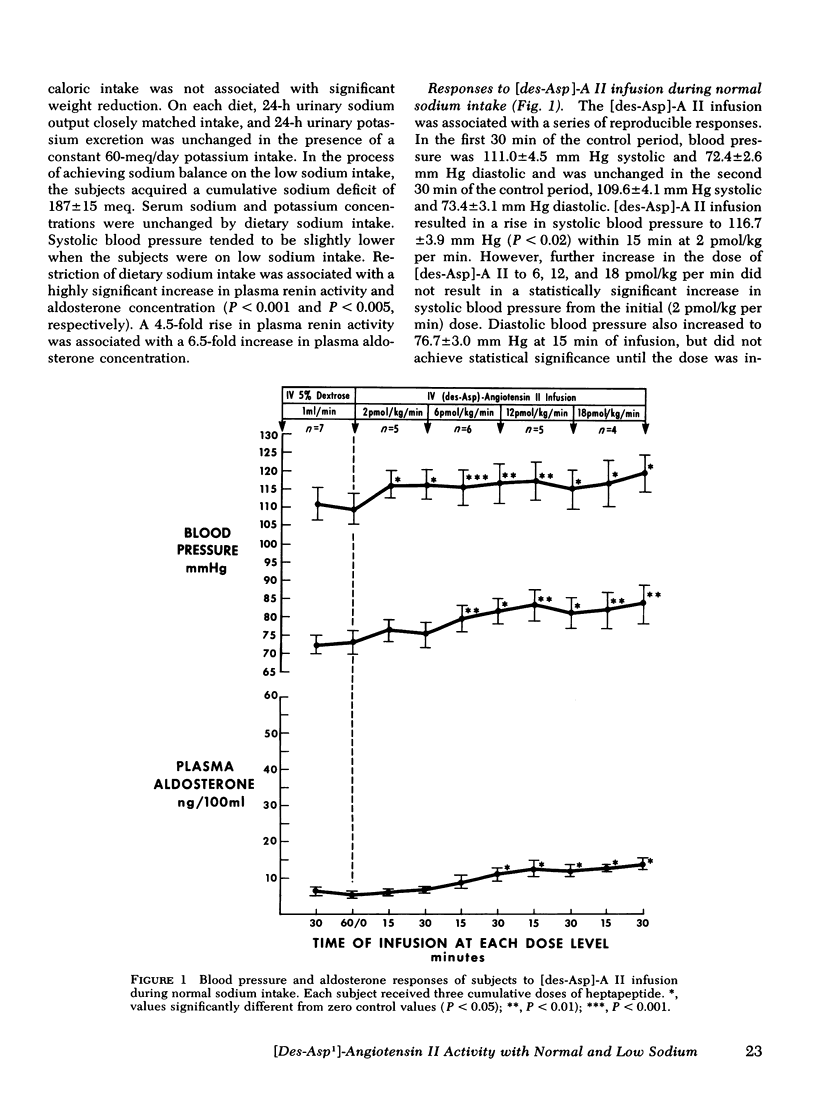
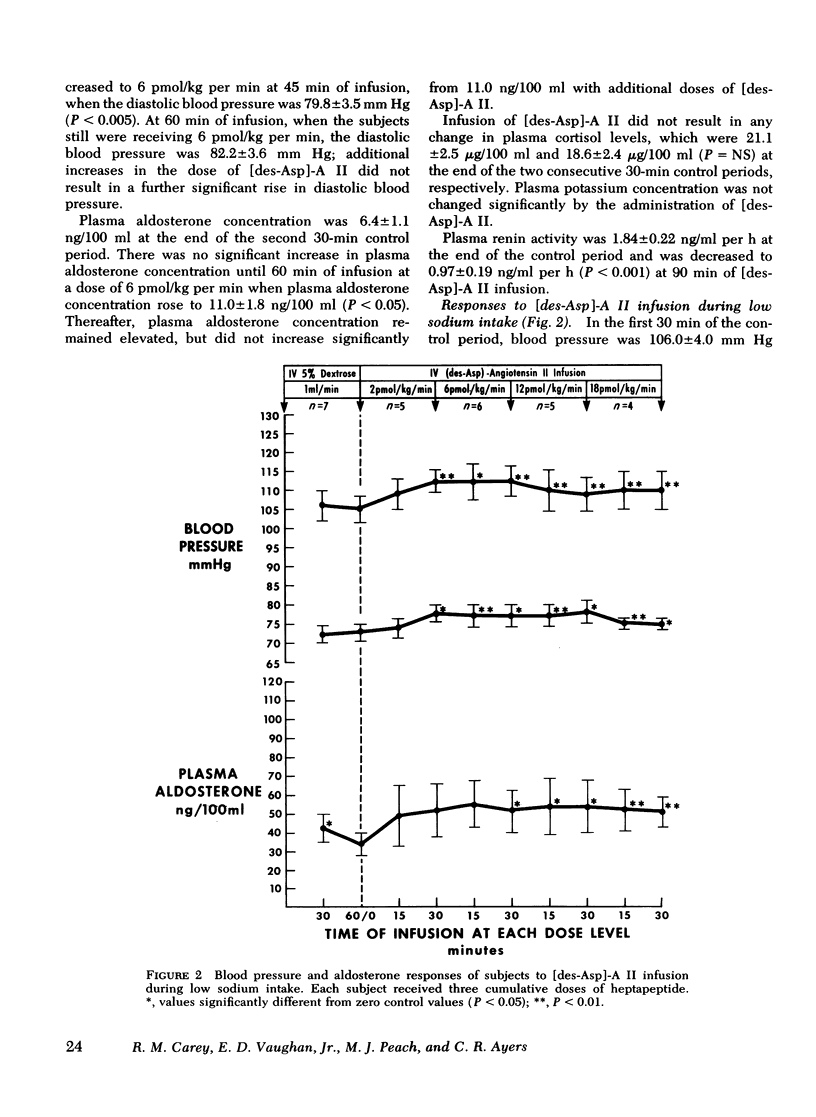
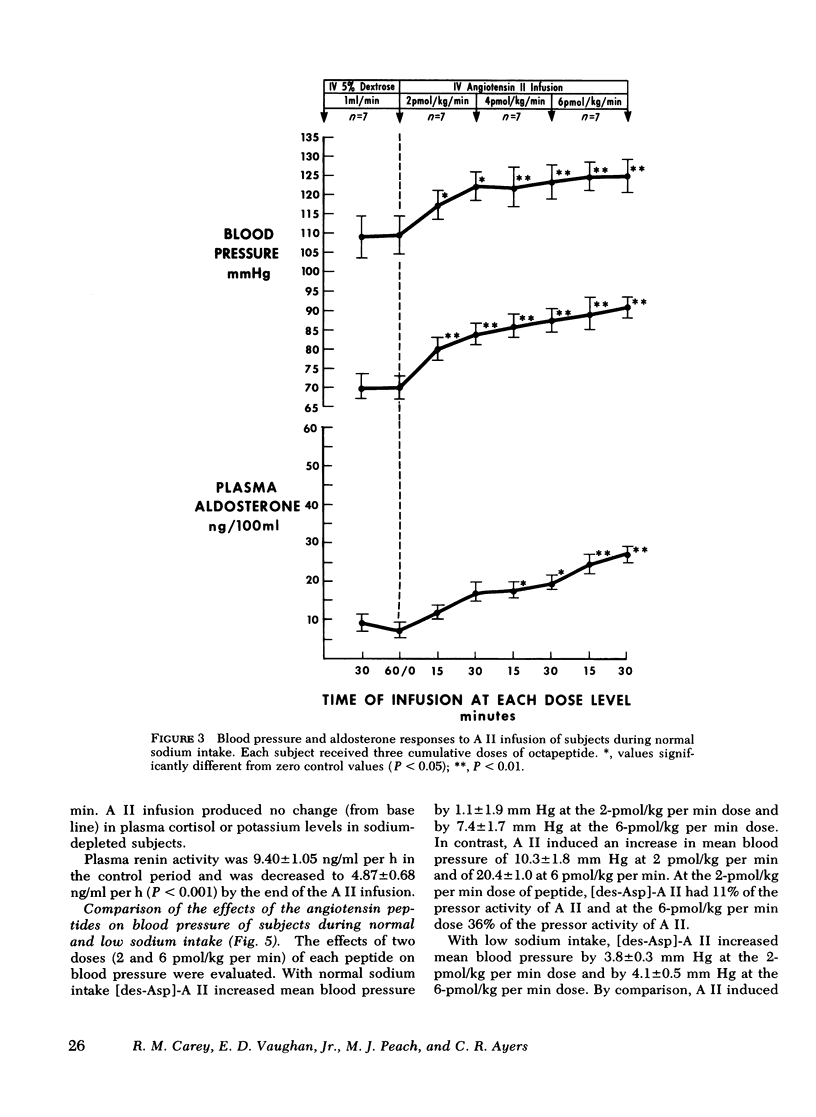
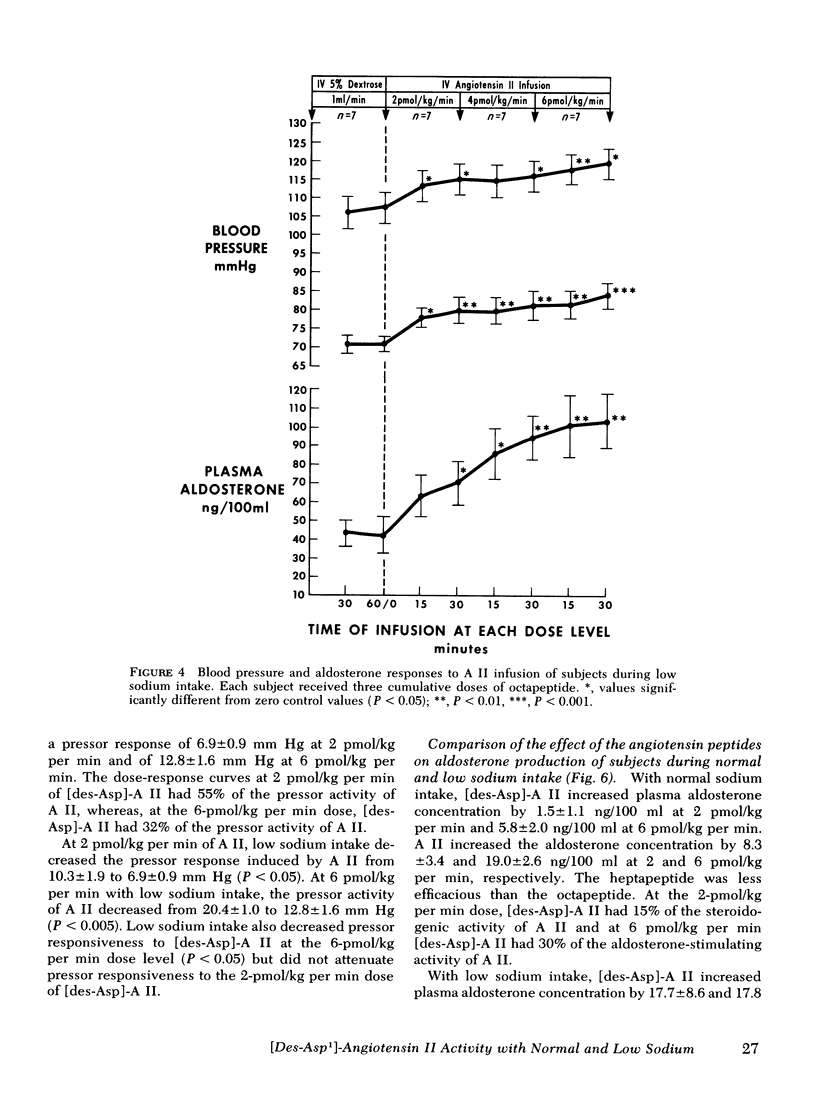
Abstract
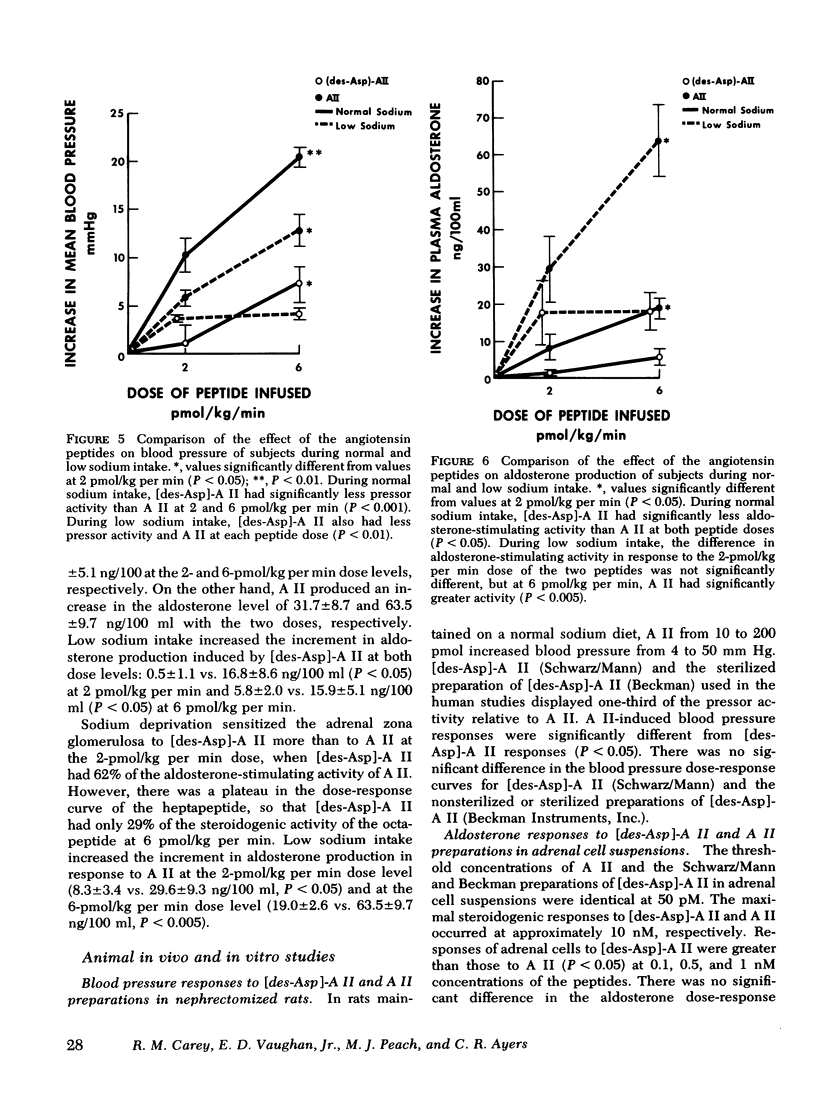
This study was designed to compare the effect of [des-Aspartyl1]-angiotensin II ([des-Asp]-A II) and angiotensin II (A II) on blood pressure and aldosterone production in man under conditions of normal and low sodium (Na) intake. Seven normal male subjects in balance on constant normal Na intake (UNa V 160.3±5.0 meq/24 h) for 5 days received A II and [des-Asp]-A II infusions on two consecutive days; 1 mo later they were restudied after 5 days of low Na intake (UNa V 10.5±1.6 meq/24 h). Each dose was infused for 30 min, sequentially. During normal Na intake, [des-Asp]-A II from 2 to 18 pmol/kg per min increased mean blood pressure from 85.2±3 to 95.3±5 mm Hg and plasma aldosterone concentration from 5.2±1.1 to 14.3±1.9 ng/100 ml. During low Na intake, the same dose of [des-Asp]-A II increased mean blood pressure from 83.7±3 to 86.7±3 mm Hg and plasma aldosterone concentration from 34.4±6.0 to 51.0±8.2 ng/100 ml. In contrast, A II from 2 to 6 pmol/kg per min during normal Na intake increased mean blood pressure from 83.3±4 to 102.3±4 mm Hg and plasma aldosterone concentration from 7.0±2.2 to 26.8±2.0 ng/100 ml; during low Na intake, A II increased mean blood pressure from 83.0±3 to 96.0±4 mm Hg and plasma aldosterone concentration from 42.0±9.7 to 102.2±15.4 ng/100 ml. A II and [des-Asp]-A II were equally effective in suppressing renin release. Plasma cortisol and Na and K concentration did not change.
The effects of two doses (2 and 6 pmol/kg per min) of each peptide on blood pressure and aldosterone production were evaluated. During normal Na intake, [des-Asp]-A II had 11-36% of the pressor activity and 15-30% of the steroidogenic activity of A II. Na deprivation attenuated the pressor response and sensitized the adrenal cortex to both peptides, but the increase in steroidogenesis was greater with [des-Asp]-A II than with A II. The dose-response curves for [des-Asp]-A II with respect to blood pressure and aldosterone production were not parallel, and although no maximum was established for A II, [des-Asp]-A II was less efficacious.
