Abstract
Endothelial dysfunction is associated with diverse cardiovascular pathologies. Here, we show a previously unappreciated role for the Abelson (Abl) family kinases (Abl and Arg) in endothelial function and the regulation of angiogenic factor pathways important for vascular homeostasis. Endothelial Abl deletion in Arg-null mice led to late-stage embryonic and perinatal lethality, with mutant mice displaying focal loss of vasculature and tissue necrosis. Loss of Abl kinases led to increased endothelial cell apoptosis both in vitro and in vivo, contributing to vascular dysfunction, infarction, and tissue damage. Mechanistically, we identify a unique dual role for Abl kinases in the regulation of angiopoietin/Tie2 protein kinase signaling. Endothelial Abl kinases modulate Tie2 expression and angiopoietin-1–mediated endothelial cell survival. These findings reveal a critical requirement for the Abl kinases in vascular development and function, which may have important implications for the clinical use of Abl kinase inhibitors.
Keywords: Abl tyrosine kinases, heart defects, fibrosis, thrombosis
Disruption of vascular homeostasis plays a key role in pathological conditions including atherosclerosis, cancer, diabetes mellitus, and inflammatory arthritis (1, 2). Endothelial function is regulated, in part, by a variety of vascular growth factors, including vascular endothelial growth factor (VEGF) and the angiopoietins (Angpt) (3). These factors signal through receptor tyrosine kinases to support endothelial cell proliferation, survival, migration, and vascular stability. The angiopoietins, of which Angpt1 and Angpt2 are best characterized, signal through the Tie2 (tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2) receptor. Angpt1 is the primary Tie2 stimulatory ligand, whereas Angpt2 functions as a context-dependent Tie2 antagonist or agonist (4, 5). Although VEGF signaling is necessary for initial vasculogenesis, angiopoietin signaling is important for subsequent vascular remodeling as well as for interaction of the endothelium with supporting mural cells (6, 7). Vascular growth factor signaling also functions to maintain homeostasis in the quiescent vasculature (8, 9). Thus, delineating the intracellular signaling mechanisms that mediate endothelial responses to these factors has important implications for understanding vascular homeostasis as well as cardiovascular diseases.
The Abl family of nonreceptor tyrosine kinases, which includes the Abl (Abl1) and Arg (Abl2) kinases, has roles in diverse cellular processes, including proliferation, survival, adhesion, and migration (10). These kinases are activated transiently and mediate cytoskeletal remodeling downstream of several growth factor receptors and following cadherin and integrin engagement (10, 11). Abl is activated constitutively as a result of the t(9;22) chromosomal translocation that produces the BCR-ABL1 fusion protein, the causal agent in chronic myelogenous leukemia (CML) (12). Global Abl/Arg-null mice die by embryonic day 10.5 (E10.5), exhibiting hemorrhage and pericardial edema, suggesting a role for these kinases in vascular development (13). It was reported that long-term treatment with imatinib (Gleevec/STI571), a pharmacological inhibitor of the Abl kinases (as well as Kit and the platelet-derived growth factor receptor) caused severe congestive heart failure in a subset of CML patients (14). This cardiotoxicity was attributed to Abl inhibition in cardiomyocytes, leading to endoplasmic reticulum stress and cell death. Similarly, global Abl deletion (C57BL/6J genetic background) led to cardiomyocyte dysfunction, heart enlargement, and perinatal lethality (15). However, cardiomyocyte-specific restoration of Abl expression did not rescue viability, suggesting a critical role for Abl kinase in additional cell types. Notably, several case reports of patients treated with the second-generation BCR-ABL1 kinase inhibitor nilotinib have detailed the occurrence of vascular occlusive events (16, 17), suggesting potential vascular dysfunction following Abl kinase inhibition. In vitro studies have demonstrated a requirement for the Abl kinases in mediating both endothelial barrier-promoting effects of sphingosine-1-phosphate and barrier-disrupting effects of VEGF and inflammatory mediators (18, 19). However, the role of the Abl kinases in the endothelium has not yet been examined using genetic models.
Here, we demonstrate a crucial role for the Abl kinases in the vasculature, using endothelial Abl knockout mice. Loss of endothelial Abl kinases resulted in lethality at late embryonic and perinatal stages of development, with focal regions of vascular loss and tissue necrosis/apoptosis. Further, we demonstrate increased endothelial cell apoptosis in these embryos, as well as in Abl/Arg-knockdown endothelial cells in vitro. Notably, our studies reveal an unexpected link to angiopoietin/Tie2 signaling, with loss of endothelial Abl kinases leading to decreased Tie2 expression, diminished Tie2 receptor signaling, and loss of Angpt1-mediated survival. Further, we find that Abl kinases are activated by Angpt1/Tie2 signaling. Together, these findings reveal bidirectional signaling linking Abl kinases and Tie2, which is critical for endothelial cell survival and function.
