Abstract
The evolution of transcriptional regulatory networks entails the expansion and diversification of transcription factor (TF) families. The forkhead family of TFs, defined by a highly conserved winged helix DNA-binding domain (DBD), has diverged into dozens of subfamilies in animals, fungi, and related protists. We have used a combination of maximum-likelihood phylogenetic inference and independent, comprehensive functional assays of DNA-binding capacity to explore the evolution of DNA-binding specificity within the forkhead family. We present converging evidence that similar alternative sequence preferences have arisen repeatedly and independently in the course of forkhead evolution. The vast majority of DNA-binding specificity changes we observed are not explained by alterations in the known DNA-contacting amino acid residues conferring specificity for canonical forkhead binding sites. Intriguingly, we have found forkhead DBDs that retain the ability to bind very specifically to two completely distinct DNA sequence motifs. We propose an alternate specificity-determining mechanism whereby conformational rearrangements of the DBD broaden the spectrum of sequence motifs that a TF can recognize. DNA-binding bispecificity suggests a previously undescribed source of modularity and flexibility in gene regulation and may play an important role in the evolution of transcriptional regulatory networks.
Keywords: transcription factor binding site motif, protein–DNA interactions
The regulation of gene expression by the interaction of sequence-specific transcription factors (TFs) with target sites (cis-regulatory elements) near their regulated genes is a central mechanism by which organisms interpret regulatory programs encoded in the genome to develop and interact with their environment. The emergence of new species has depended in part on the evolution of the network of interactions by which an organism's TFs control gene expression. Much attention has been paid to changes in cis-regulatory sequences over evolutionary time, because these changes can result in incremental modifications of organismal phenotypes without large-scale rewiring of transcriptional regulatory networks that would result from changes in TF DNA-binding specificity (1). Nevertheless, TFs and their DNA-binding specificities have changed over time (2). Gene duplication, followed by divergence of the resulting redundant TFs, has resulted in the emergence of families of paralogous TFs with diversified DNA-binding specificities and functions (3). Thus, identifying mechanisms by which related DNA-binding domains (DBDs) have acquired novel specificities is important for understanding TF evolution.
The forkhead box (Fox) family of TFs spans a wide range of species and is one of the largest classes of TFs in humans. In metazoans, Fox proteins have vital roles in development of a variety of organ systems, metabolic homeostasis, and regulation of cell-cycle progression, and fungal Fox proteins are involved in cell-cycle progression and the expression of ribosomal proteins. The Fox family of TFs shares a conserved DBD that is structurally identifiable as a subgroup of the much larger winged helix superfamily, which includes both sequence-specific DNA-binding proteins and linker histones, which appear to bind DNA nonspecifically (4, 5). Proteins with unambiguous sequence homology to the forkhead domain are present throughout opisthokonts—the phylogenetic grouping that includes all descendants of the last common ancestor of animals and fungi—but have diverged so extensively over approximately 1 billion years of evolution that distantly related Fox proteins are not generally alignable outside the forkhead domain (6, 7). Moreover, distantly related Fox-like domains have been found in Amoebozoa, a sister group to opisthokonts (8). Three distinct subfamilies (Fox1–3) of fungal Fox proteins have been identified. Metazoan Fox proteins are classified into 19 subfamilies (FoxA–S), some of which have been further subdivided on phylogenetic grounds.
The Fox domain itself is ∼80–100 amino acids (aa) in length and, like other winged helix domains, comprises a bundle of three α-helices connected via a small β-sheet to a pair of loops or “wings.” In available structures of forkhead domain–DNA complexes, helix 3 forms a canonical recognition helix positioned in the major groove of the DNA target site by the helical bundle, whereas the wings, which often contain a poorly alignable region rich in basic residues, lie along the adjacent DNA backbone (9–13).
Several groups have studied the evolutionary history of the family using multiple sequence alignment and phylogenetic inference methods; however, the results of these studies are in many cases inconsistent. Published forkhead phylogenies lack statistical support for deep branches and the relative positions of forkhead subfamilies, especially of the fungal groups (14, 15). Thus, the relationships among Fox genes have remained unclear.
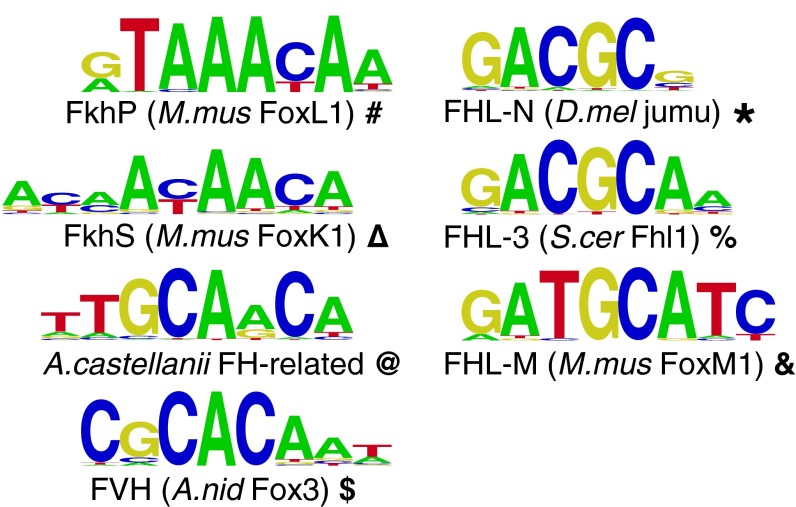
In separate studies, the DNA-binding specificities of various forkhead proteins have been examined. In most cases, in vitro binding has been observed to variants of the canonical forkhead target sequence RYAAAYA (16–21), which we refer to as the forkhead primary (FkhP) motif (Fig. 1). A similar variant, AHAACA, has been observed during in vitro selection (SELEX) (17) and protein-binding microarray (PBM) experiments (20); this specificity appears to be common to several Fox proteins, and we refer to it as the forkhead secondary (FkhS) motif (22). However, a SELEX study of the FoxN1 TF mutated in the famous nude mouse identified an entirely different sequence, ACGC, as its preferred binding site (23). The closely related Mus musculus FoxN4 has been shown to bind ACGC in vivo (24). A PBM survey of Saccharomyces cerevisiae TFs identified a very similar sequence, GACGC, as the binding site of the Fox3 factor Fhl1 (19); we therefore refer to the GACGC site as the FHL motif (Fig. 1).
