that PER2 suppression increases the prevalence of two different types of BCSCs (44) (SI Appendix, Fig. S14). Because PER2 has influence on BCSC prevalence and, most importantly, their EMT property, it represents a potential target for therapeutic development.
Epidemiological studies have suggested that altered circadian rhythms may be a crucial risk factor contributing to tumorigenesis, but the underlying mechanisms are unknown (11, 48, 49). Many physiological functions are modulated by the circadian system via chromatin remodeling (50–52). Disruption of circadian outputs may promote tumorigenesis via epigenetic modification. Thus, a disrupted circadian rhythm, such as in shift workers and frequent flyers, may contribute to tumor initiation, and then the hypoxic tumor microenvironment further induces PER2 degradation and gene suppression to promote tumor growth and malignancy. Overall, our findings provide a mechanistic link between the circadian clock and tumorigenesis and a basis to further elucidate the central role of PER2 and its clinical significance.
Materials and Methods
All breast tissue specimens are from BioBank of the Tri-Service General Hospital (Taipei, Taiwan). All patients were given informed consent, which was approved by the institutional review board of the Tri-Service General Hospital. Animal care and experiments were approved by the Institutional Animal Care and Utilization Committee of Academia Sinica (IACUC#080085). All data points of cell line experiments were performed in at least triplicate, and all experiments were performed at least three times with similar results. One representative result is shown. Detailed materials and methods used in this research are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Paul E. Verslues (Institute of Plant and Microbial Biology, Academia Sinica) for critical reading of the manuscript and Ms. Meng-Han Wang, Kai-Lin Peng, Mr. Chun-Chin Chen, Wen-Ting Lo, Alfie Chen, Dr. Li-Jung Juan, Ruey-Hwa Chen, Hungwen Chen, Shiaw-Wei Tyan, Heng-Hsiung Wu, and Shih-Chia Huang for kind assistance throughout this study. This work was supported by an internal research grant of Academia Sinica and an Academia Sinica Postdoctoral Research Fellowship (to W.W.H.-V.). E.Y.-H.P.L. is supported by National Cancer Institute Grant CA137102 and the BCRF-35127 fund.
Footnotes
Conflict of interest statement: According to the UCI policy, W.-H.L. declares that he serves as a member of Board of Directors of the biotech company, GeneTex. This arrangement has been reviewed and approved by UCI conflict of interest committee.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222684110/-/DCSupplemental.
References
- 1.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: Implications for biological rhythms. J Biol Rhythms. 2001;16(3):196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 3.Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8(10):790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 4.Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21(6):470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- 5.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 6.Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oishi K, et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278(42):41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 8.Schibler U. The daily timing of gene expression and physiology in mammals. Dialogues Clin Neurosci. 2007;9(3):257–272. doi: 10.31887/DCNS.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipski E, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94(9):690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 10.Sack RL, et al. American Academy of Sleep Medicine Circadian rhythm sleep disorders: Part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schernhammer ES, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93(20):1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67(21):10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 13.Cao Q, et al. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69(19):7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Climent J, et al. Deletion of the PER3 gene on chromosome 1p36 in recurrent ER-positive breast cancer. J Clin Oncol. 2010;28(23):3770–3778. doi: 10.1200/JCO.2009.27.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C, Weaver DR, Reppert SM. Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol Cell Biol. 2004;24(2):584–594. doi: 10.1128/MCB.24.2.584-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Im JS, Jung BH, Kim SE, Lee KH, Lee JK. Per3, a circadian gene, is required for Chk2 activation in human cells. FEBS Lett. 2010;584(23):4731–4734. doi: 10.1016/j.febslet.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Gery S, et al. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22(3):375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010;24(4):345–357. doi: 10.1101/gad.564110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimaldi B, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12(5):509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 21.Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia. 2007;9(10):797–800. doi: 10.1593/neo.07595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjöblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 23.Chen ST, et al. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26(7):1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 24.Hwang-Verslues WW, et al. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30(21):2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 25.Fu L, Lee CC. The circadian clock: Pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3(5):350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 26.Hua H, et al. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97(7):589–596. doi: 10.1111/j.1349-7006.2006.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua H, et al. Inhibition of tumorigenesis by intratumoral delivery of the circadian gene mPer2 in C57BL/6 mice. Cancer Gene Ther. 2007;14(9):815–818. doi: 10.1038/sj.cgt.7701061. [DOI] [PubMed] [Google Scholar]
- 28.Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26(57):7916–7920. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki K, Wakabayashi M, Hara Y, Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes to Cells. 2010;15(4):351–358. doi: 10.1111/j.1365-2443.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 30.Gu X, et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012;19(3):397–405. doi: 10.1038/cdd.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May CD, et al. Epithelial-mesenchymal transition and cancer stem cells: A dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13(1):202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang MH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12(10):982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 34.Karolchik D, et al. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004;32(Database issue):D493–D496. doi: 10.1093/nar/gkh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemeyer T, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26(1):362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messeguer X, et al. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18(2):333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 37.Farré D, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31(13):3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang MH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 39.Fujita N, et al. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113(2):207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 40.You A, Tong JK, Grozinger CM, Schreiber SL. CoREST is an integral component of the CoREST- human histone deacetylase complex. Proc Natl Acad Sci USA. 2001;98(4):1454–1458. doi: 10.1073/pnas.98.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy L, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89(3):373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 42.Wu CY, Tsai YP, Wu MZ, Teng SC, Wu KJ. Epigenetic reprogramming and post-transcriptional regulation during the epithelial-mesenchymal transition. Trends Genet. 2012;28(9):454–463. doi: 10.1016/j.tig.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Vanselow K, Kramer A. Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:167–176. doi: 10.1101/sqb.2007.72.036. [DOI] [PubMed] [Google Scholar]
- 44.Hwang-Verslues WW, et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS ONE. 2009;4(12):e8377. doi: 10.1371/journal.pone.0008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128(1):59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loncaster JA, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: Correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61(17):6394–6399. [PubMed] [Google Scholar]
- 47.Guo W, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control. 2006;17(4):539–545. doi: 10.1007/s10552-005-9010-9. [DOI] [PubMed] [Google Scholar]
- 50.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16(5):462–467. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahar S, Sassone-Corsi P. Metabolism and cancer: The circadian clock connection. Nat Rev Cancer. 2009;9(12):886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 52.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S, Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: The interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41(1):81–86. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.