Results
Embryonic Lethality of AblECKO; Arg−/− Mice.
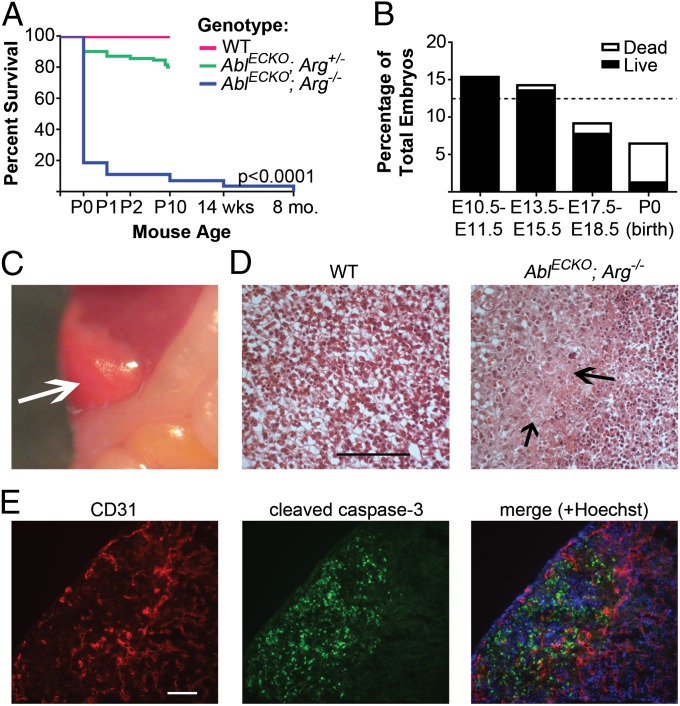
To evaluate the vascular function of the Abl kinases, we generated mice with endothelial inactivation of the Abl kinase by crossing mice carrying a floxed Abl allele (Ablflox/flox mice) on an Arg−/− background to Tie2-Cre mice. A near-complete loss of both Abl mRNA and protein was observed in endothelial cells of both embryos and adult mice (Fig. S1 A–D). No Cre-mediated recombination or Abl depletion was observed in nonendothelial/nonhematopoietic cells (Fig. S1 B–D). Strikingly, loss of the endothelial Abl kinases resulted in significant lethality. Most endothelial Abl/Arg-null pups (Ablflox/flox; Arg−/−; Tie2-Cre+/−, hereafter referred to as AblECKO; Arg−/− or mutant) died at birth, with 90% mortality by the end of postnatal day 1 (Fig. 1A). Further, the total number of AblECKO; Arg−/− mice born was reduced (6.5%) compared with the expected Mendelian ratio (12.5%), suggesting that ∼50% died during embryonic development. Although mutant embryo viability largely was unaffected at earlier stages of cardiovascular development, a decreased number of AblECKO; Arg−/− embryos was observed at later stages (E17.5–E18.5), and mutant embryo viability was decreased (Fig. 1B). Surviving late-stage mutant embryos were indistinguishable from wild-type (Ablflox/flox; Arg+/+; Tie2-Cre−/−) littermates, with no defects in gross vascular morphology (Fig. S1E). In addition, no changes in overall vascular patterning were observed in the heart or lungs (Fig. S1 F and G), nor was smooth muscle cell coverage of vessels affected (Fig. S1H). However, over 50% (42 of 77) of AblECKO; Arg−/− embryos displayed focal areas of hepatic necrosis of varying severity, typically localized to the periphery of the lobes (Fig. 1 C and D). Costaining of liver sections for the endothelial marker CD31 [platelet endothelial cell adhesion molecule (PECAM-1)] and apoptosis marker cleaved caspase-3 showed dramatic reduction in vascular density and extensive apoptosis in the peripheral necrotic areas (Fig. 1E), suggesting a loss of vascular perfusion in these regions.
Fig. 1.
Embryonic lethality of AblECKO; Arg−/− mice. (A) Survival of AblECKO; Arg−/− (mutant) mice compared with Ablflox/flox; Arg+/+; Tie2-Cre−/− (wild-type, WT) and Ablflox/flox; Arg+/−; Tie2-Cre+/− (AblECKO; Arg+/−) littermates (n = 462 total pups examined). P0, postnatal day 0 (birth). (B) Survival of mutant embryos at various stages of gestation, relative to expected Mendelian frequency (12.5%, indicated by the dashed line). (C) E18.5 mutant liver, displaying peripheral necrosis (arrow). (D) H&E-stained sections of E18.5 WT and AblECKO; Arg−/− livers, demonstrating necrotic areas in mutant liver (arrows). Scale bar: 100 μm. (E) E18.5 mutant liver section costained for CD31 (endothelial cell marker, red) and cleaved caspase-3 (apoptosis marker, green). Scale bar: 50 μm.
