Abstract
Fish stocks experiencing high fishing mortality show a tendency to mature earlier and at a smaller size, which may have a genetic component and therefore long-lasting economic and biological effects. To date, the economic effects of such ecoevolutionary dynamics have not been empirically investigated. Using 70 y of data, we develop a bioeconomic model for Northeast Arctic cod to compare the economic yield in a model in which life-history traits can vary only through phenotypic plasticity with a model in which, in addition, genetic changes can occur. We find that evolutionary changes toward faster growth and earlier maturation occur consistently even if a stock is optimally managed. However, if a stock is managed optimally, the evolutionary changes actually increase economic yield because faster growth and earlier maturation raise the stock’s productivity. The optimal fishing mortality is almost identical for the evolutionary and nonevolutionary model and substantially lower than what it has been historically. Therefore, the costs of ignoring evolution under optimal management regimes are negligible. However, if fishing mortality is as high as it has been historically, evolutionary changes may result in economic losses, but only if the fishery is selecting for medium-sized individuals. Because evolution facilitates growth, the fish are younger and still immature when they are susceptible to getting caught, which outweighs the increase in productivity due to fish spawning at an earlier age.
Keywords: Atlantic cod, genetic adaptations, harvest control rule, marine governance, adaptive management
Life-history theory, experiments, and field-based studies strongly suggest that fishing is capable of inducing genetic adaptations, especially when it removes individuals with characteristics such as large body size (1–5). Even if fishing is not size-selective, high fishing mortality may be sufficient to induce genetic change (6, 7). It is difficult to predict how genetic changes at the individual level affect population-level properties. Genetic adaptations may, in principle, be beneficial for the state of a stock, by enabling individuals to invest more into reproduction and growth (1, 8). As a consequence, the stock may become more productive, allowing exploited populations to withstand higher fishing mortalities than they could in the absence of such adaptation, possibly permitting higher yields. However, although an individual’s increased reproductive investment leads to larger gonads, this happens at the expense of slower postmaturation growth. Maturing earlier may also reduce fecundity, because individuals are smaller when they reproduce (9). Moreover, adapting to fishing may bear a cost of maladaptation, resulting in increased natural mortality (10, 11). Therefore, fisheries-induced evolution (FIE) may reduce yield (2, 4, 12, 13) and may even imply a “Darwinian debt” (14) to be paid back by future generations, at least if genetic changes are difficult to reverse (1, 15, 16). Clearly, FIE has the potential for causing positive and negative effects on key stock properties such as spawning stock biomass (SSB) and yield, making its economic effect ambiguous. It is also an open question whether the expected size of the economic effects are substantial, largely because any evolutionary changes are closely intertwined with ecological effects. For example, the release of density dependence when population biomass is fished down could be an important driver of phenotypic change (1, 17, 18) and might override effects of FIE on yield. However, the economic consequences of FIE and its effects on optimal fishing mortalities in wild populations have yet to be determined. Here, we ask how evolutionarily informed management differs from classical fisheries management. First, we determine how an evolving fish population should be optimally managed. Second, we analyze how these management strategies differ compared with optimal management derived for a population whose development is purely determined by ecological processes. Third, we ask how substantial the losses are if a fishery’s manager—unaware of any evolutionary changes—manages an evolving population as if it were not evolving. Fourth, we analyze how FIE affects the performance of the fishery that is not optimally managed, but heavily exploited.
Northeast Arctic (NEA) cod is currently the world’s largest stock of Atlantic cod (Gadus morhua) and provides substantial ecosystem services. The stock’s fishery is an important economic resource for Norway and Russia, with annual catches by Norway being worth more than 500 million US dollars in 2010, and Russia obtaining about the same revenue. Traditionally, harvesting focused on adult cod at the stock’s spawning grounds along the Norwegian coast. From the 1930s, when industrial trawlers were introduced in the stock’s feeding grounds in the Barents Sea, immature fish came under substantial fishing pressure, and total fishing mortality increased (19). Evolutionary changes have been predicted to be a factor in explaining the observed declines in age and length at maturation in NEA cod, although the predicted extent has varied among studies (17, 20, 21).
