Abstract
The d- and l-isomers of glyceraldehyde are equally effective in the inhibition of SS erythrocyte sickling in vitro. The following compounds at a concentration of 20 mM were ineffective in inhibiting sickling: glyceraldehyde-3-phosphate, d-erythrose, d-ribose, d-fructose, d-glucose, d-sucrose, dihydroxyacetone, and methylglyoxal. Glyceraldehyde does not reverse the sickling of cells in the deoxy state. The properties of purified hemoglobin after treatment with glyceraldehyde and of the hemoglobin isolated from treated cells are very similar; these results suggest that glyceraldehyde itself is the reactive species within the erythrocyte. Erythrocyte glutathione is decreased by treatment in vitro with the aldehyde.
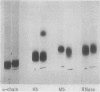
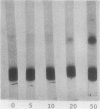
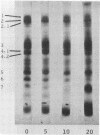
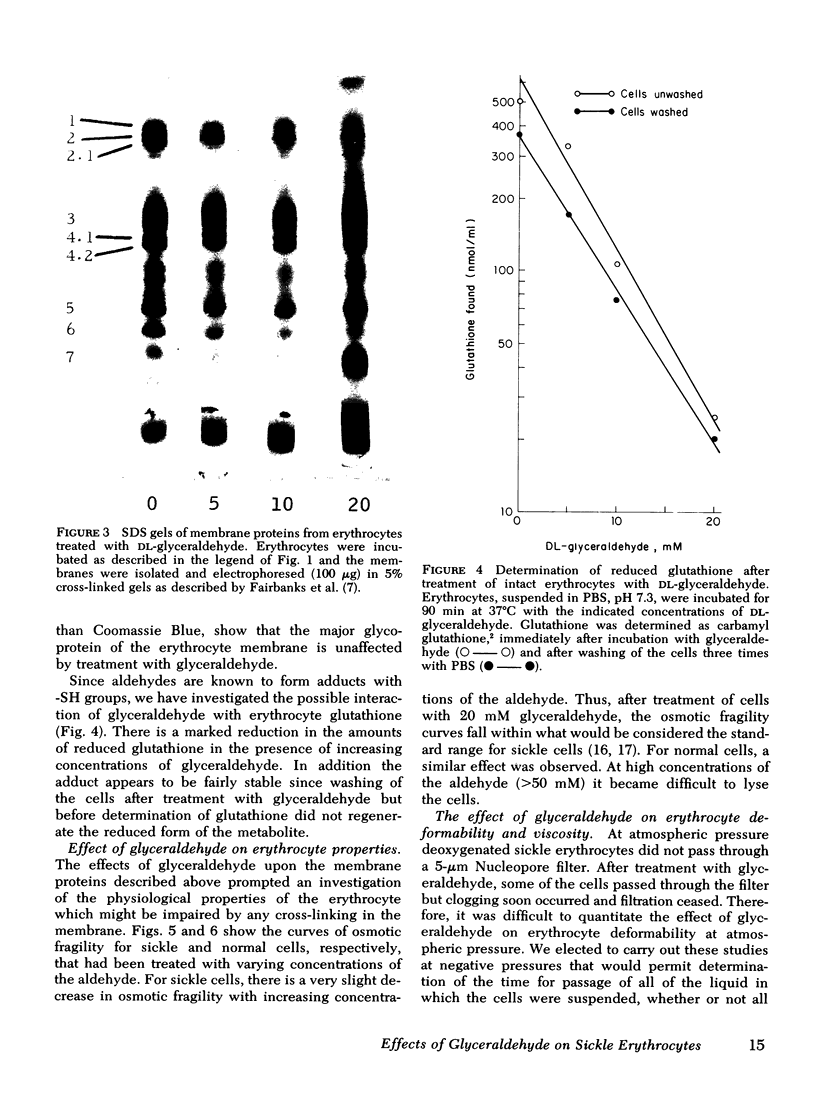
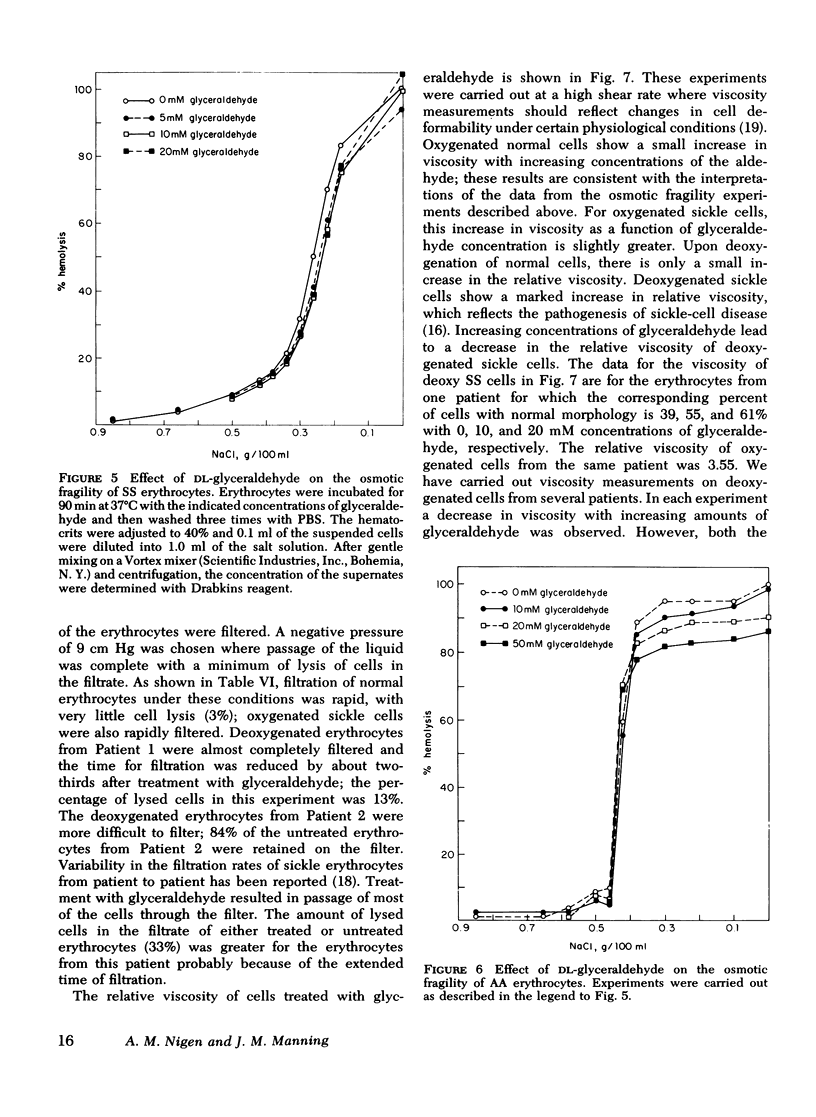
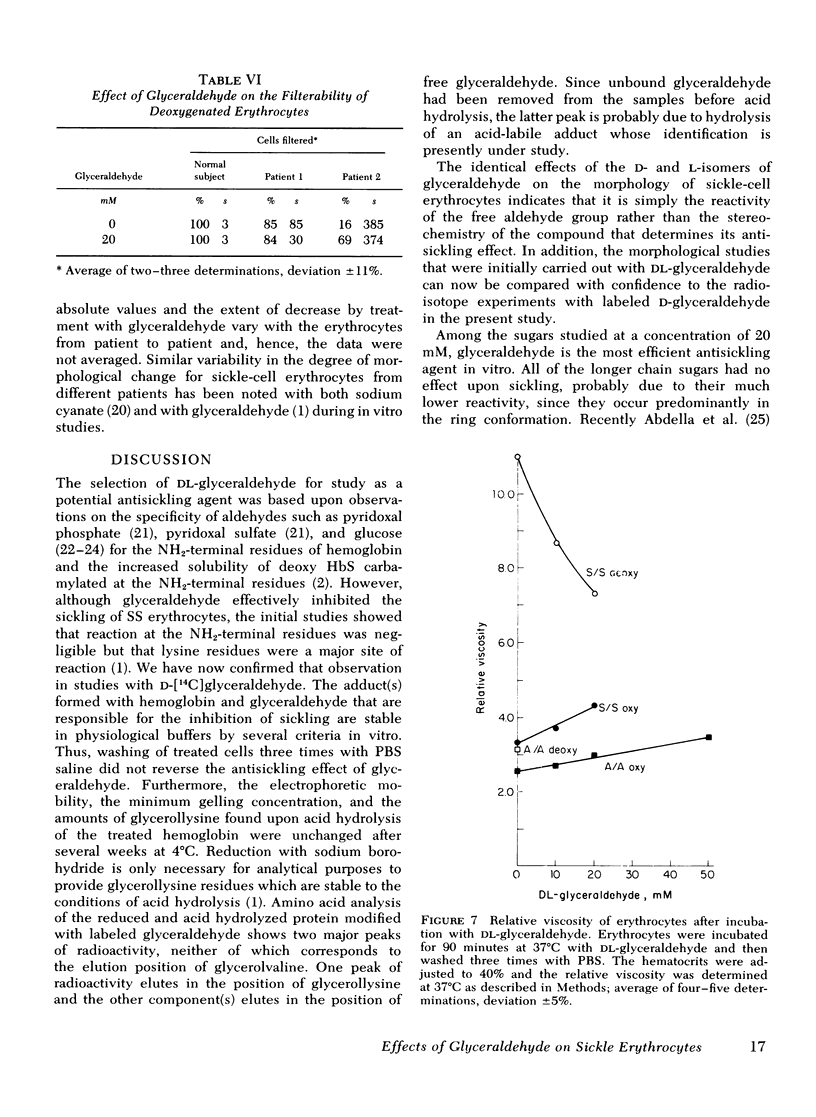
Relatively high concentrations of glyceraldehyde (50 mM) lead to a small amount (3%) of cross-linking between hemoglobin monomers as well as to some cross-linking of erythrocyte membrane proteins. Lower concentrations of dl-glyceraldehyde (5-20 mM), which reduce the sickling of erythrocytes in vitro, lead to proportionally less cross-linking of hemoglobin. Cells that have been treated with those concentrations of the aldehyde show little change in their osmotic fragility, exhibit improved filtration properties, and have a lowered viscosity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella P. M., Ritchey J. M., Tam J. W., Klotz I. M. Glycosylation of hemoglobin S by reducing sugars and its effect on gelation. Biochim Biophys Acta. 1977 Feb 22;490(2):462–470. doi: 10.1016/0005-2795(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Apple M. A., Ludwig F. C., Greenberg D. M. Selective cancer growth inhibition in mice by dihydroxypropanal without concomitant inhibition of bone marrow or other normal tissue. Oncology. 