Abstract
Classic brown fat and inducible beige fat both dissipate chemical energy in the form of heat through the actions of mitochondrial uncoupling protein 1. This nonshivering thermogenesis is crucial for mammals as a defense against cold and obesity/diabetes. Cold is known to act indirectly through the sympathetic nervous systems and β-adrenergic signaling, but here we report that cool temperature (27–33 °C) can directly activate a thermogenic gene program in adipocytes in a cell-autonomous manner. White and beige fat cells respond to cool temperatures, but classic brown fat cells do not. Importantly, this activation in isolated cells is independent of the canonical cAMP/Protein Kinase A/cAMP response element-binding protein pathway downstream of the β-adrenergic receptors. These findings provide an unusual insight into the role of adipose tissues in thermoregulation, as well as an alternative way to target nonshivering thermogenesis for treatment of obesity and metabolic diseases.
Keywords: Ucp1, cold sensing
Maintaining body temperature in a cold environment is key for the survival of euthermic animals. In mammals, it is achieved by both shivering and nonshivering thermogenesis. Nonshivering thermogenesis is mainly mediated by brown adipose tissue, in which chemical energy is dissipated in the form of heat through the actions of uncoupling protein 1 (UCP1) in mitochondria (1). In addition to its function to prevent hypothermia, brown fat has also been of interest as a defense against obesity and diabetes. Indeed, ablation of brown fat through the use of a UCP1 promoter-driven toxin has shown that loss of brown adipose tissue (BAT) causes an increased propensity to weight gain and diabetes in experimental animals (2). Similarly, ablation of UCP1 itself causes increased fat accumulation and insulin resistance in mice, at least at thermoneutrality (3). Because of the recent realization that adult humans have active brown fat (4–6), UCP1-mediated thermogenesis has becoming a compelling subject for research in obesity and other metabolic disorders.
In addition to the “classic” brown fat that is formed during embryonic development, certain white fat depots contain cells that can activate UCP1 and a broad thermogenic program (7–11). This also occurs in response to the β-adrenergic stimulation that is downstream of cold exposure. These cells have been called beige (12) or brite adipocytes (13). Recently, it has been shown that these beige cells have a developmental origin (14, 15) and molecular signature (12) that is distinct from that of classic brown adipocytes. Although classic brown cells come from a muscle-like myogenic factor 5 (myf5)-positive lineage, the beige cells come from a myf5-negative lineage. Interestingly, the UCP1-positive adipose tissue of adult humans seems closer in its gene expression pattern to the beige cells of mice than to the classic brown cells (12).
Cold is a powerful environmental signal to activate thermogenesis in vivo. According to the best studied pathway, cold is first sensed by the sensory nerves in peripheral tissues. This information is then received and processed in the hypothalamus, which controls the activity of the sympathetic nervous system (SNS), leading to release of norepinephrine (NE) onto brown and beige fat cells. NE works through G protein-coupled β-adrenergic receptors (β-ARs) on adipocytes to activate the cAMP/Protein Kinase A/cAMP response element-binding protein signaling cascade that controls the transcription of the thermogenic gene program.
Thus, although cold is the natural stimulus for the activation of thermogenesis, the prevailing model is that it exerts its effect on adipocytes indirectly (i.e., via the central nervous system and SNS). Here, we report that cold can directly act on beige and white adipocytes in a cell-autonomous manner to activate the expression of a thermogenic gene program. This provides a unique view to the understanding and control of adaptive thermogenesis.
Results
β-AR–Independent Thermogenic Pathway in Subcutaneous Fat.
Mice that lack all three β-adrenergic receptors (β-less mice) have been shown to have severely impaired thermogenesis in their interscapular BAT in response to cold (16). Recently, it has become clear that certain white adipose depots can activate a thermogenic program once exposed to prolonged cold or β-adrenergic stimulation. Increasing evidence suggests that these cells play a significant role in thermogenesis and energy homeostasis (17); however, the extent to which the thermogenic capacity of these cells is dependent on β-adrenergic signaling is unclear.