We develop a bioeconomic model to investigate if and how FIE affects economic yield (Fig. 1). Our model is a comprehensive compilation of a life-history model for a harvested species with economic components relying on individual vessel data, making this a unique empirically derived bioeconomic model for investigating genetic adaptations to harvesting. This model has been specifically built for NEA cod to investigate the ecological and evolutionary effects of exploitation on the changes in maturation that occurred after fishing mortality was intensified in the 1930s in the feeding grounds (17). To match the observed trends in the biological model as closely as possible, we recreated the historical selection pressure to determine the evolvability (i.e., the coefficient of genetic variation) in the life-history traits (17). Although we focus on the feeding ground fishery in the Barents Sea, we also included fishing in the spawning grounds at the historic levels between 1932 until 2005, and at a constant rate after 2006. Hence, we consider the spawning ground fishery to be beyond the control of the manager. The biological model component is built on the individual-based ecogenetic model framework developed by Dunlop et al. (1), describing four evolving life-history traits capturing key aspects of growth, maturation, and reproduction (Table S1). Changes in life-history traits may be driven both by ecological processes, such as phenotypic plasticity and density dependence, and genetic processes. To evaluate whether accounting for FIE requires a special harvest strategy, we also analyze a nonevolutionary version of the biological model in which the genetic traits cannot evolve. We therefore compare a nonevolutionary model, in which changes in populations are driven only by phenotypic plasticity, with an evolutionary model that allows, in addition, for genetic adaptations. The economic model component consists of production and cost functions estimated specifically for the Norwegian cod trawler fleet (Table S1). We incorporate a demand function, also estimated from empirical data, to account for how total catch affects the price of landings (22). Our model incorporates feedbacks between the stock development and the economic gains through an optimal harvest control rule (HCR), which is constrained by the two parameters Bmax and Fmax (Fig. 1). This shape makes it directly comparable to the HCR that is being implemented for NEA cod since 2004 (23, 24). We search for the parameter combination that gives the highest net present value (NPV) of fleet profits. We derive HCRs that are optimized in either the evolutionary or nonevolutionary versions of the model.
Fig. 1.
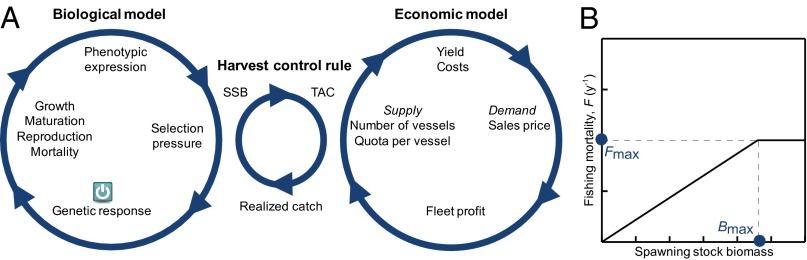
An overview of the bioeconomic model. (A) The biological and economic model components are coupled by the harvest control rule (HCR). The individual-based biological model describes the evolution of key life-history traits if genetic changes are allowed to occur in the model. The economic model accounts for the supply and demand side of the fishery, as well as for fleet profit generated. (B) The shape of the HCR depends on two parameters: above the level Bmax of spawning stock biomass, the maximum fishing mortality Fmax is allowed. Between Bmax and a biomass level of zero, fishing mortality linearly decreases from Fmax to zero. The structure of this HCR is in agreement with that advised in 2004 by the International Council for the Exploration of the Sea for the Northeast Arctic cod fishery.
Results
We first compare the emerging properties of the evolutionary model with the nonevolutionary model, when both are managed according to what an HCR recommends that has been optimized for fleet profits (Table 1, Evolution vs. Ecology). We find that the optimal fishing mortality is almost identical for the evolutionary and nonevolutionary models and substantially lower than what it has been historically. Despite this, the emerging biomass levels and the total allowable catch (TAC) are higher in the evolutionary model, indicating that evolution indeed makes the stock more productive, permitting higher yields for the same fishing mortality. Overall, the NPV of the fishery is higher when evolution occurs, even though the total effect is very small. Given that the recommended fishing mortalities are almost identical, the loss of disregarding any evolutionary effects is negligible, and the NPV is still higher if evolution occurs and is ignored by managers (Table 1, Evolution ignored). The key message here is that a low fishing mortality is optimal, regardless of whether genetic changes occur. This prediction holds for different discount rates (Table S2), when sales prices are assumed to be independent of the total catch, and when the price that can be obtained per kilogram of cod rises with the weight of the fish (Table S3).
Table 1.
Optimal HCR for the evolutionary model (evolution) and nonevolutionary model (ecology)
| Model | F | TAC | SSB | NPV |
| Evolution | 0.34 | 469 (60) | 767 (163) | 25.4 |
| Ecology | 0.35 | 443 (48) | 643 (118) | 25.3 |
| Evolution ignored | 0.35 | 470 (60) | 735 (155) | 25.4 |
Values shown are averages for 1932–2100 of fishing mortality (F), total allowable catch (TAC), spawning stock biomass (SSB), with temporal SDs in parentheses, and net present value (NPV) is given for a discount rate of 2%. “Evolution ignored” uses the evolutionary model with the ecologically optimal harvest control rule (HCR). Units: F (y−1), TAC and SSB (1,000 tonnes), NPV (in billions, US dollars).