Fig. 1.
DNA binding-site motifs bound by forkhead domain proteins. A representative member of each class of binding site discussed in the text is shown. Bold symbols are used to represent binding specificities in subsequent figures.
Previous work on differences in forkhead DNA-binding specificity has focused on preferential recognition of FkhP and FkhS variants by forkhead proteins (17, 18). Contrary to the common mechanism of varying specificity by changing amino acid residues that make base-specific DNA contacts (25), the positions in the forkhead recognition helix that make base-specific contacts are conserved across proteins with different binding specificities (9, 17). In subdomain swap experiments, a 20-aa region immediately N-terminal to the recognition helix was shown to switch DNA-binding specificities between forkhead proteins (17). Interestingly, this region has been shown by NMR to adopt different secondary structures in forkheads with distinct DNA-binding specificities (26). However, a similar analysis of sequence features conferring binding to the FHL motif has not been performed.
The observation of binding to such different sequences—RYAAAYA and GACGC—within widely diverged members of the Fox family raises the question of how the binding specificity of these proteins has evolved. We have addressed this question using a combined phylogenetic and biochemical approach. We conducted a phylogenetic analysis of Fox domains from 10 metazoans, 30 fungi, and 25 protists (Dataset S1). We chose these species based on their evolutionary importance and annotation level (27) (Fig. S1). For example, we included Spizellomyces punctatus and Fonticula alba because they are very close to the root of fungi and a closely related outgroup, respectively. We considered conserved splice junctions along with multiple sequence alignment to infer the phylogeny. We assayed DNA-binding specificity in vitro using universal PBM technology, in which a DNA-binding protein is applied to a double-stranded DNA microarray containing 32 replicates of all possible 8-bp sequences (8-mers) and is fluorescently labeled, permitting the exhaustive cataloguing of the range of sequences that a protein can recognize (28). We analyzed the binding specificities of 30 forkhead proteins, combining published data for 9 proteins with data for 21 proteins that we characterized for this study (Dataset S2). We focused on proteins from clades in which we had previously observed alternate binding specificities and clades of unknown specificity. By using two orthogonal means of evaluating the same proteins, we obtained a much richer picture of the evolutionary trajectory of changes in TF DNA-binding specificity than either analysis alone could provide.
Results
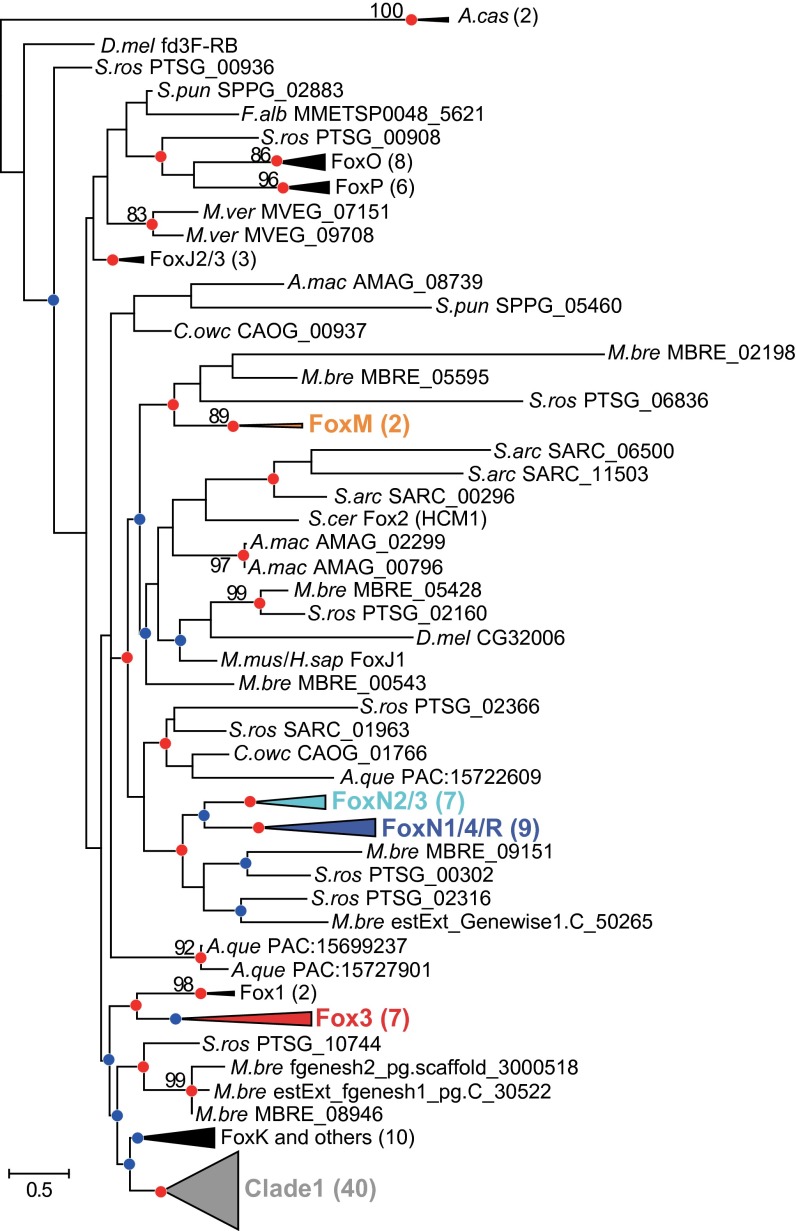
The published observation of approximately the same alternate binding motif (FHL) for metazoan FoxN1/4 and fungal Fox3 suggests the parsimonious hypothesis that they derive from a common FHL-binding ancestral protein in the last common ancestor of opisthokonts. To explore this hypothesis, we performed phylogenetic inference on a broad group of Fox domain sequences (Materials and Methods), spanning 623 genes from 65 species (Dataset S1 and Fig. S1). We included two distantly related forkhead domains from the opisthokont sister group Amoebozoa as an outgroup. After removing partial domain sequences and those identical throughout the Fox domain, we used 529 Fox domain sequences (340 nonredundant; Dataset S1). We constructed a complete maximum likelihood (ML) tree of all nonredundant Fox domain sequences (Fig. S2). For each branch, the approximate likelihood-ratio test (aLRT) and 100 bootstrap replicates were used to evaluate support for inferred relationships (Materials and Methods). For presentation purposes, we constructed a ML tree of 262 (133 nonredundant) Fox domains from selected informative species (Fig. 2 and Dataset S1).