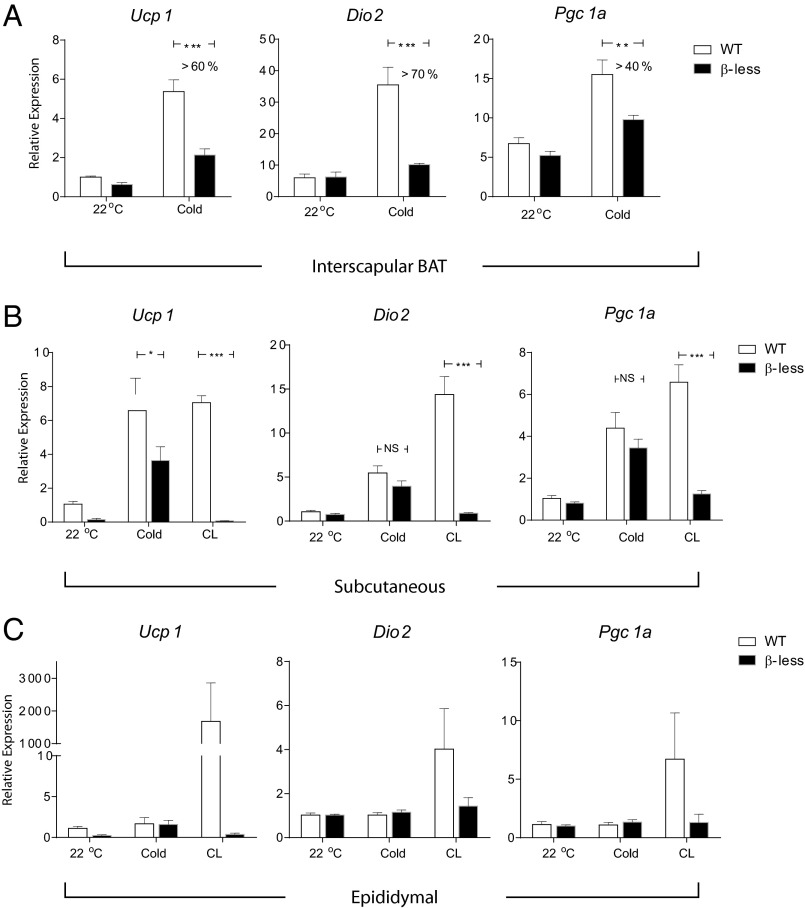
Prolonged exposure to 4 °C can be fatal to β-less mice (16); therefore, a milder cold challenge was given by exposing these animals to 10 °C for 20 h. As expected, the thermogenic gene expression [including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1a), Ucp1, and type II iodothyronine deiodinase (Dio2)] in the classic (interscapular) BAT was severely diminished in the β-less mice compared with the wild-type controls (Fig. 1A). Interestingly, and in contrast, the induction of thermogenic genes in the s.c. (inguinal) fat in the β-less mice was largely preserved on cold exposure (Fig. 1B), suggesting there is a β-AR–independent pathway that controls thermogenic gene expression in the s.c. fat. In the visceral (epididymal) fat, although a β3 agonist caused a β-AR-dependent thermogenic response, cold exposure did not lead to significant induction of thermogenic gene expression, as it did in the s.c. fat, suggesting the β-AR–independent thermogenic response is limited to the s.c. fat (Fig. 1C).
Fig. 1.
Cold induces a β-adrenergic–independent thermogenic gene program in s.c. adipose tissue. qPCR analysis of Ucp1, Dio2, and Pgc1a mRNA in interscapular brown fat (A), s.c. fat (inguinal) (B), and visceral fat (epididymal) (C) from β-less and WT mice. For cold exposure, mice were individually housed in 10 °C for 20 h. As a positive control, 24-h treatment of CL316243 (1 mg/kg, twice daily) was given. Data are presented as mean ± SEM (n = 6–8 in each group).
One obvious difference between s.c. and visceral fat depots is their anatomical locations. The s.c. fat serves as an insulating layer beneath the skin, and this makes the s.c. fat more likely to directly experience changes in environmental temperature. For example, it has been reported that temperature in s.c. fat can drop by 15–20 °C with acute cold exposure in humans (18). In contrast, visceral fat is located deeper in the abdominal cavity, which certainly experiences much less fluctuation in temperature. We thus hypothesized that the environmental temperature could directly contribute to differences in the regulation of the thermogenic program in these two fat depots.
Cool Temperature Induces a Thermogenic Program in White Adipocytes.