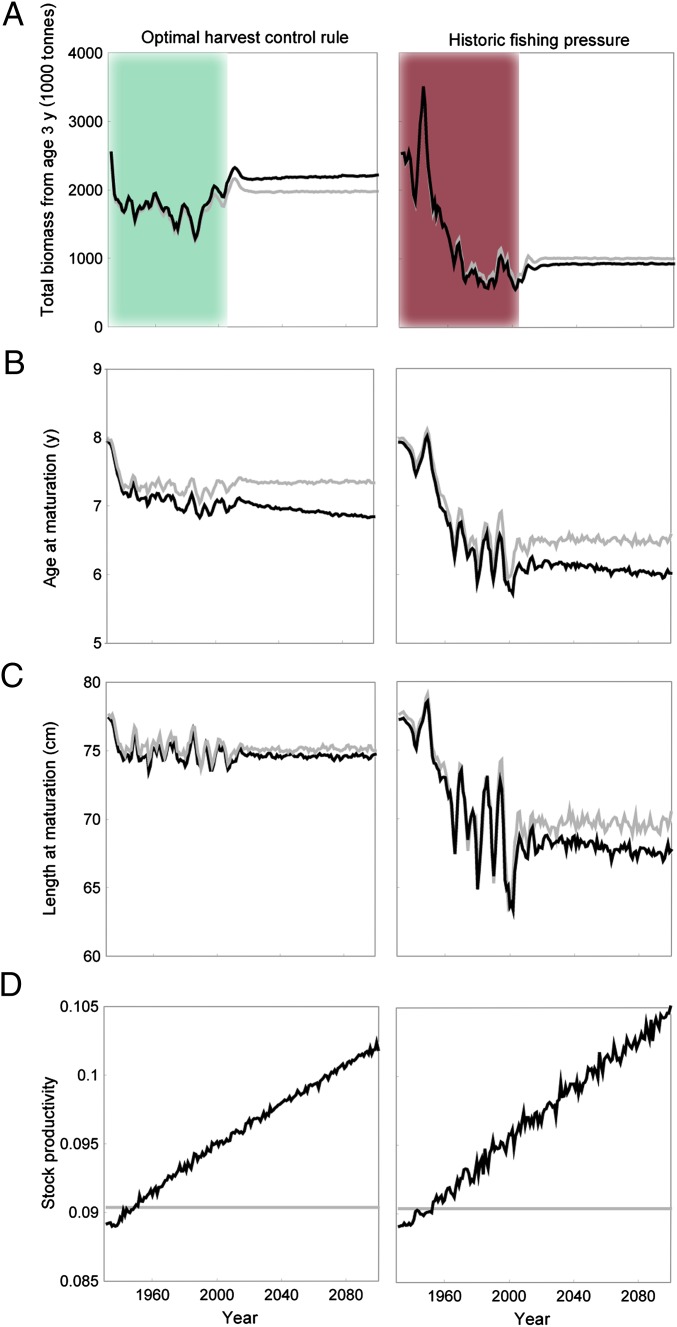
Given that fishing mortality has not been low for the NEA cod fishery in the past, and considering that worldwide most fisheries are still far from being managed optimally, we also investigate how evolution affects the stock when it is overexploited. To do so, we use historic fishing mortalities between 1932 and 2006, and the average fishing mortality afterwards to simulate a scenario of high fishing pressure; this is then contrasted with a counterfactual scenario that analyzes how the fate of the fishery would have developed if an optimal HCR had been introduced already in 1932 (as given in Table 1, Evolution). We find that using an optimal HCR leads to higher biomass levels in the evolutionary model, compared with the case where only ecological effects are present. The opposite is true for the scenario of historically high fishing mortality, where biomass is slightly lower in the evolutionary model (Fig. 2A). As a result, the corresponding TAC and NPV are also slightly lower when evolution occurs and fishing mortality is high (Table S4).
Fig. 2.
The first scenario (Left) is based on an optimal harvest control rule (HCR) maximizing fleet profit (green shading shows the period for which we have data), and the second scenario (Right) is based on the observed historic fishing mortalities for 1932–2005 (red shading), and from 2006 onward follows the average fishing mortality for 1946–2005. For each scenario, the emerging properties for an evolutionary model (black) are compared with those of the corresponding nonevolutionary model (gray). (A) Total biomass from age 3 y is lower in the evolutionary model when fishing mortality is high, but higher in the evolutionary model when the optimal HCR is used. (B) Predicted age at maturation and (C) length at maturation is lower in the evolutionary model than in the nonevolutionary model. The historic scenario predicts age and length at maturation to fall to between ages 6 and 7 y, and 60 and 70 cm in 2005, in agreement with the observed data. (D) Stock productivity, i.e., mean gonad mass divided by total spawning stock biomass, increases when evolution occurs, and more if fishing mortality is high.