In summary, (a) [des-Asp]-A II has biologic activity in man, (b) [des-Asp]-A II is less efficacious than A II in stimulating aldosterone production, (c) Na deprivation sensitizes the adrenal cortex more markedly to [des-Asp]-A II than A II, and (d) dose-response curves for the two peptides differ, suggesting the possibility that they act at different receptor sites in vascular smooth muscle and the adrenal cortex.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES R. P., BORKOWSKI A. J., SICINSKI A. M., LARAGH J. H. PROLONGED INFUSIONS OF ANGIOTENSIN II AND NOREPINEPHRINE AND BLOOD PRESSURE, ELECTROLYTE BALANCE, AND ALDOSTERONE AND CORTISOL SECRETION IN NORMAL MAN AND IN CIRRHOSIS WITH ASCITES. J Clin Invest. 1965 Jul;44:1171–1186. doi: 10.1172/JCI105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair-West J. R., Coghlan J. P., Denton D. A., Funder J. W., Scoggins B. A., Wright R. D. The effect of the heptapeptide (2-8) and hexapeptide (3-8) fragments of angiotensin II on aldosterone secretion. J Clin Endocrinol Metab. 1971 Apr;32(4):575–578. doi: 10.1210/jcem-32-4-575. [DOI] [PubMed] [Google Scholar]
- Bravo E. L., Khosla M. C., Bumpus F. M. Action of (1-des(aspartic acid), 8-isoleucine) angiotensin II upon the pressor and steroidogenic activity of angiotensin II. J Clin Endocrinol Metab. 1975 Mar;40(3):530–533. doi: 10.1210/jcem-40-3-530. [DOI] [PubMed] [Google Scholar]
- Bravo E. L., Khosla M. C., Bumpus F. M. The role of angiotensins in aldosterone production. Circ Res. 1976 Jun;38(6 Suppl 2):104–107. doi: 10.1161/01.res.38.6.104. [DOI] [PubMed] [Google Scholar]
- Brecher P. I., Pyun H. Y., Chobanian A. V. Studies on the angiotensin II receptor in the zona glomerulosa of the rat adrenal gland. Endocrinology. 1974 Oct;95(4):1026–1033. doi: 10.1210/endo-95-4-1026. [DOI] [PubMed] [Google Scholar]
- Bunag R. D., Page I. H., McCubbin J. W. Inhibition of renin release by vasopressin and angiotensin. Cardiovasc Res. 1967 Jan;1(1):67–73. doi: 10.1093/cvr/1.1.67. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Brooks S. N., Pettinger W. A. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science. 1974 May 31;184(4140):994–996. doi: 10.1126/science.184.4140.994. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Peach M. J. Inhibition of induced aldosterone biosynthesis with a specific antagonist of angiotensin II. Proc Natl Acad Sci U S A. 1974 Feb;71(2):341–344. doi: 10.1073/pnas.71.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. H., Davis J. O., Lohmeier T. E. Des-1-Asp-angiotensin II. Possible intrarenal role in homeostasis in the dog. Circ Res. 1975 Jul;37(1):30–34. doi: 10.1161/01.res.37.1.30. [DOI] [PubMed] [Google Scholar]
- Freeman R. H., Davis J. O., Lohmeier T. E., Spielman W. S. Evidence that des-Asp1 angiotensin II mediates the renin-angiotensin response. Circ Res. 1976 Jun;38(6 Suppl 2):99–103. doi: 10.1161/01.res.38.6.99. [DOI] [PubMed] [Google Scholar]
- Hollenberg N. K., Chenitz W. R., Adams D. F., Williams G. H. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest. 1974 Jul;54(1):34–42. doi: 10.1172/JCI107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. M., Goodfriend T. L., McCallum Z. T., Dempsey P. J., Cooper T. Inotropic agents in hypoxic cat myocardium: depression and potentiation. Circ Res. 1972 Feb;30(2):196–204. doi: 10.1161/01.res.30.2.196. [DOI] [PubMed] [Google Scholar]