Fig. 2.
ML phylogenetic tree of forkhead domains. This compact tree was constructed for presentation purposes from a representative subset of phylogenetically informative species: metazoans mouse, fly, and sponge; choanoflagellates Salpingoeca rosetta and Monosiga brevicollis; Capsaspora owczarzaki and Sphaeroforma arctica from Ichthyosporea; S cerevisiae from Dikarya; Allomyces macrogynus from Blastocladiomycota; S. punctatus from Chytridiomycota; Mortierella verticillata from Mortierellomycotina; F. alba from Nucleariida; and Acanthamoeba castellanii from Amoebozoa. Nodes supported with strong likelihood ratios are indicated with red circles (aLRT ≥ 99%) or blue circles (aLRT ≥ 95%); bootstrap support values are shown for nodes with ≥80% support. Clades containing alternate binding specificities are highlighted in color (see text). Importantly, the groupings of subfamilies in this tree and the complete tree with all Fox domains are almost identical to each other (Fig. S2).
Various portions of the phylogeny could be determined with high confidence. Our analysis recovered the previously identified subfamily relationships between Fox proteins and also identified a previously unobserved fungal group (Fox4) not represented in S. cerevisiae. However, the structure of the deep portions of the Fox tree could not be resolved for two major reasons. First, the number of alignable positions within the Fox domain is too small to resolve the phylogenetic history of such a broadly and deeply diverged family, and regions outside the domain are not alignable among distantly related members. Second, some Fox genes appear to have evolved through gene conversion and/or crossover events (15), as evinced by the appearance of species-specific Fox domain signatures.
The ML tree inferred here strongly supports the hypothesis of Larroux et al. that a monophyletic group of forkhead domains (which they refer to as clade I) emerged in the common ancestor of metazoans (14) (aLRT value = 0.9999, bootstrap value = 4%; Fig. 2). Additionally, there is a splice site between amino acid positions 46 and 47 in the Pfam Fork_head domain hidden Markov model (HMM) (29) conserved in various clade II forkhead proteins across kingdoms; no clade I genes share this splice site, further supporting the monophyly of clade I in metazoans.
Surprisingly, there is no support for a tree topology in which metazoan FoxN and fungal Fox3 subfamilies form a monophyletic, FHL-binding clade. A tree containing a FoxN+3 clade (Fig. S3A) is significantly less likely than the observed tree (P < 10−8, likelihood ratio test), and likelihood maximization using this tree as a starting tree separates the FoxN and Fox3 clades (Fig. S3 B and C). Moreover, we see separate, well-supported clades (aLRT values ≥ 0.99) combining each of these groups with others that bind only the FkhP and FkhS motifs (Fig. 2). This result suggests that FHL binding capacity evolved twice independently within the family and led us to examine these two subgroups in more detail.
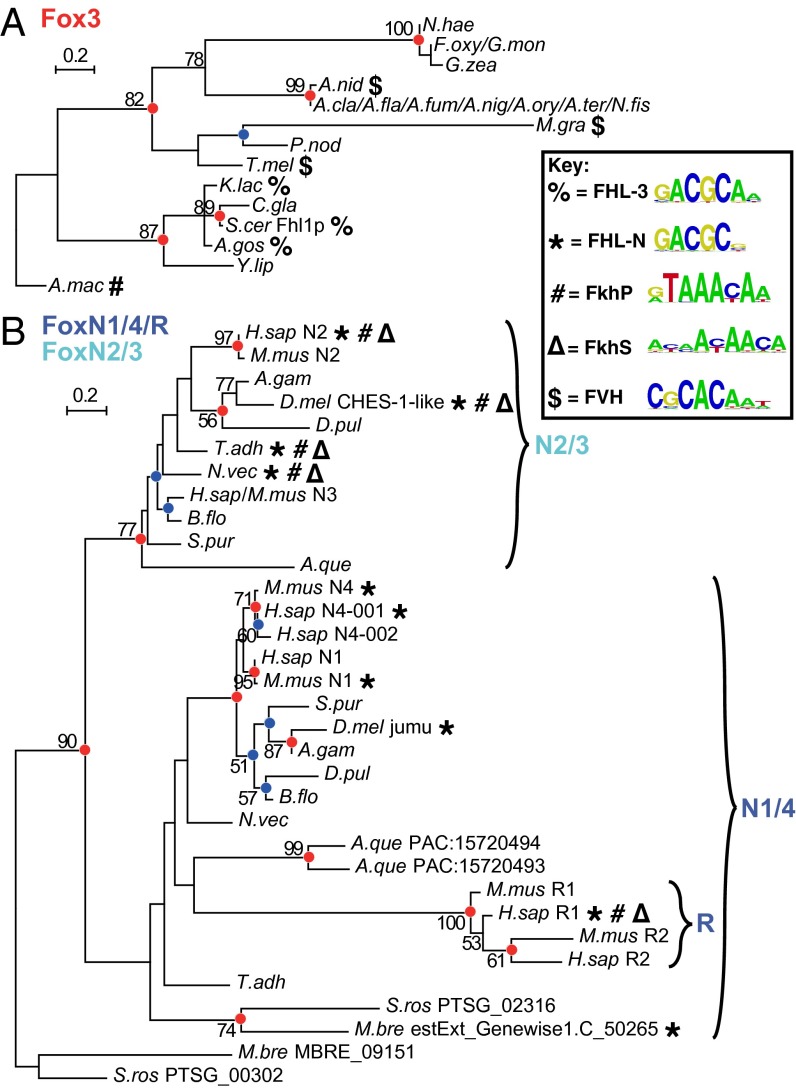
A phylogenetic tree constructed from only fungal Fox3 domains (Fig. 3A) is much more stable than the larger, more complex tree, with acceptable bootstrap support at major branch points; moreover, it follows the species tree closely (Fig. S1), suggesting radiation of a family of orthologs. The most basally diverged member of this group, Allomyces macrogynus Fox3, binds only the canonical FkhP and FkhS motifs (Figs. 3A and 4), providing experimental support for the hypothesis that FHL binding arose within the Fox3 clade after its divergence from other forkhead domains. The remaining Fox3 proteins considered here fall into two distinct groups. Those most closely related to Fhl1 (S. cerevisiae Fox3) show the same FHL-binding specificity, binding the FkhP,S motifs no better than non-forkhead proteins (percent signs in Fig. 3A). Members of the other group, including Aspergillus nidulans Fox3, bind another motif entirely, which we term the Forkhead Variant Helix (FVH) motif (dollar signs in Fig. 3A; see also Fig. 1), with no specific binding to either the FkhP,S or FHL motifs.