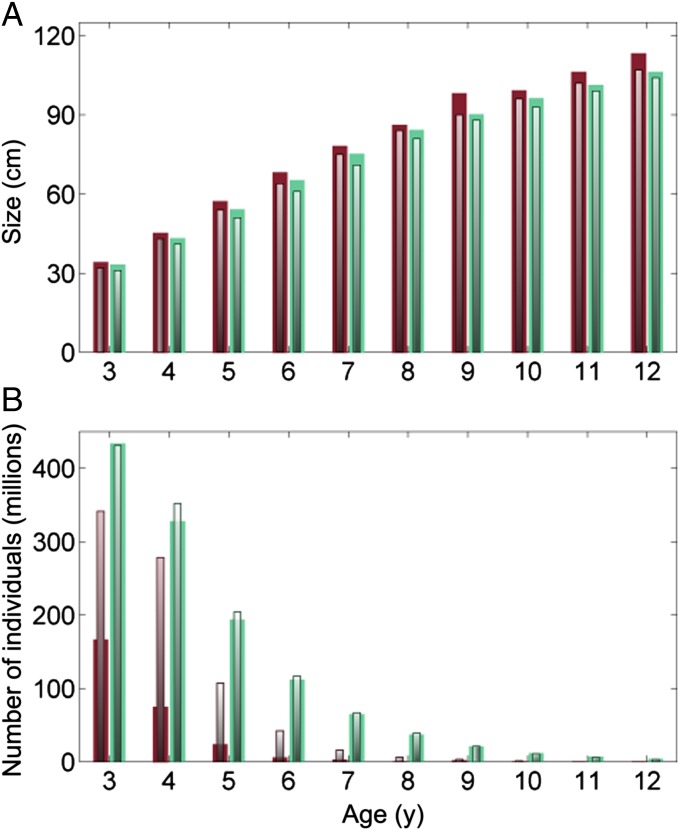
It is not immediately obvious why evolution has a positive effect on the fishery if fishing mortality is set optimally, but a negative effect if fishing mortality is high. Inspecting key life-history traits reveals that age at maturation declines over time in all scenarios (Fig. 2B), and although this also occurs in the nonevolutionary model (solely as a result of phenotypic plasticity), the decline is even more severe when evolution takes place. A decline in length at maturation occurs in all scenarios as well, and is even more pronounced if fishing mortality is high (Fig. 2C). Despite reduced age and length at maturation, the reproductive output per unit of SSB, a measure of the stock’s productivity, is increasing over time when evolution occurs (Fig. 2D). To better understand the population structure, we take a closer look at the age composition at the simulation endpoints (Fig. 3). We find that despite individual fish being smaller at maturation, the size at a given age is consistently larger for the evolutionary model compared with the nonevolutionary model, irrespective of the fishing mortality being optimal or high (Fig. 3A). Indeed, the underlying genetic trait changes show that the evolving population invests more in intrinsic somatic growth capacity and reproduction, resulting in overall larger body sizes and higher reproductive output (Fig. 3A; Fig. S1). Looking closer at the age structure of the fish stock makes it immediately clear that the evolutionary loss occurs because the number of individuals in each age class is much lower if fishing mortality is high and evolution occurs (Fig. 3B). The fish grow quicker and mature earlier in the evolutionary scenario when fishing pressure is high, but these genetic changes do not pay off in terms of population biomass, TAC or NPV, because fish are also younger (and still immature) when they are potentially caught by trawlers, which in the model spare all fish below the minimum size limit of 45 cm. It might seem surprising that these genetic changes toward faster growth occur, given that this makes the fish more vulnerable to fishing at an earlier age. However, faster growth also means maturing earlier, which enables individuals to have a higher probability to reproduce and pass on genes before being captured by the fishery.
Fig. 3.
Ecoevolutionary dynamics and age truncation. The optimal HCR scenario is shown by green bars, and the scenario with historic (high) fishing mortality is indicated with red bars. The evolutionary model outcome is shown with full bars, and the nonevolutionary one is shown by gray inner bars. (A) The mean size is larger for all age classes if evolution occurs. (B) The number of individuals in each age class is much lower if evolution occurs, but only if fishing mortality is high.