As Tie2-Cre also drives Abl inactivation in hematopoietic cells, we additionally examined hematopoietic progenitor cells in mutant fetal livers. No differences in percentages of lineage-negative (Lin−)/Kit+ hematopoietic progenitors or erythroid marker TER-119/CD71 double-positive progenitors were observed in mutant embryos compared with wild-type littermates (Table S1). Thus, together our data suggest that loss of Abl kinases in endothelial cells results in lethality linked to loss of vascular function late in development.
Cardiac Enlargement and Scarring in AblECKO; Arg+/− Mice.
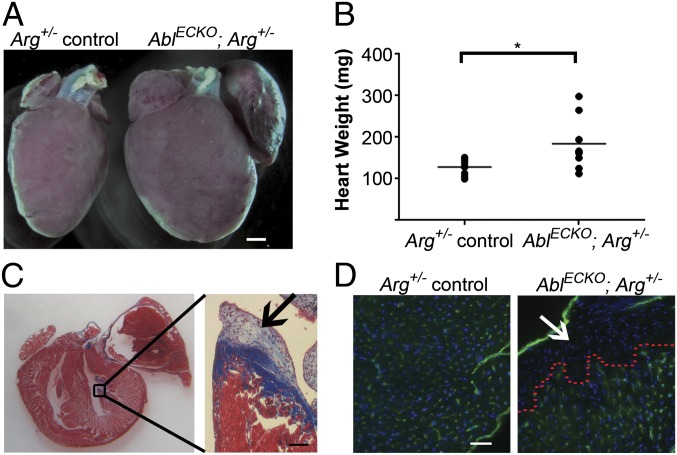
To examine the role of endothelial Abl kinases in vascular structure and function in adult mice, we used endothelial Abl-deficient mice on an Arg+/− background (Ablflox/flox; Arg+/−; Tie2-Cre+/−, hereafter referred to as AblECKO; Arg+/−), which survive to adulthood. Approximately 15% (17 of 118) of these AblECKO; Arg+/− mice were severely runted (body weights less than 75% of Ablflox/flox; Arg+/−; Tie2-Cre−/− littermate controls) and displayed dramatic cardiovascular phenotypes by 2–3 mo of age. The hearts of these mice were enlarged, typically with prominent dilation of the left atrium (Fig. 2 A and B). Histological analysis of cardiac tissue sections demonstrated localized regions of collagen deposition and scarring in the left ventricle (Fig. 2C). Although overall capillary density in the heart and skeletal muscle of AblECKO; Arg+/− mice was not significantly different from Arg+/− controls (Fig. S2 A and B), a near-complete loss of blood vessels was observed in the scarred regions (Fig. 2D). These findings are consistent with the localized loss of vasculature observed in the livers of AblECKO; Arg−/− embryos (Fig. 1E) and suggest a critical role for the Abl kinases in vascular maintenance and function. Interestingly, these AblECKO; Arg+/− mice also displayed thickening of the right ventricular wall, which correlated with cardiomyocyte hypertrophy (Fig. S3 A and B).
Fig. 2.
Cardiac enlargement and scarring in AblECKO; Arg+/− mice. (A) Stereoscopic image of hearts from 8-wk-old Arg+/− control (Ablflox/flox; Arg+/−; Tie2-Cre−/−) and AblECKO; Arg+/− mice, demonstrating heart enlargement and a dilated left atrium in an AblECKO; Arg+/− mouse. Scale bar: 1 mm. (B) Quantification of weights of hearts from Arg+/− control and AblECKO; Arg+/− mice (lines indicate mean values, n = eight mice per genotype; *P < 0.05). (C) Trichrome staining of AblECKO; Arg+/− heart, displaying scarring in the left ventricle (magnified in Inset, arrow). Scale bar: 50 μm. (D) CD31 staining (green) of AblECKO; Arg+/− left ventricle. A complete loss of capillaries was observed in the scarred region (above red dotted line, arrow). Scale bar: 20 μm.
Lung Fibrosis and Thrombosis in AblECKO; Arg+/− Mice.