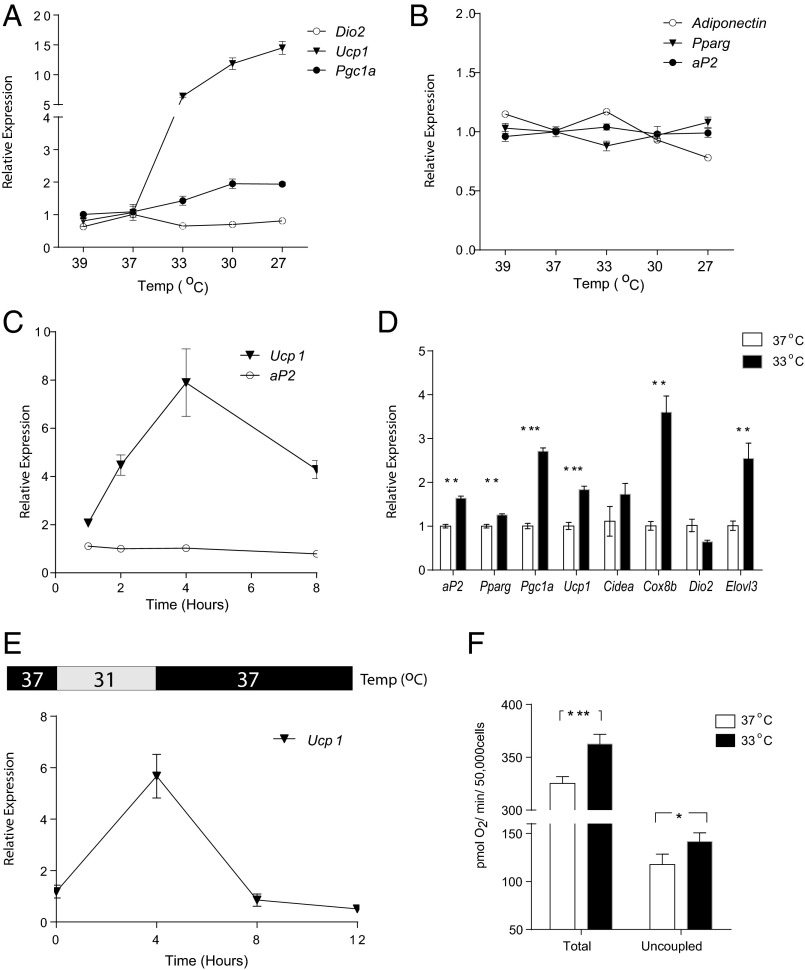
We tested this idea in an in vitro system with a clonal, immortalized fat cell line. We exposed fully differentiated 3T3-F442A adipocytes to a range of temperatures (27–39 °C) in culture. Interestingly, 4 h of cooling (27–33 °C) led to a robust increase of Ucp1 and Pgc1a gene expression (Fig. 2A); no significant changes were observed in the expression of genes that serve as markers of adipocyte differentiation such as adipocyte Protein 2 (aP2), peroxisome proliferator-activated receptor gamma (Pparg), and Adiponectin (Fig. 2B). Importantly, raising the temperature to 39 °C did not have the same effect on gene expression, suggesting that the induction of thermogenic genes is not a nonspecific response to the stress of nonphysiological temperatures. Moreover, as the buffering capacity of bicarbonate buffer can be sensitive to temperature, we compared the induction of gene expression in Hepes-based media with that in sodium-bicarbonate media and found no difference (Fig. S1A). This indicates that pH changes were unlikely to be responsible for the temperature-induced gene expression changes.
Fig. 2.
Cool temperatures induce a thermogenic program in isolated white adipocytes. Cultured and fully differentiated 3T3-F442A adipocytes were exposed to 39 °C, 37 °C, 33 °C, 30 °C, and 27 °C for 4 h before mRNA was harvested (Materials and Methods). The mRNA expression of thermogenic genes (A) and adipose markers (B) were measured by qPCR. (C) 3T3-F442A adipocytes were exposed to 31 °C for 1, 2, 4, and 8 h before mRNA was harvested and analyzed by qPCR. The values were normalized to cells kept in 37 °C from the same times. (D) 3T3-F442A adipocytes were differentiated and maintained in 37 °C or 33 °C for 10 d (day 0–10). mRNA was analyzed by qPCR for thermogenic gene expression. (E) 3T3-F442A adipocytes were first exposed to 31 °C for 4 h, and then the temperature was changed back to 37 °C for an additional 4–8 h. mRNA was analyzed by qPCR and normalized to cells kept in 37 °C from the same times. (F) Total and uncoupled respiration (as oxygen consumption rate) in 3T3-F442A adipocytes were measured after 6 h incubation in 37 °C or 31 °C. Data are presented as mean ± SD (n = 6 in each group).