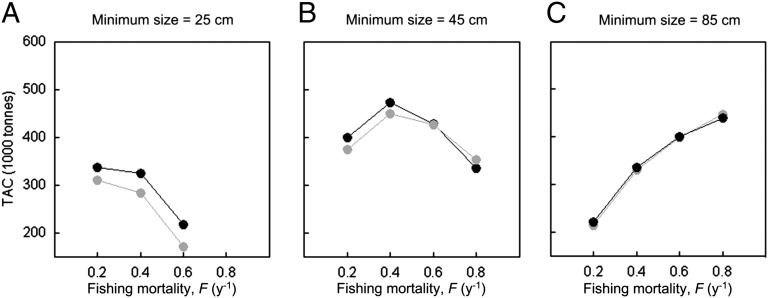
If certain environmental conditions are responsible for the evolutionary loss, it may be sufficient to tweak the environment to avoid or reverse these losses. Indeed, we find that changing the minimum size limit is sufficient to avoid any evolutionary costs (Fig. 4). With a very low minimum size limit, evolution is unambiguously good for the fishery, because it leads to individual growth that is fast enough to negate any detrimental effects of early maturation on TACs (Fig. 4A). As expected, evolution has little effect on the TAC when the minimum size limit is high, because selection acting on maturation and growth is weaker, so and there is little difference between the evolutionary and nonevolutionary predictions (Fig. 4C; Fig. S2). Therefore, the loss in NPV due to evolution only occurs for intermediate minimum size limits, where the beneficial effects of growing faster are overriden by making those fish more vulnerable that are larger, but also younger and still immature (Table S5).
Fig. 4.
(A–C) Total allowable catch (TAC) under different minimum size limits and for different constant fishing mortalities, F. The evolutionary model (black) predicts higher TAC than the nonevolutionary model (gray) when selection also acts on very young fish. For a minimum size limit of 85 cm, the two model predictions become essentially indistinguishable. At the intermediate minimum size limit of 45 cm, the TAC is highest for the evolutionary model when fishing mortality is low, but as fishing intensity increases, the TAC is smaller for the evolutionary model.
In this study, the coefficient of genetic variation was set at a level that resulted in the best fit to empirical observations in age and length at maturation (Table S1), but we nonetheless investigated the effect of this parameter (the evolvability of traits) on model predictions. As expected (1, 16, 25, 26), higher genetic variance resulted in fish maturing at even younger ages and smaller sizes, while also growing faster. Consequently, higher TACs can be obtained when the evolvability is high, suggesting that stronger evolutionary forces can have a positive effect on the fishery (Fig. S3).
Discussion
Our model predicts that evolutionary change occurs even if fishing mortality is low, which implies that a management strategy aimed at avoiding genetic change might not be feasible. At the same time, we find that fisheries-induced evolution is not necessarily bad for the fishery, and most of the time even beneficial—especially a fishery that is managed according to what is ecologically optimal can safely ignore any evolutionary effects, at least for the stock and under the conditions that we are considering. This finding is very surprising and in contrast to much of the existing literature, which tends to sketch a gloomy picture of the potential consequences of FIE. It is also comforting that fishing can cause evolution of faster growth, allowing the population to withstand higher harvest pressure and prevent stock collapse (Fig. S3). Nonetheless, the life-history changes we predict could have management implications because they affect important indicators that are commonly used to assess the state of the stock. Evolution tends to increase the ratio between SSB and total biomass (Fig. S4), which could mask a decreasing trend in total biomass and affect the stock-recruitment relationship (26); this may have important management implications when biomass levels approach SSB-based limit reference points (27, 28). Even more worrisome is our finding that evolutionary effects tend to be more important when a fish stock is overexploited and the fishery is intermediately size-selective. Admittedly, such an exploitation regime is a special case, but unfortunately the one that, worldwide, most fisheries are facing. Surprisingly, an economic cost of evolution under these conditions does not materialize because of a drop in reproductive output or, as many might expect, because of a reduction in growth or size-at-age (29). On the contrary, evolution here promoted faster growth, yet still could exact an economic cost. These results underscore the importance of management taking into account the detailed age and size structure of the stock (30–32).
Although we find that selectively removing individuals of intermediate size may result in economic losses due to evolutionary change, we do not find any evidence that targeting only large fish results in evolutionary loss (Fig. 4). These findings may shed light on the discussion whether harvesting should be balanced or selective (33). In this study, we assume a knife-edge selectivity in our model (34, 35), so different gear types with other selectivity patterns remain to be explored by further research. Although gear regulations can, in principle, be easily changed, our findings may also hint at broader problems. If predation is size-selective, evolutionary changes may affect natural mortality which may lead to similar consequences as fishing mortality (10, 11). Investigating how FIE acts in concert with natural mortality, climatic changes, or other driving forces remains to be explored, especially in the light of recovery potential (16).
