Although the viscoelastic properties of naturally occurring biopolymers have been studied for some time, their structural and molecular origins are only now being elucidated. In PNAS, a report by Münster et al. (1) describes experimental studies of the origins of the nonlinear mechanical response of fibrin and collagen with large deformations. These results have biological and clinical significance because they provide some ideas about the origin of the mechanical properties of the extracellular matrix and blood clots, which may be shared by other fibrous biopolymers.
Three-dimensional extracellular matrices are scaffolds that influence the shape, development, and behavior of tissues, as well as provide an organized lattice within which cells can migrate and interact. The vertebrate extracellular matrix consists of glycosaminoglycans and fibrous proteins, including collagen, with embedded cells. These fibrous networks bear most of the mechanical loads that act on tissues, making them flexible and protecting cells, yet allowing their movement. Fibrin is a special type of extracellular polymeric protein network formed in clotted blood to provide a structural support for hemostatic clots and obstructive thrombi that experience mechanical loads imposed by blood flow and wound stretching. These biopolymer networks are subject to both small and very large deformations, and their mechanical behavior is essential for biological functions.
Both fibrin and collagen are branched networks of fibers, the structural and mechanical properties of which can vary greatly, with the lengths, thicknesses, and densities of the fibers and branch points modulated by the conditions of formation. These networks are viscoelastic polymers, which means that they have both elastic and inelastic (viscous or plastic) properties. An elastic material deforms quickly with applied stress, maintains constant deformation under load, and immediately regains its initial shape when the stress is removed. In contrast, an inelastic material shows delays in responding to applied stress and undergoes creep during sustained application of constant stress. This dual nature of biopolymers is central to their behavior, functions, and pathologies. For example, stiff fibrin clots have been associated with coronary heart disease and other thrombotic pathologies (2, 3), whereas less-stiff clots have been associated with bleeding (4). Similarly, inelastic deformations of fibrin may enhance the ability of clots to stem bleeding because of decreased permeability and allow the restoration of obstructed flow in vessels, as well as reducing embolization. Collagen is the most abundant protein in mammals and its stiffness is essential to the functioning of many tissues, including tendons, ligaments, skin, and blood vessels, and its inelastic properties allow remodeling of these structures.
Fibrin is highly extensible polymer, which means that under stress blood clots will tend to stretch rather than break. Even fibrin clots that have been covalently cross-linked by the transglutaminase factor XIIIa can be stretched three- to fourfold before they rupture (5). Fibrin also experiences a large decrease in volume with extension (negative compressibility or densification), and the resulting increase in density helps to stem bleeding, and thrombi will be reduced in size, preventing obstruction of vessels. In contrast, collagen is much less extensible and does not experience large changes in volume with stretching. It now appears that partial unfolding of some fibrin domains is necessary to account for the viscoelastic properties of fibrin clots (5, 6). The γ-nodule unfolds first, with the α-helical coiled-coil acting as a highly elastic molecular spring to store the mechanical energy and undergoing a conformational change to β-sheet (7, 8). Forced molecular unfolding of collagen has also been shown to be a potential mechanism for stress accommodation during deformation (9, 10).
At small deformations of these biopolymers, stress is directly proportional to strain and the slope of the curve, or the elastic modulus (stiffness), is constant. At large strains, the modulus of the polymer increases greatly (11, 12), a phenomenon called “strain hardening,” which may be important biologically because fibrin or collagen polymers will be compliant at normal strain levels and then become stiffer at larger deformations that could otherwise threaten the integrity of these materials. Structural changes underlying the elastic properties of fibrin and collagen polymers occur at different, yet interconnected, spatial levels: namely the molecular level, individual fibers, fiber network, and macroscopic (5, 13, 14).