As right ventricular hypertrophy may occur as a consequence of altered pulmonary circulation and hypertension (20), we examined the lungs of AblECKO; Arg+/− mice. The lungs of these mice were enlarged and dense, with prominent white fibrous areas (Fig. S3 C and D). Histological analysis of pulmonary structure showed extensive interstitial fibrosis (Fig. S3E), along with fibrin deposition in the airways (Fig. S3 F and G), indicating prior hemorrhage and defective pulmonary vascular integrity. We also observed a dramatic loss of vascular density, as assessed by staining for the endothelial marker von Willebrand factor (vWF) (Fig. S3G). An abundance of hemosiderin-laden macrophages was detected in the lungs of AblECKO; Arg+/− mice (Fig. S3H); the presence of these cells has been associated with heart failure (21). Thus, defective left ventricular function may contribute to the observed lung abnormalities, by producing congestion of the lung vasculature, with resulting leakage of red blood cells. Interestingly, no overt cardiac and pulmonary phenotypes were observed in E18.5 AblECKO; Arg−/− embryos (Fig. S4 A and B), suggesting that these defects develop later in adult AblECKO; Arg+/− mice, potentially as a result of cumulative injury or stress in the adult vasculature. Importantly, Arg protein levels were comparable in Arg+/− control and AblECKO; Arg+/− mice (Fig. S5 A and B), and no cardiac hypertrophy or pulmonary fibrosis was observed in adult Arg−/− mice (Fig. S4 C–G), suggesting that the observed cardiovascular phenotypes result from endothelial Abl rather than Arg depletion in AblECKO; Arg+/− adult mice.
The occurrence of liver necrosis in AblECKO; Arg−/− embryos and left ventricular scarring in adult AblECKO; Arg+/− mice suggests that loss of endothelial Abl kinases results in localized defects in tissue perfusion, with infarctions leading to tissue death and scarring. In this regard, we observed sporadic thrombi in lung and liver microvessels of AblECKO; Arg+/− adult mice, which were not seen in vessels of Arg+/− control mice. These thrombi stained positively for vWF, fibrin (Fig. S6A), and the platelet marker CD41 (integrin αIIb) (Fig. S6B). Importantly, these thrombi were observed in mice without any outward cardiac pathology, suggesting that their occurrence is not a secondary effect of compromised cardiac function. Histological analysis revealed abnormally swollen endothelial cells adjacent to a lung thrombus (Fig. S6C, Right), consistent with endothelial injury contributing to thrombosis. These findings suggest that loss of Abl kinases perturbs vascular function as a result of endothelial cell damage or death.
Increased Apoptosis Following Loss of Endothelial Abl Kinases.
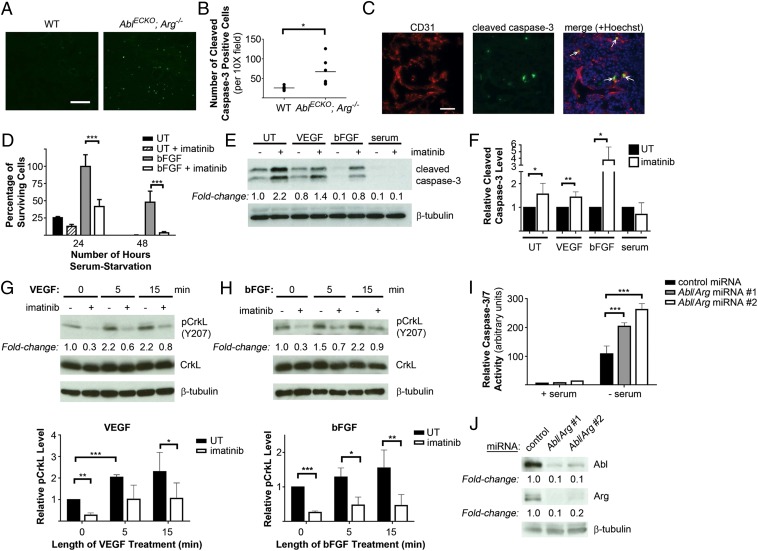
Global Abl/Arg knockout mice displayed increased apoptosis in all tissues (13), suggesting that the Abl kinases may have an important prosurvival function. Thus, we examined whether loss of Abl kinases affected endothelial cell viability in vivo. Indeed, a significant increase in cleaved caspase-3–positive cells, as well as numerous CD31/cleaved caspase-3 double-positive endothelial cells, was observed in AblECKO; Arg−/− embryo lungs (Fig. 3 A–C), demonstrating that loss of endothelial Abl kinases led to increased apoptosis. This finding is consistent with the extensive apoptosis detected in AblECKO; Arg−/− embryo livers (Fig. 1E). To confirm that the observed endothelial cell apoptosis represented a cell-autonomous phenotype, we examined the role of the Abl kinases in endothelial cell survival in vitro. Treatment of primary human umbilical vein endothelial cells (HUVECs) with the Abl pharmacological inhibitor imatinib decreased cell viability and increased levels of apoptosis following serum starvation (Fig. 3 D–F). The prosurvival effects of both VEGF and basic fibroblast growth factor (bFGF) also were decreased in the presence of the Abl inhibitor. Treatment with either VEGF or bFGF led to increased Abl kinase activation, as assessed by the phosphorylation of CrkL at Y207, an Abl-specific phosphorylation site (22) (Fig. 3 G and H), suggesting that the Abl kinases might modulate prosurvival signaling downstream of the VEGF and bFGF receptors. Interestingly, imatinib treatment did not increase apoptosis in HUVECs maintained in serum-containing medium (Fig. 3 E and F), suggesting that the Abl kinases may support survival specifically under stress conditions such as nutrient deprivation, as well as downstream of proangiogenic factors. Similarly, microRNA (miRNA)-mediated Abl/Arg knockdown in HUVECs led to increased apoptosis in response to serum-starvation stress (Fig. 3 I and J). These findings demonstrate an important prosurvival role for the Abl kinases in endothelial cells.