Although our biological model is complex, the optimal HCR was constrained by two parameters, resembling the shape of the HCR currently adopted for NEA cod. It would be interesting to see to what extent our results carry over to a simpler biological model that could then be used for more flexible optimization routines treating the minimum size limit, for example, as a choice variable. Another interesting avenue is to separately optimize HCRs for the NEA cod’s feeding and spawning grounds. Previous research has found predictions for FIE to differ depending on whether management actions target feeding or spawning grounds (36). Here, we focused on the fishery in the stock’s feeding grounds and kept the fishing mortality at observed levels in the stock’s spawning grounds to mimic the historic selection pressure on mature fish, while parsimoniously asking what can be changed for the trawler fleet in the Barents Sea.
Together, our results show that the economic consequences of FIE are rather small, and mostly beneficial, largely because of the positive effects of fishing on growth. This prediction is made possible because of the crucial ecoevolutionary feedbacks among biomass, growth, and maturation, and the inclusion of growth as an evolving trait. Models that do not include these crucial factors might incorrectly predict a larger economic cost of evolution. Regardless, low fishing mortality is the key for successful management. Today, many fish stocks are still far from being managed in an ecologically optimal way. In such a case, our model predicts that FIE enables the stock to withstand higher harvests, but only if fishing mortality is not intermediately size-selective; otherwise, FIE may reduce economic yield and make the stock less viable. Admittedly, these evolutionary costs are small, but they may just be enough to push a fish stock from the state of overexploitation into collapse.
Materials and Methods
Our bioeconomic model consists of two model components: the biological model, describing the life cycle of NEA cod, and the economic model, describing details such as cost and demand for the NEA cod trawl fishery. Each of these components have been specifically estimated and calibrated for this stock by using data from 1932 to 2007 (Table S1). A more extensive model description can be found in SI Materials and Methods: Model and Data Description.
Biological Model.
The biological model component is individual-based and has been developed in ref. 17, building on the ecogenetic modeling framework derived in ref. 1. The model describes each individual’s growth, maturation, reproduction, and mortality in each year and follows the fate of ∼50,000 superindividuals (37, 38). If a fish reproduces, genetic traits are inherited by offspring and expressed phenotypically. Mortality acts on these phenotypic traits, resulting in selection that may cause a genetic response in the life-history traits (Fig. 1A). We studied two versions of our model, an evolutionary and a nonevolutionary version, each modeling their respective population of individuals to compare a population that has the propensity to evolve with a population that does not evolve. We consider the evolution of four quantitative life-history traits: maturation tendency given by the (i) slope and (ii) intercept of a probabilistic maturation reaction norm (20), (iii) growth capacity, and (iv) reproductive investment. The genetic traits evolve independently, and we therefore do not account for pleiotropy or genetic linkage between traits. Our model has limitations, but thanks to the data availability for NEA cod, we are able to include estimates of the initial mean life-history trait values and annual exploitation rates, as well as parameters specifying the stock-recruitment relationship (describing fecundity and newborn mortality) and the density dependence of growth on stock biomass (17). Furthermore, a growth-survival tradeoff is included; the strength of this tradeoff was determined by matching the ecological properties for data on age and length at maturation, phenotypic growth, and biomass from 1932 to 1950 in the nonevolutionary version of the model after reaching demographic equilibrium (17). In the evolving population, the coefficient of genetic variation (CVz,G) has been determined empirically for each trait (17) by matching trends in age and length at maturation over a 74-y period (from 1932 to 2005). In this calibration, the historic selection pressure was mimicked by using annual harvest probabilities in the feeding and spawning grounds from 1932 until 2005. The resultant CVz,G has been found to be lower than what was assumed in previous studies using the same modeling framework but not based on specific stocks (1, 16, 25, 26), as was the case here. For the nonevolving population, which is only driven by ecological processes, the CVz,G is equal to zero.
Economic Model and Harvest Control Rule.