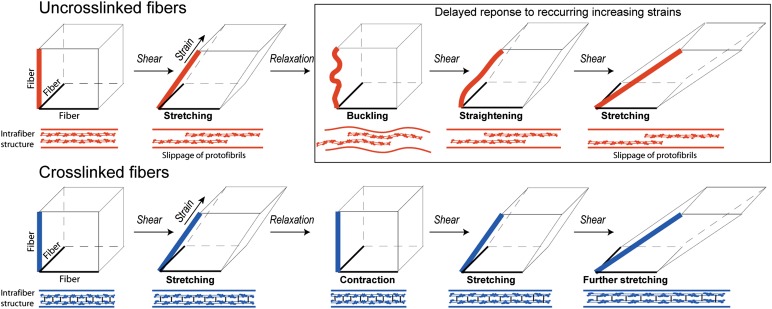
To get insight into the structural changes underlying deformations of fibrous networks, especially the plastic or irreversible changes, Münster et al. (1) combine rheological measurements with fluorescent confocal microscopy to visualize deformations of individual fibers. The originality of the rheological measurements is based on applying repeated shear with stepwise increasing strains, which allowed study of the dependence of mechanical response on loading history. Alternating repeated deformations are physiologically relevant because they reproduce, e.g., recurring muscular motions as well as pulsatory blood flow and vessel wall oscillations. The stress-strain curves of fibrin and collagen networks displayed remarkable changes in response to repeated large-strain loading. These changes looked much like weakening because with every new step of deformation, the same strain was reached at a smaller stress. However, when the repeated stress-strain cycles were corrected for a shift of the onset of strain-hardening, the superimposed curves had the same slopes, indicating the same stiffness of the networks, so the loadings do not weaken the networks. What actually changed after repeated increasing deformations was only the absolute strain at which the strain-hardening began. This delay is attributed to the interplay between persistent lengthening and buckling of individual fibers as a result of each stretching event, so that every new deformation starts only after the buckled filaments are stretched again to higher strain (Fig. 1).
Fig. 1.
The mechanism for the delayed nonlinear response to recurring increasing shear strains of a viscoelastic protein polymer with a high degree of plasticity, exemplified by fibrin. Schematic representation of repeated shear deformation with increasing strain of 3D networks built of uncross-linked and cross-linked fibers. The lower left corner of the cube represents a branch point made up of three fibers, one of which is oriented in the direction of strain. The diagrams below each cube represent the internal structure of a fibrin fiber, showing two protofibrils. An uncross-linked fiber (red) lengthens irreversibly upon shear and buckles upon relaxation. The elongation is caused by the slippage of protofibrils past each other within the fiber. On repeated shear, the buckled fiber is straightened to the same point without stretching and additional delayed lengthening occurs only at higher strain. The cross-linked fiber (blue) lengthens reversibly upon shear and restores the initial length upon relaxation. The slippage of protofibrils within the fiber is precluded by covalent crosslinking and the fiber stretches because of molecular elongation and/or unfolding.
The structural changes following repeated deformations of fibrin were observed at two spatial scales, in bulk under shear and in a single fiber that was stretched in the lateral direction, as in previous studies (15). Thus, the changes that occur in the fibers with large strains were directly visualized. If a fiber was in or close to the direction of strain, it was stretched and elongated. If a fiber was oriented perpendicular to the direction of strain, it buckled, consistent with a spontaneous decrease of stress over time without any observed changes in the network structure. The authors concluded that stress relaxation occurred within the fibers, not as a result of network rearrangement. Collagen networks show similar viscoelastic behavior but cannot be visualized as easily.
A clue to the mechanism of adaptation of individual fibrin and collagen fibers to loading conditions was found when the fibrin or collagen molecules making up the polymers were covalently cross-linked, using either factor XIIIa for fibrin or a chemical cross-linking agent for collagen. In both proteins, the cross-linking completely abrogated the changes in stress-strain response to subsequent deformations. Because the covalent cross-linking precluded slippage of protofibrils within a fibrin fiber, as well as tropocollagen filaments within a collagen fibril, this slippage was proposed to be the main molecular mechanism for the plasticity observed in bulk and single-fiber experiments (Fig. 1).
Although the slippage of protofibrils past each other makes sense in the interpretation of the results, there is another possibility [referred to in Münster et al.’s report (1)] that applies to fibrin but probably not collagen. There could be rupture of knob-hole bonds that are responsible for the polymerization (16–18), because these bonds are reversible, giving rise to relaxation with no change in elastic modulus. Another possibility is that the flexible αC regions could contribute to the observed plasticity of fibrin (19). Because these mechanisms are not likely for collagen networks, they may only be partial explanations for fibrin or they might be responsible for some of the differences between the behavior of collagen and fibrin networks.