Fig. 3.
Increased apoptosis following loss of endothelial Abl kinases. (A and B) Staining of lung sections from E18.5 Ablflox/flox; Arg+/+; Tie2-Cre−/− (wild-type, WT) and AblECKO; Arg−/− (mutant) embryos for cleaved caspase-3 (green), revealing an increased number of apoptotic cells in mutant lungs, quantified in B (lines indicate mean values, n = six embryos per genotype). Scale bar: 100 μm. (C) Costaining of E18.5 mutant lungs for CD31 (red) and cleaved caspase-3 (green), demonstrating double-positive apoptotic endothelial cells (arrows). Scale bar: 20 μm. (D) Viability of primary HUVECs serum starved (untreated, UT) or supplemented with bFGF (10 ng/mL) in serum-free medium, with or without imatinib (10 μM). Values are expressed relative to viability of bFGF-treated cells 24 h after serum starvation. Data are presented as means ± SD (n = 3). (E and F) Analysis of cleaved caspase-3 levels (apoptosis) in HUVECs serum starved and either left untreated (UT) or supplemented with VEGF (100 ng/mL) or bFGF (10 ng/mL), or maintained in serum-containing medium (serum), with or without imatinib. Cleaved caspase-3 levels in imatinib-treated HUVECs are quantified in F, relative to levels in vehicle-treated cells (UT). Data are presented as means ± SD (n = 6). (G and H) Assessment of Abl kinase activation, as determined by phospho-CrkL(Y207) levels, following stimulation of serum-starved HUVECs with either (G) VEGF or (H) bFGF with or without imatinib. Results are quantified in the bottom panels; data are presented as means ± SD (n = 3). (I) Analysis of caspase-3/7 activity in HUVECs expressing control or Abl/Arg miRNAs either maintained in serum-containing medium (+ serum) or serum starved (− serum) for 24 h. Data are presented as means ± SD (n = 3). (J) Assessment of Abl/Arg knockdown upon miRNA expression in HUVECs. *P < 0.05; **P < 0.01; ***P < 0.001.
Abl Kinases Regulate Tie2 Expression and Signaling.
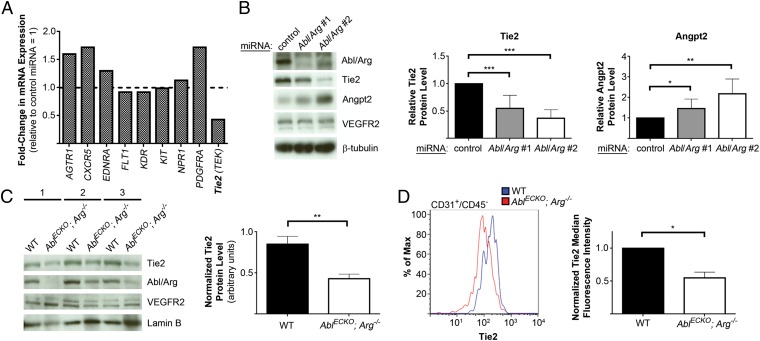
To determine the pathways whereby Abl kinases affect endothelial cell survival, we examined gene expression differences in HUVECs following Abl/Arg knockdown, using a real-time RT-PCR array. Although mRNA expression of most of the endothelial receptors analyzed was unchanged, Abl/Arg knockdown led to a greater than twofold reduction in Tie2 (also known as Tek) mRNA levels (Fig. 4A). Given the important role of Angpt1/Tie2 signaling in mediating endothelial cell survival and vascular integrity (23, 24), we examined the effects of Abl/Arg loss of function on this pathway. Using two Abl/Arg knockdown constructs, we confirmed by real-time RT-PCR (Fig. S7A) and Western blot analyses (Fig. 4B) that both Tie2 mRNA and protein levels were markedly decreased following Abl/Arg knockdown. Interestingly, levels of Angpt2 were increased following loss of Abl/Arg (Fig. 4B and Fig. S7C), whereas Angpt1 mRNA levels were decreased (Fig. S7D). No consistent difference in Tie1 receptor expression was observed in cells lacking Abl kinases (Fig. S7B). Importantly, decreased levels of Tie2 protein also were observed in AblECKO; Arg−/− embryo liver tissue (Fig. 4C), as well as in liver endothelial cells from E18.5 AblECKO; Arg−/− embryos (Fig. 4D), whereas VEGF receptor 2 levels were unchanged (Fig. 4C). Moreover, a similar decrease in Tie2 expression was observed in primary endothelial cells isolated from Ablflox/flox; Arg+/+: Tie2-Cre−/− mice following Arg knockdown and in vitro Abl depletion by adenoviral Cre transduction (Fig. S7E).