Fig. 3.
Detailed analysis of Fox3 and FoxN subfamilies. ML phylogenetic trees for Fox domains from a broader range of species for fungal Fox3 (A) and holozoan FoxN/R (B) clades. Red and blue circles indicate node support as in Fig. 2. Bold symbols represent binding capacity for different motif classes as defined in Fig. 1.
Fig. 4.
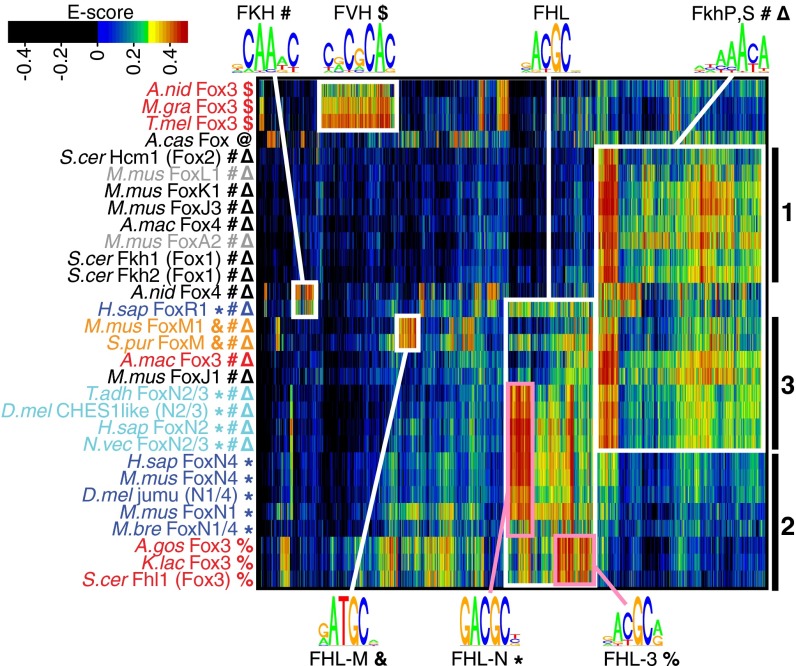
Biclustering of Fox domain binding data reveals multiple functional classes. E-score binding profiles were clustered both by protein (rows) and by contiguous 8-mer (columns) for any 8-mer bound (E score ≥ 0.35) by at least one assayed Fox protein. Fox domains fall into functional classes (bold symbols represent binding capacity for different motifs as defined in Fig. 1) that do not uniformly correlate with phylogeny (protein names are colored by phylogenetic grouping as in Fig. 2). Cluster 1 (black bar) comprises proteins specific only for the FkhP,S motifs, cluster 2 proteins are specific only for FHL variants, and cluster 3 proteins have more complex specificity; see text for details. Sequence motifs shown were generated by alignment of the indicated clusters of 8-mers and are for visualization purposes only.
Similarly, the phylogeny of the holozoan FoxN subfamily is relatively stable (Fig. 3B). Our analysis supports the existence of a fundamental split into FoxN1/4 and N2/3 clades, with FoxR [initially called N5 (30)] placed within the N1/4 group (14). As expected, FoxN1 and other N1/4 proteins are highly specific for the FHL motif. Surprisingly, all FoxN2/3 proteins assayed by PBMs exhibited high sequence specificity for both the FkhP,S and FHL motifs (Fig. 4). For example, the top two 8-mers [ranked by PBM enrichment (E) score, which indicates the preference of a protein for every possible 8-mer (28)] bound by the Drosophila melanogaster FoxN2/3 protein checkpoint suppressor homologue (CHES-1–like) are ATAAACAA and GTAAACAA, perfectly matching the FkhP consensus, and the next two are the FHL matches GACGCTAA and GACGCTAT. FoxR1 also shows bispecificity, despite presumably arising from an FHL-specific N1/4 ancestor. To our knowledge, such bispecificity for two seemingly unrelated sequence motifs by a single DBD (i.e., excluding proteins with multiple DNA-binding subdomains) has not been observed previously.
Consistent with the hypothesis that FHL binding arose independently in the fungal Fox3 and holozoan FoxN groups, we observed slight variations between the versions of the FHL motif bound by each of these two groups. Specifically, all tested FHL-binding Fox3 proteins strongly preferred A immediately 3′ to the core GACGC, which we refer to as the FHL-3 motif, whereas FHL motifs from FoxN/R proteins all strongly disfavored A in that position, a variant we refer to as the FHL-N motif (Fig. 1). Similarly, Homo sapiens FoxR1 (which appears to have regained FkhP,S binding from an FHL-only ancestor) strongly preferred a C at position 2 of the FkhP motif, whereas other FkhP-binding Fox domains strongly preferred T at that position (Fig. 4 and Fig. S4).