1970;24(3):210–222. doi: 10.1159/000224521. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yung S. Chemical modifications that inhibit gelation of sickle hemoglobin. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1504–1505. doi: 10.1073/pnas.71.4.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Guinto E. The reduction of glyceraldehyde by human erythrocytes. L-hexonate dehydrogenase activity. J Clin Invest. 1974 May;53(5):1258–1264. doi: 10.1172/JCI107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L. Ligand-induced conformational dependence of hemoglobin in sickling interactios. J Mol Biol. 1971 Sep 14;60(2):263–270. doi: 10.1016/0022-2836(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Furia F. G., Miller D. R., Cerami A., Manning J. M. The effects of cyanate in vitro on red blood cell metabolism and function in sickle cell anemia. J Clin Invest. 1972 Mar;51(3):566–574. doi: 10.1172/JCI106845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERSON C. P., Jr, SHEIN S. C., HAM T. H., FLEMING E. M., CASTLE W. B. Studies on the destruction of red blood cells. IX. Quantitative methods for determining the osmotic and mechanical fragility of red cells in the peripheral blood and splenic pulp; the mechanism of increased hemolysis in hereditary spherocytosis (congenital hemolytic jaundice) as related to the functions of the spleen. AMA Arch Intern Med. 1956 Jan;97(1):1–38. doi: 10.1001/archinte.1956.00250190017001. [DOI] [PubMed] [Google Scholar]