Fig. 4.
Decreased Tie2 expression following Abl/Arg knockdown. (A) Real-time RT-PCR array analysis of gene expression in HUVECs expressing control or Abl/Arg miRNAs. mRNA expression levels in Abl/Arg-knockdown HUVECs are shown, relative to levels in cells expressing control miRNA. AGTR1, angiotensin II receptor, type 1; EDNRA, endothelin receptor type A; FLT1, VEGF receptor 1; KDR, VEGF receptor 2; NPR1, natriuretic peptide receptor 1. (B) Analysis of Tie2 and Angpt2 protein levels in HUVECs expressing control miRNA or either of two Abl/Arg miRNAs, quantified at Right (means ± SD, n = 9). (C) Analysis of levels of Tie2 protein in livers from three pairs of Ablflox/flox; Arg+/+; Tie2-Cre−/− (wild-type, WT) and AblECKO; Arg−/− littermate E18.5 embryos, quantified at Right (means ± SD, normalized to lamin B levels, n = three mice per genotype). (D) Flow cytometric analysis of Tie2 levels in CD31+/CD45− endothelial cells from E18.5 WT and AblECKO; Arg−/− livers, quantified at Right (means ± SD, normalized to CD31 levels, n = three mice per genotype). *P < 0.05; **P < 0.01; ***P < 0.001.
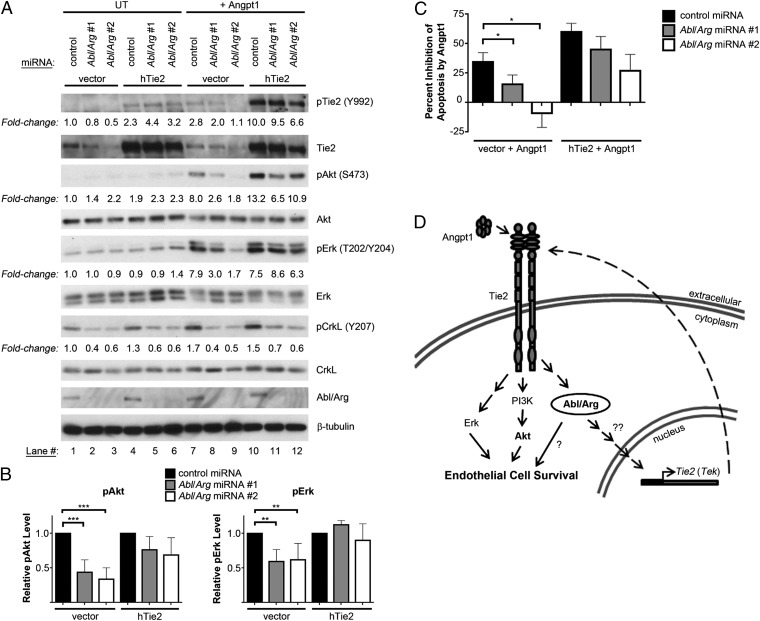
To evaluate the physiological consequences of Tie2 down-regulation in endothelial cells lacking Abl kinases, we examined the activation of downstream cellular signaling pathways. As expected, treatment of control HUVECs with angiopoietin-1 led to tyrosine phosphorylation of the Tie2 receptor, along with activation of Akt and Erk pathways (Fig. 5 A and B); activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway is required for Angpt1-mediated survival (25). Notably, the Abl kinases also were activated following Angpt1 treatment, as evidenced by increased phospho-CrkL (Y207) levels (Fig. 5A, lanes 1 and 7). Abl kinase activation also was observed following Angpt1 stimulation of both immortalized human microvascular endothelial cells (HMVECs; Fig. S8A, lanes 1 and 4) and polyoma middle T antigen (PyMT)-immortalized mouse embryo endothelial cells (Fig. S8B, lanes 1–6). These findings suggest a potential role for the Abl/Arg kinases in mediating downstream Tie2 signaling. In this regard, activation of Akt by Angpt1 was dramatically reduced in Abl/Arg-knockdown cells (Fig. 5A, lanes 7–9). Erk activation also was decreased to a lesser extent by Abl/Arg depletion. Similarly, Abl/Arg knockdown diminished Angpt1-mediated Akt activation in HMVECs, whereas Erk activation was unchanged (Fig. S8A, lanes 4–6). Importantly, although Angpt1 inhibited apoptosis following serum starvation in control HUVECs, the prosurvival effects of Angpt1 were decreased in Abl/Arg knockdown cells (Fig. 5C). Thus, down-regulation of Tie2 receptor levels following Abl/Arg knockdown decreased both Angpt1-mediated signaling and downstream antiapoptotic responses. Single Abl and Arg knockdowns demonstrated that loss of either kinase was sufficient to impair both Angpt1-mediated signaling (Fig. S8C) and survival (Fig. S8D). Interestingly, expression of exogenous Tie2 in Abl/Arg-knockdown cells largely restored Angpt1-mediated signaling (Fig. 5A, lanes 10–12) and partially rescued the antiapoptotic effects of Angpt1 (Fig. 5C). These findings suggest that the increased apoptosis observed upon loss of Abl kinase expression may partly be the result of down-regulation of Tie2 signaling.