These studies show that fibrin and collagen networks adapt to cyclical strain, thus protecting the structural integrity of the network by preventing overstretch. Furthermore, these studies suggest another function of cross-linking, in addition to stiffening clots and increase in resistance to fibrinolysis, which is to prevent such shifts under loading. These conclusions have significance for both protective hemostasis and pathological thrombosis. The mechanics of collagen is important for the migration of cells through the extracellular matrix, as in wound healing, inflammation, tissue development, and cancer metastasis. As a result, uncross-linked fibers may not be able to support enough tension for cell migration. On the other hand, cross-linked fibers may not relax their internal tension enough, affecting the ability of cancer cells to invade the surrounding extracellular matrix.
Such an automatic regulated adaptation of biopolymers to loading conditions may be a useful principle for the engineering of new biomaterials. The structural integrity of such unique materials could thus be protected against overstretch.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12197.
References
- 1.Münster S, et al. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proc Natl Acad Sci USA. 2013;110:12197–12202. doi: 10.1073/pnas.1222787110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collet JP, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary artery atherothrombosis. Arteroscler Thromb Vasc Biol. 2006;26(11):2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 3.Undas A, Ariëns RA. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31(12):e88–e99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 4.Hvas AM, et al. Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. J Thromb Haemost. 2007;5(12):2408–2414. doi: 10.1111/j.1538-7836.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: Gel stretching with protein unfolding and loss of water. Science. 2009;325(5941):741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purohit PK, Litvinov RI, Brown AE, Discher DE, Weisel JW. Protein unfolding accounts for the unusual mechanical behavior of fibrin networks. Acta Biomater. 2011;7(6):2374–2383. doi: 10.1016/j.actbio.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhmurov A, et al. Mechanism of fibrin(ogen) forced unfolding. Structure. 2011;19(11):1615–1624. doi: 10.1016/j.str.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvinov RI, Faizullin DA, Zuev YF, Weisel JW. The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys J. 2012;103(5):1020–1027. doi: 10.1016/j.bpj.2012.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautieri A, Buehler MJ, Redaelli A. Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J Mech Behav Biomed Mater. 2009;2(2):130–137. doi: 10.1016/j.jmbbm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan SM, Katti KS, Katti DR. Structural hierarchy controls deformation behavior of collagen. Biomacromolecules. 2012;13(8):2562–2569. doi: 10.1021/bm300801a. [DOI] [PubMed] [Google Scholar]
- 11.Janmey PA, Amis EJ, Ferry JD. Rheology of fibrin clots. VI. Stress relaxation, creep, and differential dynamic modulus of fine clots in large shearing deformations. J Rheol (NYNY) 1983;27(2):135–153. [Google Scholar]
- 12.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435(7039):191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 13.Piechocka IK, van Oosten AS, Breuls RG, Koenderink GH. Rheology of heterotypic collagen networks. Biomacromolecules. 2011;12(7):2797–2805. doi: 10.1021/bm200553x. [DOI] [PubMed] [Google Scholar]
- 14.Piechocka IK, Bacabac RG, Potters M, Mackintosh FC, Koenderink GH. Structural hierarchy governs fibrin gel mechanics. Biophys J. 2010;98(10):2281–2289. doi: 10.1016/j.bpj.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Carlisle CR, Sparks EA, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 2010;8(5):1030–1036. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bale MD, Müller MF, Ferry JD. Effects of fibrinogen-binding tetrapeptides on mechanical properties of fine fibrin clots. Proc Natl Acad Sci USA. 1985;82(5):1410–1413. doi: 10.1073/pnas.82.5.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu A, Schindlauer G, Ferry JD. Interaction of the fibrinogen-binding tetrapeptide Gly-Pro-Arg-Pro with fine clots and oligomers of alpha-fibrin; Comparisons with alpha beta-fibrin. Biopolymers. 1988;27(5):775–788. doi: 10.1002/bip.360270506. [DOI] [PubMed] [Google Scholar]
- 18.Chernysh IN, Nagaswami C, Purohit PK, Weisel JW. Fibrin clots are equilibrium polymers that can be remodeled without proteolytic digestion. Sci Rep. 2012;2:879. doi: 10.1038/srep00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houser JR, et al. Evidence that αC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J. 2010;99(9):3038–3047. doi: 10.1016/j.bpj.2010.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]