- Eng C. P., Bhatnagar M. K., Morgan J. F. Inhibition of mouse ascites tumors by carbohydrate combined with immunization. Can J Physiol Pharmacol. 1972 Feb;50(2):156–163. doi: 10.1139/y72-022. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gaines K. C., Salhany J. M., Tuma D. J., Sorrell M. F. Reaction of acetaldehyde with human erythrocyte membrane proteins. FEBS Lett. 1977 Mar 15;75(1):115–119. doi: 10.1016/0014-5793(77)80065-3. [DOI] [PubMed] [Google Scholar]
- HARKNESS J. A new instrument for the measurement of plasma-viscosity. Lancet. 1963 Aug 10;2(7302):280–281. doi: 10.1016/s0140-6736(63)90177-6. [DOI] [PubMed] [Google Scholar]
- HARRIS J. W., BREWSTER H. H., HAM T. H., CASTLE W. B. Studies on the destruction of red blood cells. X. The biophysics and biology of sickle-cell disease. AMA Arch Intern Med. 1956 Feb;97(2):145–168. doi: 10.1001/archinte.1956.00250200021002. [DOI] [PubMed] [Google Scholar]
- Holmquist W. R., Schroeder W. A. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2489–2503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Jensen M., Bunn H. F., Halikas G., Kan Y. W., Nathan D. G. Effects of cyanate and 2,3-diphosphoglycerate on sickling. Relationship to oxygenation. J Clin Invest. 1973 Oct;52(10):2542–2547. doi: 10.1172/JCI107445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant J. A., Steck T. L. Specificity in the association of glyceraldehyde 3-phosphate dehydrogenase with isolated human erythrocyte membranes. J Biol Chem. 1973 Dec 25;248(24):8457–8464. [PubMed] [Google Scholar]
- Koenig R. J., Cerami A. Synthesis of hemoglobin AIc in normal and diabetic mice: potential model of basement membrane thickening. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3687–3691. doi: 10.1073/pnas.72.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHAMMAD A., OLCOTT H. S., FRAENKEL-CONRAT H. The reaction of proteins with acetaldehyde. Arch Biochem. 1949 Dec;24(2):270–280. [PubMed] [Google Scholar]