The unexpected variety in Fox domain-binding specificity led us to perform additional PBM experiments on a range of Fox domains, focusing on representative proteins from other clade II groups, such as Fox4 and FoxM, and assemble them with published PBM data (Fig. S4 and Datasets S3 and S4). In addition to finding more examples of proteins that exhibit the sequence preferences described above, we also discovered a third instance of binding to an FHL-like motif. Two metazoan FoxM proteins exhibit high specificity for the FkhP and FkhS motifs, as well as for a third FHL variant, GATGC, which we refer to as FHL-M. The most preferentially bound 8-mer matching this motif is an overlapping inverted repeat, GATGCATC; human FoxM1 has previously been shown to bind overlapping multimers of the FkhP motif in vitro, which suggests that these two FoxM proteins might bind as dimers to GATGCATC. Phylogenetic analysis strongly supports an independent origin of the FoxM subfamily from FoxN (P < 10−4, likelihood ratio test; Fig. S3D), in that each subfamily is more closely related to proteins that bind only FkhP and FkhS than to each other, suggesting that this finding represents yet a third independent emergence of a form of FHL binding (FHL-M), with each one characterized by slight differences in DNA sequence preference (Fig. 1). As in the case of FoxN and Fox3, ML inference with a starting tree containing a FoxM+N clade leads to separation of the subfamilies (Fig. S3 E and F).
Biclustering of the 30 total Fox proteins and bound 8-mers according to PBM E scores reveals three major functional protein classes (Fig. 4). The first prominent cluster of proteins is characterized by specificity only for the FkhP and FkhS motifs. Binding to these motifs tracks together across proteins; the motif constructed from these 8-mers is an average over both motifs. This FkhP,S-binding cluster comprises representatives of widely varying subfamilies, including clade I (M. musculus FoxA2 and FoxL1), metazoan clade II (M. musculus FoxJ3 and FoxK1), and fungal Fox1, Fox2, and Fox4 (S. cerevisiae Fkh1, Fkh2, and Hcm1 and A. macrogynus Fox4). This broad distribution of FkhP,S binding specificity supports the hypothesis that it is the ancestral binding specificity of the entire forkhead family.
The second large cluster comprises domains that are uniquely specific for the FHL motif: holozoan FoxN1/4 and fungal Fox3 (S. cerevisiae subgroup). This cluster is further divided into holozoan and fungal groups, based on preference for the FHL-N vs. FHL-3 variants, as described above.
The third major cluster combines several proteins exhibiting broad specificity. The bispecific metazoan FoxN2/3 and FoxM subfamilies are present in this cluster, along with M. musculus FoxJ1 and A. macrogynus Fox3, both of which show strong preference for the FkhP and FkhS motifs and weaker preference for the FHL motif variants.
One of the forkhead-like domains from the nonopisthokont Acanthamoeba castellanii did not fall into any of these three clusters, because it binds another distinct motif (Fig. 1 and Fig. S4). These binding differences are associated with widespread differences in the recognition helix (Fig. 5A). Indeed, altered recognition positions (Fig. 5B) can clearly explain the non-FkhP,S specificities of the forkhead-related protein from A. castellanii and A. nidulans Fox3; furthermore, there are sufficient differences in the recognition helix of H. sapiens FoxR1 that it is perhaps surprising that its specificity is so similar to that of other Fox proteins. Surprisingly, however, the majority of specificity changes in the Fox family, including FHL binding and bispecificity, do not correlate with changes in canonical specificity-determining positions. Indeed, although H. sapiens FoxN4 is highly specific for only the FHL motif, and H. sapiens FoxN2 is bispecific and robustly binds FkhP and FkhS sites as well as the FHL motif, these two FoxNs are identical throughout the entire recognition helix; thus, the inability of FoxN4 to recognize FkhP sites is not strictly a function of the canonical DNA-contacting residues in the recognition helix.
Fig. 5.
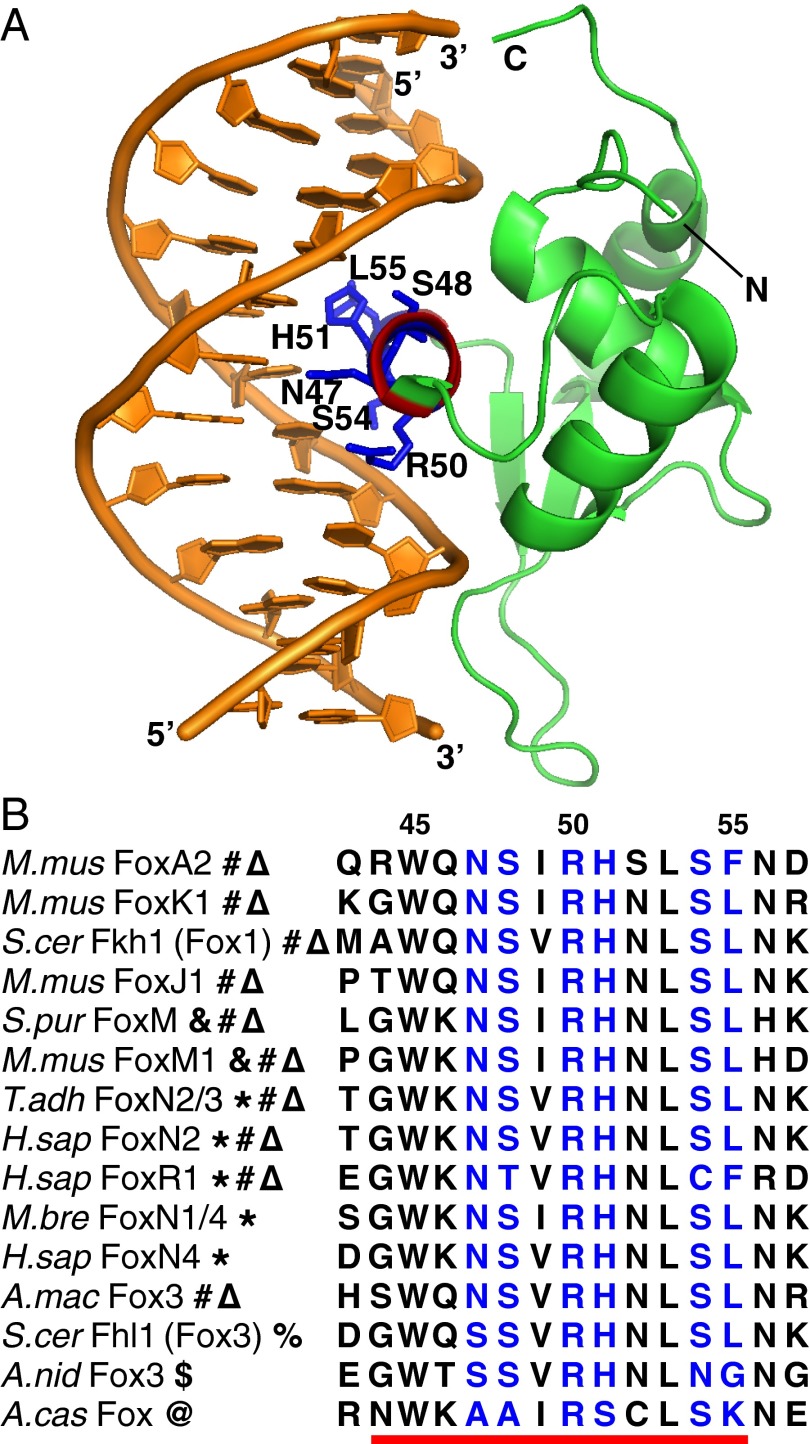
Canonical Fox base-contacting residues do not explain most alternate specificity. (A) A previous cocrystal structure of mouse FoxK1 bound to the canonical FkhP site GTAAACA [Protein Data Bank ID code 2C6Y (10)]. The recognition helix is highlighted; side chains are shown in blue and labeled for those amino acids that make base-specific contacts in at least two existing structures. (B) Protein sequence alignment of the recognition helix (red underscore) and adjacent positions for a sample of Fox domains representing various specificity classes (bold symbols represent binding capacity for different motif classes as defined in Fig. 1). Numbers above alignment represent positions within the Pfam Fork_head domain HMM.