We further characterized the temporal dynamics of the induction of thermogenic genes by cooling. In 3T3-F442A adipocytes, the increase of Ucp1 mRNA was seen as early as 1 h after temperature was changed to 31 °C, peaked at 4 h, and then stabilized at twofold after 8 h compared with control cells kept at 37 °C (Fig. 2C). In this 8-h time frame, similar changes of Ucp1 mRNA (Fig. S1B) and protein (Fig. S1C) were also observed in primary mouse adipocytes.
In more chromic studies, 3T3-F442A adipocytes were kept at cool temperature for up to 10 d, resulting in increased expression of a whole array of thermogenic genes without significant change in adipose markers (Fig. 2D). Notably, this temperature-sensitive induction is reversible: Ucp1 gene expression went back to baseline once the temperature was returned to 37 °C (Fig. 2E).
Finally, the bioenergetic consequence of the increased thermogenic gene expression was examined by measuring cellular respiration in 3T3-F442A adipocytes. As shown in Fig. 2F, 6 h of exposure to 31 °C led to a 10% increase in total respiration and a 20% increase in uncoupled respiration compared with cells kept at 37 °C. This demonstrates that the increases in thermogenic gene expression by cooling indeed have functional consequences.
Temperature-Induced Thermogenic Program Is Specific to White and Beige Adipocytes.
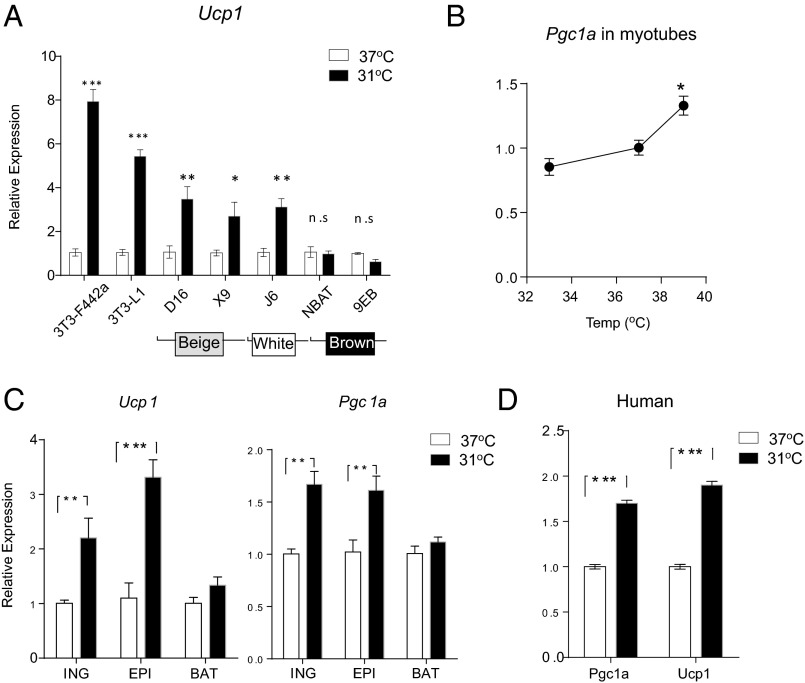
We have investigated the cell type selectivity of the temperature regulation of the thermogenic gene program. We compared 3T3-L1 and 3T3-F442A cells, two classic white adipocyte cell lines, as well as beige, white, and classic brown cell lines recently generated by our group (12). Exposure to 31 °C induced a robust increase of Ucp1 mRNA expression in all of the white and beige cell lines, but no induction was observed in the classic brown adipocytes (Fig. 3A), suggesting that the temperature-sensitive induction of Ucp1 expression is not a general characteristic of all cells involved in thermogenesis. Although UCP1 is adipose cell–specific, other temperature-sensitive thermogenic genes, such as Pgc1a, are also expressed in nonadipose cells including muscle cells; we were thus able to test the effect of different temperatures on Pgc1a expression in C2C12 myotubes. In contrast to what was observed in the white and beige adipocytes, a cool temperature (33 °C) suppressed Pgc1a mRNA expression in myotubes, whereas a very warm temperature (39 °C) mildly increased it (Fig. 3B). The latter observation is consistent with that of a previous report (19).