The economic model specifies (i) the harvest function, (ii) the profit function, (iii) the procedure for allocating fishing quotas, and (iv) the demand function. All of these functions have been estimated and derived in detail in ref. 39 and used in ref. 22. We assume a knife-edge selectivity (34, 35) that targets all fish above the size of 45 cm (17, 40). The biological and economic model components are linked through an annual feedback loop: SSB is fed into the economic model component where ultimately the TAC is determined by a HCR, and the derived TAC feeds back into the biological model component where it affects the stock size (Fig. 1, realized catch). The shape of the HCR is based on the one that has been implemented for NEA cod since 2004 (23, 24): the maximum fishing mortality Fmax is allowed above a certain SSB level, given by the parameter Bmax. Below Bmax, fishing mortality decreases linearly to the origin (Fig. 1B). We explore model simulations over a large grid of combinations of Fmax and Bmax, searching for those combinations that achieve the economic objective of maximizing the net present value of fleet profits. All results, such as those for SSB and TAC, are given for a population that has been scaled up by a factor of 100,000. Because the model is stochastic, we ran each scenario for 15 independent replicates, and then averaged across these, presenting the mean in the tables and figures.
Historic Fishing Pressure.
The observed harvest pressure in the feeding ground increased steadily from the 1930s to the middle of the 1960s and remained high until mid-2000. In the historic fishing scenarios, we use observed fishing mortalities from 1932 to 2005 and then assume a constant fishing mortality in the feeding ground (0.68 y−1) being maintained from 2006 into the future. This constant (0.68 y−1) is an average of the historic fishing mortality between 1946 and 2005 and is higher than what is considered to be precautionary for NEA cod (0.4 y−1) (40).
Supplementary Material
Acknowledgments
Valuable comments and feedback were provided by K. Enberg, J. Grasman, J. A. Hutchings, C. Jørgensen, C. T. Marshall, E. Nævdal, L. Nøstbakken, P. Sandberg, and D. van Soest. We thank two anonymous reviewers for constructive comments on earlier versions of this manuscript. We thank M. Heino for help in developing the biological model and M. Heino, O. R. Godø, O. S. Kjesbu, P. Sandberg, and the Knipovich Polar Research Institute of Marine Fisheries and Oceanography (PINRO) for access to unpublished results and data. We gratefully acknowledge the Research Computing Services at the University of Oslo and The Norwegian Metacenter for Computational Science (NOTUR) for support and access to computing resources required for this study. Financial support for this project was provided by the European Commission through the Specific Targeted Research Project FinE (A.R., E.S.D., and U.D.), the Marie Curie Research Training Network FishACE (E.S.D. and U.D.), and an Intra-European Fellowship (to A.R.), as well as the Norwegian Research Council (A.M.E., E.S.D., and N.C.S.), the Netherlands Organisation for Scientific Research (A.R.), the European Science Foundation (U.D.), the Austrian Science Fund (U.D.), the Austrian Ministry of Science and Research (U.D.), and the Vienna Science and Technology Fund (U.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212593110/-/DCSupplemental.
References
- 1.Dunlop ES, Heino M, Dieckmann U. Eco-genetic modeling of contemporary life-history evolution. Ecol Appl. 2009;19(7):1815–1834. doi: 10.1890/08-1404.1. [DOI] [PubMed] [Google Scholar]
- 2.Hutchings JA. Avoidance of fisheries-induced evolution: Management implications for catch selectivity and limit reference points. Evolutionary Applications. 2009;2(3):324–334. doi: 10.1111/j.1752-4571.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Mol Ecol. 2008;17(1):294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- 4.Jørgensen C, et al. Ecology: Managing evolving fish stocks. Science. 2007;318(5854):1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- 5.Carlson SM, et al. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius) Ecol Lett. 2007;10(6):512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe DMT, Hendry AP. Life history change in commercially exploited fish stocks: An analysis of trends across studies. Evol Appl. 2009;2(3):260–275. doi: 10.1111/j.1752-4571.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roff DA. The Evolution of Life Histories: Theory and Analysis. New York: Chapman & Hall; 1992. [Google Scholar]
- 8.Andersen KH, Brander K. Expected rate of fisheries-induced evolution is slow. Proc Natl Acad Sci USA. 2009;106(28):11657–11660. doi: 10.1073/pnas.0901690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall CT, Needle CL, Yaragina NA, Ajiad AM, Gusev E. Deriving condition indices from standard fisheries databases and evaluating their sensitivity to variation in stored energy reserves. Can J Fish Aquat Sci. 2004;61(10):1900–1917. [Google Scholar]