- Kono T., Oseko F., Shimpo S., Nanno M., Endo J. Biological activity of des-asp1-angiotensin II (angiotensin III) in man. J Clin Endocrinol Metab. 1975 Dec;41(06):1174–1177. doi: 10.1210/jcem-41-6-1174. [DOI] [PubMed] [Google Scholar]
- LARAGH J. H., ANGERS M., KELLY W. G., LIEBERMAN S. Hypotensive agents and pressor substances. The effect of epinephrine, norepinephrine, angiotensin II, and others on the secretory rate of aldosterone in man. JAMA. 1960 Sep 17;174:234–240. doi: 10.1001/jama.1960.03030030014003. [DOI] [PubMed] [Google Scholar]
- Laragh J. H., Baer L., Brunner H. R., Buhler F. R., Sealey J. E., Vaughan E. D., Jr Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972 May;52(5):633–652. doi: 10.1016/0002-9343(72)90054-x. [DOI] [PubMed] [Google Scholar]
- MATTINGLY D. A simple fluorimetric method for the estimation of free 11-hydroxycorticoids in human plasma. J Clin Pathol. 1962 Jul;15:374–379. doi: 10.1136/jcp.15.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. F., HAll M. M., Khairallah P. A. A comparison of the effects of angiotensin II and heptapeptide on smooth muscle (vascular and uterine). Eur J Pharmacol. 1976 Sep;39(1):101–107. doi: 10.1016/0014-2999(76)90117-5. [DOI] [PubMed] [Google Scholar]
- Oelkers W., Brown J. J., Fraser R., Lever A. F., Morton J. J., Robertson J. I. Sensitization of the adrenal cortex to angiotensin II in sodium-deplete man. Circ Res. 1974 Jan;34(1):69–77. doi: 10.1161/01.res.40.4.69. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Ackerly J. A. Angiotensin antagonists and the adrenal cortex and medulla. Fed Proc. 1976 Nov;35(13):2502–2507. [PubMed] [Google Scholar]
- Peach M. J., Sarstedt C. A., Vaughan E. D., Jr Changes in cardiovascular and adrenal cortical responses to angiotensin III induced by sodium deprivation in the rat. Circ Res. 1976 Jun;38(6 Suppl 2):117–121. doi: 10.1161/01.res.38.6.117. [DOI] [PubMed] [Google Scholar]
- Sarstedt C. A., Vaughan E. D., Jr, Peach M. J. Selective inhibition by des-1-Asp-8-lle-angiotensin ii of the steroidogenic response to restricted sodium intake in the rat. Circ Res. 1975 Sep;37(3):350–358. doi: 10.1161/01.res.37.3.350. [DOI] [PubMed] [Google Scholar]
- Semple P. F., Boyd A. S., Dawes P. M., Morton J. J. Angiotensin II and its heptapeptide (2-8), hexapeptide (3-8), and pentapeptide (4-8) metabolites in arterial and venous blood of man. Circ Res. 1976 Nov;39(5):671–678. doi: 10.1161/01.res.39.5.671. [DOI] [PubMed] [Google Scholar]
- Semple P. F., Morton J. J. Angiotensin II and angiotensin III in rat blood. Circ Res. 1976 Jun;38(6 Suppl 2):122–126. doi: 10.1161/01.res.38.6.122. [DOI] [PubMed] [Google Scholar]
- Shade R. E., Davis J. O., Johnson J. A., Gotshall R. W., Spielman W. S. Mechanism of action of antiotensin II and antidiuretic hormone on renin secretion. Am J Physiol. 1973 Apr;224(4):926–929. doi: 10.1152/ajplegacy.1973.224.4.926. [DOI] [PubMed] [Google Scholar]
- Steele J. M., Jr, Neusy A. J., Lowenstein J. The effects of des-Asp1-angiotensin II on blood pressure, plasma aldosterone concentration, and plasma renin activity in the rabbit. Circ Res. 1976 Jun;38(6 Suppl 2):113–116. doi: 10.1161/01.res.38.6.113. [DOI] [PubMed] [Google Scholar]
- Taub K. J., Caldicott W. J., Hollenberg N. K. Angiotensin antagonists with increased specificity for the renal vasculature. J Clin Invest. 1977 Mar;59(3):528–535. doi: 10.1172/JCI108668. [DOI] [PMC free article] [PubMed] [Google Scholar]