Discussion
The previously unappreciated diversity in DNA-binding specificity of Fox domain TFs that we have discovered raises the question of how specificity has evolved in this family. We have presented evidence that major changes in specificity have occurred separately in three different Fox subfamily lineages. In fungal Fox3 proteins, two different alternate specificities (FHL-3 and FVH) have arisen, with alteration of the canonical recognition positions in the FVH-binding, but not the FHL-3-binding, proteins. In metazoan FoxM proteins, binding to the canonical FkhP and FkhS sites has been supplemented with binding to a very different site, the FHL-M motif, with the same proteins binding well to both motifs. In addition, in the holozoan FoxN subfamily, some proteins (FoxN2/3) exhibit this kind of bispecificity for two very different motifs (FkhP,S and FHL-N), whereas others (FoxN1/4) have completely lost the ability to bind the classic forkhead site (FkhP,S) in favor of the FHL-N motif. Finally, a derived subfamily unique to vertebrates (FoxR) appears to have regained specificity for a variant of the canonical FkhP motif from a more recent, exclusively FHL-specific ancestor. Formally, it is possible that lineages containing only proteins that bind only the FkhP,S sequences are derived from a more promiscuously binding ancestor with loss of FHL binding; however, this model would require a much larger number of specificity changes than the model that we put forth here. Moreover, each instance of specificity change inferred from phylogenetic analyses is corroborated by minor but consistent differences in the motifs that have arisen; for example, all FoxN proteins bind to a version of the FHL motif that is distinguishable from the very similar FHL motif of fungal Fox3 proteins by preferences at a flanking position.
Our strategy of combining phylogenetic inference with comprehensive assays of DNA-binding specificity permits us to study the evolution of DNA-binding specificity in more detail using information from these complementary approaches. The monophyly of clade I, for example, is supported both by a high-confidence node in the inferred phylogeny and by the observed uniformity of binding specificity within this group. In the absence of phylogenetic analyses, the observation of an alternate specificity (GAYGC) appearing three times in different Fox domain subfamilies would lead to a parsimonious hypothesis that one ancestral FHL-binding forkhead domain arose before the last common ancestor of metazoa and fungi and gave rise to fungal Fox3 and metazoan FoxM and N groups. However, this hypothesis is strongly refuted by ML phylogenetic inference, which instead suggests independent origins of all three groups of alternate-specificity proteins. Further support for this surprising model comes from the observation that fine differences in FHL specificity distinguish these three groups, as discussed above.
This model raises the question of how such similar alternate specificities could have arisen independently in three different forkhead lineages. In the group of Fox3 proteins from fungi related to A. nidulans, the alteration in specificity to the FVH motif with concomitant loss of binding to FkhP,S sequences might be due to the extensive changes observed in the recognition helix. However, the appearances of the FHL motif variants during forkhead evolution, whether along with FkhP binding in bispecific proteins or as a replacement, do not correlate with any changes at amino acid positions known to specify FkhP binding and suggest an alternate mechanism for changes in DNA-binding specificity.
We propose that the existence of bispecific proteins that bind both FkhP,S and FHL sequences with high specificity points to a possible explanation—that some Fox domain proteins that bind strongly to the FkhP site can achieve an alternate conformation that supports recognition of the FHL motif. It is intriguing, in the context of this observation, that both M. musculus FoxJ1 and A. macrogynus Fox3 show weak binding to a subset of FHL-containing 8-mers and exhibit binding similarity to bispecific factors that bind much more strongly and specifically to the FHL motif (Fig. 4). We suggest that the Fox domain can adopt an alternate DNA-binding mode and thus possesses an inherent “evolvability” of DNA sequence specificity that has permitted the emergence of FHL binding multiple independent times.