Fig. 3.
The temperature-induced thermogenic program is specific to white and beige adipocytes. (A) Multiple fat cell lines were exposed to 31 °C for 4 h before Ucp1 mRNA expression was measured by qPCR. (B) Differentiated C2C12 myotubes were exposed to 33 °C, 37 °C, or 39 °C for 4 h. mRNA was harvested and analyzed by qPCR for Pgc1a expression. Primary mouse adipocytes from inguinal, epididymal, and interscapular brown fat (C) and primary human adipocytes from s.c. fat (D) were exposed to 31 °C for 4 h. mRNA expression of Ucp1 and Pgc1a was measured by qPCR. Data are presented as mean ± SD (n = 6 in each group).
To test whether the temperature responsiveness of adipocytes could be observed in nonimmortalized cells, thermogenic gene expression was studied in primary adipocytes derived from various depots. Consistent with the observations in the cell lines, primary cells differentiated from precursors in the inguinal (s.c.) and epididymal (visceral) fat were capable of increasing their Ucp1 and Pgc1a mRNA expression by two- to threefold in response to cool temperature (Fig. 3C). No comparable induction was seen in primary brown adipocytes derived from the interscapular depot. Importantly, a similar increase in Ucp1 and Pgc1a mRNA expression was also observed in cool-exposed human primary adipocytes derived from s.c. fat (Fig. 3D).
Temperature-Induced Thermogenic Program Is Independent of the Canonical cAMP/CREB Signaling Pathway.
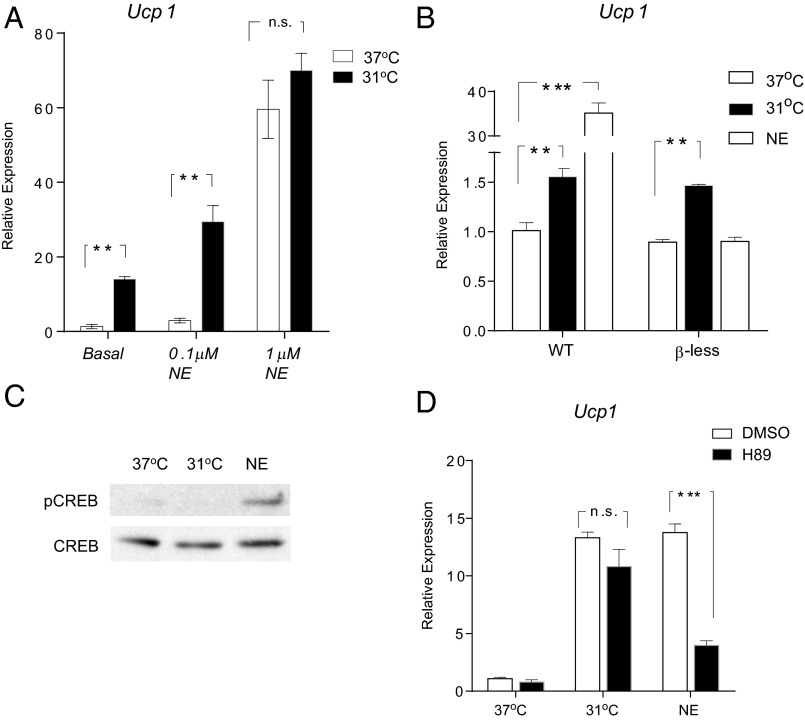
Induction of the cold-responsive thermogenesis program in classic brown and beige adipocytes in vivo is strongly influenced by the SNS and by the resulting function of the NE/β-AR/PKA/CREB axis. To investigate the interaction between this cell-autonomous, temperature-induced thermogenic program and the canonical pathway, 3T3-F442A adipocytes were treated with NE at 37 °C or at 31 °C. Interestingly, we found that cold and low-dose NE (0.1 μM) had a synergistic effect on inducing Ucp1 mRNA expression; however, this effect can be masked by a higher dose of NE (1 μM) (Fig. 4A).