- Nigen A. M., Manning J. M. Inhibition of erythrocyte sickling in vitro by DL-glyceraldehyde. Proc Natl Acad Sci U S A. 1977 Jan;74(1):367–371. doi: 10.1073/pnas.74.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigen A. M., Njikam N., Lee C. K., Manning J. M. Studies on the mechanism of action of cyanate in sickle cell disease. Oxygen affinity and gelling properties of hemoglobin S carbamylated on specific chains. J Biol Chem. 1974 Oct 25;249(20):6611–6616. [PubMed] [Google Scholar]
- Nigen A. M., Peterson C. M., Gillette P. N., Manning J. M. Determination of the blood concentrations of cyanate after intravenous administration to patients with sickle-cell disease. J Lab Clin Med. 1974 Jan;83(1):139–146. [PubMed] [Google Scholar]
- Riddle V., Lorenz F. W. Nonenzymic, polyvalent anion-catalyzed formation of methylglyoxal as an explanation of its presence in physiological systems. J Biol Chem. 1968 May 25;243(10):2718–2724. [PubMed] [Google Scholar]
- Rieber E. E., Veliz G., Pollack S. Red cells in sickle cell crisis: observations on the pathophysiology of crisis. Blood. 1977 Jun;49(6):967–979. [PubMed] [Google Scholar]
- Van Heyningen R. The metabolism of D-glyceraldehyde by the lens. Biochem J. 1969 Apr;112(2):211–220. doi: 10.1042/bj1120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar P. S., Hards J. M., Brooks D. E., Hagenberger B., Seaman G. V. Physicochemical effects of aldehydes on the human erythrocyte. J Cell Biol. 1972 Jun;53(3):809–818. doi: 10.1083/jcb.53.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]