Allostery is a widespread and fundamental phenomenon in biological regulation, and in principle the use of alternate binding modes to recognize multiple sequence motifs could result in alternate protein interaction surfaces of a TF, thus creating a new regulatory role for the alternate binding motifs as allosteric effectors of interactions with cofactors (31, 32). Exploring the mechanisms of such regulatory consequences will require an approach combining structural studies of distinct TF–DNA complexes, such as those identified here, with in vivo analyses of binding-site utilization and function. This previously undescribed phenomenon of DNA-binding bispecificity suggests an alternate source of modularity and flexibility in the structure of TFs and transcriptional regulatory networks. Improved understanding of the evolution of TF binding specificity will provide insights into the evolution of transcriptional regulatory networks, which ultimately will shed light on the processes underlying the evolution of new body plans and environmental responses.
Materials and Methods
Forkhead Sequences.
The genome sequences and annotations used in this study are summarized in Dataset S1. For each annotated protein sequence, we performed a HMM search using HMMER3 (33) with the Fork_head domain (PF00250) in the Pfam database (E value < 10−10) (29). Using the hit sequences as queries, we conducted iterative homology search using PSI-BLAST (E value < 10−10) (34). We then constructed a HMM from each multiple alignment of forkhead sequences and searched against all protein sequences again. All obtained genes are described with their identification method in Dataset S1. All sequences used for the phylogenetic analysis contain five α-helices and three β-sheets as in human FoxP2 (11).
For phylogenetic analyses, each amino acid sequence of Fox domains was aligned by using five multiple sequence alignment programs: L-INS-i program in MAFFT (35), T-Coffee (36), MUSCLE (37), Clustal Omega (38), and Clustal W (39). The accuracies of multiple sequence alignments were evaluated by FastSP (40), and the MAFFT alignment was selected by the number of homologous amino acid sites.
Phylogenetic Inference.
The amino acid replacement models of LG (41) with gamma-distributed rate variation (α = 0.881) were selected for whole forkhead domains, using the Akaike information criterion implemented in PROTTEST 3 (42). Phylogenetic trees were constructed by using the ML method in PhyML 3.0 (43) with robustness evaluated by bootstrapping (100 times) (44) and by aLRT (45, 46). The starting tree for branch swapping was obtained by using a ML tree constructed by RAxML (47). For likelihood ratio tests, two ML trees were constructed from the ML tree in Fig. 2, changing the branching pattern of Fox3 and FoxM (Fig. S3 A and B, respectively). RAxML was applied to optimize the lengths of branches and calculate ML scores (−13,422.7 for Fig. S3A and −13,414.9 for Fig. S3B). Comparing the ML score obtained from the tree in Fig. 2 (−13,406.2), P values were calculated based on the χ2 distribution with one degree of freedom.
Cloning and Protein Expression.
The DBDs of the forkhead proteins, flanked by attB recombination sites, were constructed by gene synthesis and cloned into the pUC57 vector (GenScript USA). Constructs were transferred to the pDEST15 vector, which provides an N-terminal GST tag, using the Gateway recombinational cloning system (Invitrogen). All cloned forkhead domain sequences are provided in Dataset S2. Proteins were expressed by in vitro transcription and translation using the PURExpress in vitro Protein Synthesis kit (New England BioLabs). Concentrations of the expressed GST-fusion proteins were determined by Western blots in comparison with a dilution series of recombinant GST (Sigma).
PBM Experiments and Analysis.
Double-stranding of oligonucleotide arrays and PBM experiments were performed essentially as described, except where noted in Dataset S2, using custom-designed “all 10-mer” arrays in the 4 × 44K (Agilent Technologies; AMADID #015681) or 8 × 60K (Agilent Technologies; AMADID #030236) array format (28, 48). Microarray data quantification, normalization, and motif derivation were performed as described (28, 48); some published PBM data (21) were reanalyzed for this study. DNA binding-site motif sequence logos were generated by using enoLOGOS (49). The 8-mer E-score data were collected for any contiguous 8-mer bound (E score ≥ 0.35) by at least one assayed Fox protein and clustered by using the heatmap.2 function in the gplots R package with the Manhattan distance metric.
Supplementary Material
Acknowledgments
We thank Matthew W. Brown and Iñaki Ruiz-Trillo for sharing prepublication forkhead sequences from F. alba and A. castellanii; Anastasia Vedenko and Leila Shokri for technical assistance; and Anton Aboukhalil, Shamil Sunyaev and Ivan Adzhubey for helpful discussion. This study was supported by a Japan Society for the Promotion of Science Research Fellowship for Young Scientists (to S.N.), and by National Institutes of Health Grant R01 HG003985 (to M.L.B.). J.M.R. was supported in part by National Institutes of Health Molecular Biophysics Training Grant T32 GM008313.
Footnotes
The authors declare no conflict of interest.
This article contains Supporting Information at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310430110/-/DCSupplemental.