Fig. 4.
The temperature-induced thermogenic program is independent of the canonical pathway. (A) Fully differentiated 3T3-F442A adipocytes were exposed to PBS (as “Basal”) or NE at indicated doses for 4 h at 37 °C or 31 °C. Ucp1 mRNA was measured by qPCR. (B) WT and β-less adipocytes (derived from the inguinal depots) were exposed to 31 °C or treated with 100 nM NE for 4 h before Ucp1 mRNA was measured by qPCR. (C) Fully differentiated 3T3-F442A adipocytes were exposed to 31 °C or 100 nM NE for 20 min, and cell lysates were analyzed by Western blot. (D) 3T3-F442A adipocytes were exposed to 31 °C or 100 nM NE for 4 h, with a 30-min pretreatment of either 10 μM H89 or DMSO. Ucp1 mRNA was analyzed by qPCR. Data are presented as mean ± SD (n = 6 in each group).
We then tested whether the β-ARs were required for the induction of Ucp1 mRNA in response to the cooling in culture. As expected, primary adipocytes lacking all three β-ARs (derived from β-less mice) completely lost their ability to elevate Ucp1 mRNA expression on NE stimulation compared with wild-type controls (Fig. 4B). However, these cells fully retained their ability to respond to the cooling, demonstrating definitively that the β-ARs are not required for this response (Fig. 4B).
Phosphorylation of CREB by PKA is a key step in mediating the signaling transduction between NE release by the SNS and Ucp1 gene expression. NE treatment at 100 nM robustly enhanced the phosphorylation of CREB at serine 133, as expected. However, we did not detect any increase in this phosphorylation event after exposing adipocytes for 20 min to 31 °C (Fig. 4C). Similarly, a well-studied PKA inhibitor (H89) failed to block the induction of the Ucp1 gene caused by cooling and greatly diminished the induction caused by NE stimulation (Fig. 4D). Altogether, these results strongly suggest that the temperature-induced thermogenic program described here is mediated by pathways that are independent of the canonical NE/β-AR/PKA/CREB axis.
Discussion
A key question raised by this work is how cool temperatures are directly sensed by fat cells. Agonism of the β-adrenergic receptors via the SNS is the classic and best-studied means by which cold can activate thermogenesis in brown (and beige) adipose tissues (1). In fact, agonism of the β-adrenergic systems has been shown to increase proliferation of classic brown fat cells (20, 21) as well as to increase expression of UCP1 and other thermogenic genes (10, 22). However, the broad tissue expression of the different β-ARs in many vital organ systems has limited the potential for using β-agonists as therapeutic agents in the context of obesity and other metabolic disorders. The discovery of this β-AR–independent, cell-autonomous, and temperature-sensitive thermogenic program in white adipose tissues could provide a new avenue for targeting energy balance in an in vivo setting.
The molecular mechanisms of thermosensation have mainly been studied in sensory neurons and keratinocytes. The thermo-transient receptor potential (TRP) channels, such as transient receptor potential cation channel subfamily V member 1-4 (TRPV1-4) (23-27), transient receptor potential cation channel subfamily M member 8 (TRPM8) (28, 29), and transient receptor potential cation channel subfamily A member 1 (TRPA1) (30), are the best-characterized molecular sensors for temperature in these systems. Among them, TRPV1, TRPV2, and TRPV4 have been shown to be expressed in adipocytes (31). Interestingly, both TRPV4 and TRPV1 negatively regulate a thermogenic program in fat (31). However, adipocytes lacking both TRPV1 and TRPV4 still have an intact induction of Ucp1 in response to cool temperature (Fig. S2), indicating that the temperature-sensing mechanism in adipocytes described here must be independent of TRPV1 or TRPV4. Nonetheless, given the overlapping functions of this family of proteins, it is still possible that other TRP channels might be involved in mediating the temperature sensitivity of fat cells.