Fig. 5.
Abl kinases modulate Tie2 signaling and angiopoietin-1–mediated survival. (A and B) Assessment of Angpt1-mediated activation of intracellular signaling pathways in HUVECs infected with control or Abl/Arg miRNA lentiviruses, with or without exogenous hTie2 expression, quantified in B. Cells were serum starved for 6 h, then left unstimulated (UT) or treated with Angpt1 (200 ng/mL, 15 min). (B) pAkt and pErk levels (normalized to total Akt and Erk protein) are shown as means ± SD, relative to levels in Angpt1-stimulated control miRNA-expressing cells (n = 4). (C) Analysis of levels of apoptosis in HUVECs infected with Tie2 retrovirus and Abl/Arg miRNAs following 24-h serum starvation in the presence of Angpt1 (200 ng/mL). Values are expressed as percent inhibition of apoptosis by Angpt1 relative to serum-starved (nonsupplemented) control cells. Data are presented as means ± SEM (n = 3). (D) Model for the dual role of the Abl family kinases in angiopoietin/Tie2 signaling. The Abl kinases positively regulate Tie2 (Tek) mRNA expression and are required for maximal Angpt1-mediated prosurvival signaling primarily through the PI3K/Akt and, to a lesser extent, Erk signaling pathways. The Abl kinases also are activated downstream of the Tie2 receptor. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Our findings have uncovered a crucial role for the endothelial Abl kinases in the vasculature, as loss of endothelial Abl/Arg kinase expression resulted in embryonic and perinatal lethality. Loss of endothelial Abl kinases had an adverse impact on vascular function, resulting in localized loss of vascular density and resultant cell death in affected tissues (necrosis/apoptosis). Interestingly, even partial loss of endothelial Abl kinase expression produced focal loss of cardiac vasculature and myocardial injury. The localized nature of the observed vascular loss and tissue damage, along with the normal overall vascular density, branching, and patterning observed in AblECKO; Arg−/− mice, suggests that loss of endothelial Abl/Arg kinases likely adversely affects vascular maintenance and stability, rather than vessel formation. Given that Tie2-Cre–mediated recombination occurs in most endothelial cells by E9.5 (26), it is possible that subtle structural defects during vessel formation might contribute to the phenotypes observed later in development in mutant embryos. However, our finding that loss of the Abl kinases sensitizes endothelial cells to stress-induced apoptosis in vitro suggests that the sporadic and focal nature of the observed phenotypes may result from vascular damage due to localized endothelial apoptosis in response to cumulative vascular stresses in the absence of the Abl kinases.
Our demonstration of a critical requirement for the Abl kinases in the vasculature is particularly notable considering the cardiotoxicity previously observed in a subset of patients upon chronic Abl kinase inhibition using imatinib (14, 27, 28). Additional case reports detail instances of interstitial lung disease of unknown origin in some imatinib-treated cancer patients (29). Although the incidence of these events appears low and imatinib generally is well tolerated, our findings demonstrate a crucial role for the Abl kinases in normal vascular development and function, which may have implications for the clinical use of Abl kinase inhibitors such as imatinib and nilotinib.
Unexpectedly, the current study also reveals bidirectional links between the Abl kinases and angiopoietin/Tie2 signaling in the endothelium. Loss of endothelial Abl/Arg kinase expression decreased Tie2 receptor levels and led to a shift in angiopoietin levels, with enhanced Angpt2 levels and decreased Angpt1 levels. Consequently, loss of Abl kinases decreased Angpt1/Tie2 signaling and diminished the prosurvival effects of Angpt1. Our finding that Abl kinases are activated following Angpt1 stimulation supports a dual role for Abl kinases in the regulation of angiopoietin/Tie2 signaling, through the control of receptor/ligand expression, as well as the modulation of downstream prosurvival signaling pathways (Fig. 5D). Loss of Tie2 impairs endothelial cell survival in vivo (23). Angpt1/Tie2 signaling also supports vascular stability and inhibits inflammatory endothelial barrier dysfunction and adhesion molecule expression (30). Taken together, our findings support an important role for the Abl kinases in Angpt1/Tie2-mediated vascular homeostasis. As alterations in the angiopoietin/Tie2 pathway have been implicated in diverse vascular pathologies (31–33), a potential role for the Abl family kinases in modulating Tie2 signaling during the progression of these disorders merits further investigation.