References
- 1.Carroll S, Grenier J, Weatherbee S. From DNA to Diversity. Malden, MA: Blackwell Science; 2001. [Google Scholar]
- 2.Gasch AP, et al. Conservation and evolution of cis-regulatory systems in ascomycete fungi. PLoS Biol. 2004;2(12):e398. doi: 10.1371/journal.pbio.0020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teichmann SA, Babu MM. Gene regulatory network growth by duplication. Nat Genet. 2004;36(5):492–496. doi: 10.1038/ng1340. [DOI] [PubMed] [Google Scholar]
- 4.Gajiwala KS, et al. Structure of the winged-helix protein hRFX1 reveals a new mode of DNA binding. Nature. 2000;403(6772):916–921. doi: 10.1038/35002634. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993;362(6417):219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- 6.Shimeld SM, Degnan B, Luke GN. Evolutionary genomics of the Fox genes: Origin of gene families and the ancestry of gene clusters. Genomics. 2010;95(5):256–260. doi: 10.1016/j.ygeno.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14(2):142–146. [PubMed] [Google Scholar]
- 8.Sebé-Pedrós A, de Mendoza A, Lang BF, Degnan BM, Ruiz-Trillo I. Unexpected repertoire of metazoan transcription factors in the unicellular holozoan Capsaspora owczarzaki. Mol Biol Evol. 2011;28(3):1241–1254. doi: 10.1093/molbev/msq309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 10.Tsai KL, et al. Crystal structure of the human FOXK1a-DNA complex and its implications on the diverse binding specificity of winged helix/forkhead proteins. J Biol Chem. 2006;281(25):17400–17409. doi: 10.1074/jbc.M600478200. [DOI] [PubMed] [Google Scholar]
- 11.Stroud JC, et al. Structure of the forkhead domain of FOXP2 bound to DNA. Structure. 2006;14(1):159–166. doi: 10.1016/j.str.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Littler DR, et al. Structure of the FoxM1 DNA-recognition domain bound to a promoter sequence. Nucleic Acids Res. 2010;38(13):4527–4538. doi: 10.1093/nar/gkq194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boura E, Rezabkova L, Brynda J, Obsilova V, Obsil T. Structure of the human FOXO4-DBD-DNA complex at 1.9 Å resolution reveals new details of FOXO binding to the DNA. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 12):1351–1357. doi: 10.1107/S0907444910042228. [DOI] [PubMed] [Google Scholar]
- 14.Larroux C, et al. Genesis and expansion of metazoan transcription factor gene classes. Mol Biol Evol. 2008;25(5):980–996. doi: 10.1093/molbev/msn047. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Wang Q, Zhao H, Zhang X, Pan Y. Evolutionary selection pressure of forkhead domain and functional divergence. Gene. 2009;432(1-2):19–25. doi: 10.1016/j.gene.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann E, Müller D, Knöchel W. DNA recognition site analysis of Xenopus winged helix proteins. J Mol Biol. 1995;248(2):239–254. doi: 10.1016/s0022-2836(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 17.Overdier DG, Porcella A, Costa RH. The DNA-binding specificity of the hepatocyte nuclear factor 3/forkhead domain is influenced by amino-acid residues adjacent to the recognition helix. Mol Cell Biol. 1994;14(4):2755–2766. doi: 10.1128/mcb.14.4.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierrou S, Hellqvist M, Samuelsson L, Enerbäck S, Carlsson P. Cloning and characterization of seven human forkhead proteins: Binding site specificity and DNA bending. EMBO J. 1994;13(20):5002–5012. doi: 10.1002/j.1460-2075.1994.tb06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C, et al. High-resolution DNA-binding specificity analysis of yeast transcription factors. Genome Res. 2009;19(4):556–566. doi: 10.1101/gr.090233.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badis G, et al. Diversity and complexity in DNA recognition by transcription factors. Science. 2009;324(5935):1720–1723. doi: 10.1126/science.1162327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badis G, et al. A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol Cell. 2008;32(6):878–887. doi: 10.1016/j.molcel.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, et al. Differential regulation of mesodermal gene expression by Drosophila cell type-specific Forkhead transcription factors. Development. 2012;139(8):1457–1466. doi: 10.1242/dev.069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlake T, Schorpp M, Nehls M, Boehm T. The nude gene encodes a sequence-specific DNA binding protein with homologs in organisms that lack an anticipatory immune system. Proc Natl Acad Sci USA. 1997;94(8):3842–3847. doi: 10.1073/pnas.94.8.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo H, et al. Forkhead box N4 (Foxn4) activates Dll4-Notch signaling to suppress photoreceptor cell fates of early retinal progenitors. Proc Natl Acad Sci USA. 2012;109(9):E553–E562. doi: 10.1073/pnas.1115767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehming N, Sartorius J, Kisters-Woike B, von Wilcken-Bergmann B, Müller-Hill B. Mutant lac repressors with new specificities hint at rules for protein—DNA recognition. EMBO J. 1990;9(3):615–621. doi: 10.1002/j.1460-2075.1990.tb08153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsden I, Chen Y, Jin C, Liao X. Evidence that the DNA binding specificity of winged helix proteins is mediated by a structural change in the amino acid sequence adjacent to the principal DNA binding helix. Biochemistry. 1997;36(43):13248–13255. doi: 10.1021/bi971514m. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Ezpeleta N, et al. Toward resolving the eukaryotic tree: The phylogenetic positions of jakobids and cercozoans. Curr Biol. 2007;17(16):1420–1425. doi: 10.1016/j.cub.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Berger MF, et al. Compact, universal DNA microarrays to comprehensively determine transcription-factor binding site specificities. Nat Biotechnol. 2006;24(11):1429–1435. doi: 10.1038/nbt1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punta M, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40(Database issue) D1:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh M, Katoh M. Identification and characterization of human FOXN5 and rat Foxn5 genes in silico. Int J Oncol. 2004;24(5):1339–1344. [PubMed] [Google Scholar]
- 31.Meijsing SH, et al. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324(5925):407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scully KM, et al. Allosteric effects of Pit-1 DNA sites on long-term repression in cell type specification. Science. 2000;290(5494):1127–1131. doi: 10.1126/science.290.5494.1127. [DOI] [PubMed] [Google Scholar]
- 33.Eddy SR. Accelerated Profile HMM Searches. PLOS Comput Biol. 2011;7(10):e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schäffer AA, et al. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001;29(14):2994–3005. doi: 10.1093/nar/29.14.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33(2):511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302(1):205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 37.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirarab S, Warnow T. FastSP: Linear time calculation of alignment accuracy. Bioinformatics. 2011;27(23):3250–3258. doi: 10.1093/bioinformatics/btr553. [DOI] [PubMed] [Google Scholar]
- 41.Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25(7):1307–1320. doi: 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
- 42.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27(8):1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 44.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39(4):783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 45.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 2006;55(4):539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 46.Anisimova M, Gil M, Dufayard J-F, Dessimoz C, Gascuel O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol. 2011;60(5):685–699. doi: 10.1093/sysbio/syr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 48.Berger MF, Bulyk ML. Universal protein-binding microarrays for the comprehensive characterization of the DNA-binding specificities of transcription factors. Nat Protoc. 2009;4(3):393–411. doi: 10.1038/nprot.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Workman CT, et al. (2005) enoLOGOS: A versatile web tool for energy normalized sequence logos. Nucleic Acids Res 33(Web Server issue):W389–W392. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.