Subcutaneous adipose tissue has been viewed as a white fat depot that is important for both energy storage and for thermic insulation. However, it has recently become clear that s.c. fat possesses substantial thermogenic capacity in response to cold or β-AR stimulation (7–11). In fact, there is a subset of adipocytes in this depot that can activate a robust thermogenic program; these represent a distinct cell type that has been termed beige (or brite) cells. Having a heat-producing tissue at the interface between the body and the outside environment seems likely to prevent a temperature gradient being generated from the surface to the core and thus provides a more effective way to maintain a more stable core body temperature than would otherwise be possible. In contrast, there was a small induction of Ucp1 mRNA (twofold) in the classic BAT independent of β-ARs (Fig. 1A), but it was probably not mediated by the cell-autonomous mechanism described here (Fig. 3 A and C). This could be worthy of further investigation.
Going forward, it will be important to determine whether the systems described here are robust enough to change the physiology and energy balance of an intact animal or human. In this regard, we note that the induction of thermogenic genes by cool temperatures is sufficient in isolated cells to increase both total respiration and the uncoupled fraction of respiration. Human studies have found that more brown fat and more active brown fat was present in individuals that were acutely exposed to cold (32), living in winter (4, 32) or chronically working in the cold (33). Of course, the prevailing view has been that this was mainly a result of increased SNS activity as a consequence of cold. However, it has been recently reported that the elevated thermogenesis in response to cold was not inhibited by chemical blockade of the β-ARs (34), suggesting it is at least partially independent of the β-ARs. Together with our findings here, it is possible that a substantial portion of cold-induced thermogenesis comes from a cell-autonomous effect of temperature itself on certain fat depots. Whether there is a way to capture this pathway for human therapeutics with devices or chemicals remains to be determined.
Materials and Methods
Animals.
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee of Dana–Farber Cancer Institute. Mice were kept on a standard diet (Research Diets) with 12-h light cycles. β-less mice and WT controls on a similar genetic background were provided by Brad Lowell (Beth Israel Deaconess Medical Center, Boston). Trpv4−/− mice (from Wolfgang Liedtke, Duke University, Durham, NC) (35) were crossed to Trpv1−/− mice (from Jackson Laboratory) to generate Trpv1−/−, Trpv4−/− double-knockout mice for making primary adipocytes. For cold exposure, mice were housed individually in a 10 °C incubator for 20 h with adequate food and water. For β3-agonist treatment, 1 mg/kg CL316243 was injected intraperitoneally twice daily. Seven- to 8-wk-old male mice were used in all experiments. Each group contained 7–8 animals. Experiments were performed with at least two independent cohorts.
Materials.
Phospho-CREB (9191) and CREB (9197) antibodies were from Cell Signaling. The PKA inhibitor H89 was from Cayman Chemical (10010556). CL316243 (C5976) and other chemicals were from Sigma Aldrich.
Cell Culture.
3T3-F442A adipocyte differentiation was induced by treating confluent cells with 850 nM insulin and 1 μM rosiglitazone for 2 d; cells were then maintained in 850 nm insulin for another 4 d. For immortalized beige, white, and brown cell lines, confluent cultures of clonal lines were exposed to induction DMEM/F-12 GlutaMAX (Invitrogen) containing dexamethasone (5 μM), insulin (0.5 μg/mL), isobutylmethylxanthine (0.5 mM), rosiglitazone (1 μM), T3 (1 nM), and 10% (vol/vol) FBS. From day 4 after induction, cells were maintained in media containing insulin (0.5 μg/mL), T3 (1 nM), and 10% (vol/vol) FBS until they were collected. For primary adipocytes, the stromal-vascular fraction from the inguinal fat pad of 5–6-wk-old male C57bl/6J (Jackson Laboratory) mice were prepared and differentiated for 8 d, as previously described (36). Human adipose stem cells from s.c. adipose tissue were obtained from consenting healthy donors undergoing liposuction (from Jeffrey Gimble, Pennington Biomedical Research Center, Baton Rouge, LA). Cells from 3 donors were used, and representative data were shown. Human adipose stem cells were isolated, cultured, and differentiated as described (37). C2C12 myoblasts were differentiated by treating confluent cells with DMEM with 2% (vol/vol) horse serum. The differentiation into myotubes took 3–5 d.
Cellular Cooling.