Materials and Methods
Additional experimental details are provided in SI Materials and Methods, including reagents and procedures for endothelial cell culture and viability/apoptosis assays, isolation and characterization of mouse endothelial and fetal liver cells, viral transduction, immunohistochemistry, immunoblotting, and real-time PCR analysis.
Generation of Abl Endothelial Conditional Knockout Mice.
Both Ablflox/flox mice and Tie2-Cre mice were described previously (26, 34). Ablflox/flox mice were crossed into an Arg−/− background (13) and backcrossed six generations onto the C57BL/6 genetic background. AblECKO; Arg−/− (mutant) embryos were obtained from timed matings of Ablflox/flox; Arg+/−; Tie2-Cre+/− males to Ablflox/flox; Arg+/−; Tie2-Cre−/− females. The presence of a vaginal plug was considered to be E0.5. All animal procedures used in this study were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism 5 software. Comparisons of two groups were performed using Student t tests (two-tailed). Comparisons involving multiple groups were evaluated using one-way ANOVA, followed by Bonferroni posttests. Two-way ANOVA, followed by Bonferroni posttests, was used to evaluate differences in HUVEC survival between drug treatments over time, as well as changes in mRNA expression in control vs. Abl/Arg knockdown cells. Survival of embryos of various genotypes was evaluated by log-rank (Mantel–Cox) test. For all tests, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Anthony Koleske (Yale University) for Ablflox/flox and Arg−/− mice and Masashi Yanagisawa (University of Texas Southwestern) for Tie2-Cre mice, Emily Riggs (Duke University Medical Center) for technical assistance, Laura Hale and Elizabeth Pavlisko (both from the Duke University Medical Center Department of Pathology) for evaluation of histology specimens, and Christopher Kontos and Emileigh Greuber (both of Duke University Medical Center) for helpful comments on this manuscript. We also thank the Duke University Light Microscopy Core Facility and the Duke Cancer Center Flow Cytometry Shared Resource for technical assistance. This work was supported by a Pharmaceutical Research and Manufacturers of America Foundation Predoctoral Fellowship in Pharmacology/Toxicology (to E.M.C.), an American Heart Association Predoctoral Fellowship (to E.M.C.), and National Institutes of Health Grants CA155160, CA070940, HL084102, and AI056266 (to A.M.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304188110/-/DCSupplemental.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 4.Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med. 2013;19(1):31–39. doi: 10.1016/j.molmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 7.Sato TN, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376(6535):70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 8.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendergast AM. The Abl family kinases: Mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 11.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci USA. 2007;104(45):17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 13.Koleske AJ, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21(6):1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 14.Kerkelä R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 15.Qiu Z, Cang Y, Goff SP. c-Abl tyrosine kinase regulates cardiac growth and development. Proc Natl Acad Sci USA. 2010;107(3):1136–1141. doi: 10.1073/pnas.0913131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aichberger KJ, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(7):533–539. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 17.Quintás-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12(5):337–340. doi: 10.1016/j.clml.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Aman J, et al. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126(23):2728–2738. doi: 10.1161/CIRCULATIONAHA.112.134304. [DOI] [PubMed] [Google Scholar]
- 19.Dudek SM, et al. Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell. 2010;21(22):4042–4056. doi: 10.1091/mbc.E09-10-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelkel NF, et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 21.Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest. 2004;125(2):669–682. doi: 10.1378/chest.125.2.669. [DOI] [PubMed] [Google Scholar]
- 22.Burton EA, Plattner R, Pendergast AM. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 2003;22(20):5471–5479. doi: 10.1093/emboj/cdg512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones N, et al. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2(5):438–445. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448(2-3):249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 25.Kim I, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 27.Park YH, et al. BNP as a marker of the heart failure in the treatment of imatinib mesylate. Cancer Lett. 2006;243(1):16–22. doi: 10.1016/j.canlet.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Turrisi G, et al. Congestive heart failure during imatinib mesylate treatment. Int J Cardiol. 2010;145(1):148–150. doi: 10.1016/j.ijcard.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Peerzada MM, Spiro TP, Daw HA. Pulmonary toxicities of tyrosine kinase inhibitors. Clin Adv Hematol Oncol. 2011;9(11):824–836. [PubMed] [Google Scholar]
- 30.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98(8):1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhandari V, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12(11):1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim HS, Blann AD, Chong AY, Freestone B, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: Implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27(12):2918–2924. doi: 10.2337/diacare.27.12.2918. [DOI] [PubMed] [Google Scholar]
- 33.Parikh SM, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25(26):6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.