Fully differentiated adipocytes were kept in a 37 °C incubator with 10% (vol/vol) CO2 before experiments. Cells grew in 12-well or 6-well plates. Eight to 12 h before cooling treatment, medium was refreshed with prewarmed DMEM [supplemented with 10% (vol/vol) FBS, 850 nM insulin, and 20 mM Hepes at 37 °C]. For cooling, culture plates were taken out from the home incubator (37 °C) and immediately transferred to water baths inside CO2 incubators set at different temperatures for the indicated time. The temperature in each water bath was calibrated by thermometer.
qPCR and Western Blotting.
Total RNA from cultured cells or tissues was isolated using the TRIzol method (Invitrogen) combined with Qiagen RNAEasy minicolumns, according to the manufacturer’s instructions. For quantitative PCR (qPCR) analysis, RNA was reverse transcribed, using the ABI high-capacity cDNA synthesis kit, and was used in quantitative PCR reactions containing SYBR-green fluorescent dye (ABI). Relative expression of mRNAs was determined after normalization with TATA-binding protein (TBP) levels using the ΔΔCt method. For Western blot analysis, cells were lysed in RIPA buffer (0.5% Nonidet P-40, 0.1% sodium deoxycholate, 150 mM NaCl, 50 mM Tris-Cl at pH 7.5). Lysates were resolved by SDS-PAGE, transferred to PVDF membrane (Millipore), and probed with the indicated antibodies.
Cellular Respiration.
Oxygen consumption rate was determined with 3T3-F442A adipocytes, using a XF24 analyzer (Seahorse Bioscience). Briefly, at day 6 of differentiation, cells were plated into V7-PS plates coated with Cell-Tak adhesive (BD Bioscience) at 50,000 cells per well. On day 7, cells were either kept in 37 °C or transferred to a 31 °C incubator for 6 h. Growth medium was replaced with 37 °C unbuffered DMEM 15 min before the measurement to allow equilibrium. Measurement was at 37 °C, using 2–2–2-min intervals. Oligomycin (5 μg/mL) was used to probe uncoupled respiration. The baseline respiration before adding oligomycin was defined as “total respiration.”
Statistics.
Student t test was used for single comparisons. Unless otherwise specified, *P < 0.05, **P < 0.01, ***P < 0.001, and not significant (n.s.) P > 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Brad Lowell for providing the β-less mice, Dr. Wolfgang Liedtke for providing Trpv4−/− mice, and Dr. Jeffery Gimble for providing human adipose cells. We also thank Dr. Sandra Kleiner, Dr. Rana Gupta, Dr. Jorge Ruas, Dr. Patrick Seale, Dr. Jennifer Estall, Dr. Shingo Kajimura, and Dr. Rajan Sah for discussion. Support was provided by National Institutes of Health Interdisciplinary Training Grant DK071507 (to L.Y.) and National Institutes of Health Grant DK094824 (to J.W.) DK31403, and DK080261 (to B.M.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310261110/-/DCSupplemental.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366(6457):740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 7.Cousin B, et al. Occurrence of brown adipocytes in rat white adipose tissue: Molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 8.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21(6):465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 9.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102(2):412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himms-Hagen J, et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279(3):C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 11.Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol. 2005;25(18):8311–8322. doi: 10.1128/MCB.25.18.8311-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrovic N, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48(7):424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachman ES, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013;27(3):234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33(1):25–29. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CT, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol. 2012;112(3):354–361. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Géloën A, Collet AJ, Guay G, Bukowiecki LJ. Beta-adrenergic stimulation of brown adipocyte proliferation. Am J Physiol. 1988;254(1 Pt 1):C175–C182. doi: 10.1152/ajpcell.1988.254.1.C175. [DOI] [PubMed] [Google Scholar]
- 21.Bronnikov G, Houstĕk J, Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J Biol Chem. 1992;267(3):2006–2013. [PubMed] [Google Scholar]
- 22.Himms-Hagen J, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266(4 Pt 2):R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 23.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 24.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 25.Peier AM, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296(5575):2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 26.Güler AD, et al. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 28.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 29.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 30.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 31.Ye L, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151(1):96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46(4):339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- 34.Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: Possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96(4):E598–E605. doi: 10.1210/jc.2010-1957. [DOI] [PubMed] [Google Scholar]
- 35.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA. 2003;100(23):13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kajimura S, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460(7259):1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu G, et al. Adipogenic differentiation of adipose-derived stem cells. Methods Mol Biol. 2011;702:193–200. doi: 10.1007/978-1-61737-960-4_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.