- 10.Jørgensen C, Fiksen Ø. Modelling fishing-induced adaptations and consequences for natural mortality. Can J Fish Aquat Sci. 2010;67(7):1086–1097. [Google Scholar]
- 11.Swain DP. Life-history evolution and elevated natural mortality in a population of Atlantic cod (Gadus morhua) Evol Appl. 2011;4(1):18–29. doi: 10.1111/j.1752-4571.2010.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hard JJ, et al. Evolutionary consequences of fishing and their implications for salmon. Evol Appl. 2008;1(2):388–408. doi: 10.1111/j.1752-4571.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland WJ. Evolution and fisheries. Nature. 1990;344(6269):814–815. [Google Scholar]
- 14.Dieckmann U, Heino M, Rijnsdorp AD. The dawn of Darwinian fishery management. ICES Insight. 2009;46:34–43. [Google Scholar]
- 15.Conover DO, Munch SB, Arnott SA. Reversal of evolutionary downsizing caused by selective harvest of large fish. Proc Biol Sci. 2009;276(1664):2015–2020. doi: 10.1098/rspb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enberg K, Jorgensen C, Dunlop ES, Heino M, Dieckmann U. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evol Appl. 2009;2(3):394–414. doi: 10.1111/j.1752-4571.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eikeset AM, Dunlop ES, Heino M, Stenseth NC, Dieckmann U (2010) Is evolution needed to explain historical maturation trends in Northeast Atlantic cod? PhD thesis (University of Oslo, Oslo)
- 18.Eikeset AM, Richter AP, Diekert FK, Dankel DJ, Stenseth NC. Unintended consequences sneak in the back door: Making wise use of regulations in fisheries management. In: Belgrano A, Fowler CW, editors. Ecosystem Based Management for Marine Fisheries: An Evolving Perspective. Cambridge, UK: Cambridge Univ Press; 2011. pp. 183–217. [Google Scholar]
- 19.Godø OR. Fluctuation in stock properties of north-east Arctic cod related to long-term environmental changes. Fish Fish. 2003;4(2):121–137. [Google Scholar]
- 20.Heino M, Dieckmann U, Godø OR. Estimating reaction norms for age and size at maturation with reconstructed immature size distributions: A new technique illustrated by application to Northeast Arctic cod. ICES J Mar Sci. 2002;59(3):562–575. [Google Scholar]
- 21.Heino M, Dieckmann U, Godø OR. Reaction norm analysis of fishery-induced adaptive change and the case of the Northeast Arctic cod. ICES CM Series, Report Y:14. 2002 Available at http://brage.bibsys.no/imr/handle/URN:NBN:no-bibsys_brage_3050. [Google Scholar]
- 22.Eikeset AM, et al. A bio-economic analysis of harvest control rules for the Northeast Arctic cod fishery. Mar Pol. 2013;39:172–181. doi: 10.1016/j.marpol.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bogstad B, et al. (2005) Harvest control rules for management of fisheries on Cod and Haddock and optimal long term optimal harvest in the Barents Sea ecosystem. Report of the Basic Document Working Group (BDWG) to the Joint Norwegian–Russian Fisheries Commission. Available at www.regjeringen.no/upload/kilde/fkd/prm/2005/0084/ddd/pdfv/262877-vedlegg_11_bdwg-2005_final.pdf.
- 24. International Council for the Exploration of the Sea (ICES) (2011) Report of the ICES Advisory Committee, 2011. ICES Advice (ICES, Copenhagen)
- 25.Dunlop ES, Baskett ML, Heino M, Dieckmann U. Propensity of marine reserves to reduce the evolutionary effects of fishing in a migratory species. Evol Appl. 2009;2(3):371–393. doi: 10.1111/j.1752-4571.2009.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enberg K, Jørgensen C, Mangel M. Fishing-induced evolution an changing reproductive biology of fish: The evolution of steepness. Can J Fish Aquat Sci. 2010;67(10):1708–1719. [Google Scholar]
- 27.Marshall CT, Needle CL, Thorsen A, Kjesbu OS, Yaragina NA. Systematic bias in estimates of reproductive potential of an Atlantic cod (Gadus morhua) stock: Implications for stock-recruit theory and management. Can J Fish Aquat Sci. 2006;63(5):980–994. [Google Scholar]
- 28.Heino M, et al. Can fisheries-induced evolution shift reference points for fisheries management? ICES Journal of Marine Science. 2013;70(4):707–721. [Google Scholar]
- 29.Conover DO, Munch SB. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297(5578):94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- 30.Diekert FK, Hjermann DO, Naevdal E, Stenseth NC. Spare the young fish: Optimal harvesting policies for North-East Arctic Cod. Environ Resour Econ. 2010;47(4):455–475. [Google Scholar]
- 31.Sinclair AF, Swain DP, Hanson JM. Measuring changes in the direction and magnitude of size-selective mortality in a commercial fish population. Can J Fish Aquat Sci. 2002;59(2):361–371. [Google Scholar]
- 32.Tahvonen O. Economics of harvesting age-structured fish populations. J Environ Econ Manage. 2009;58(3):281–299. [Google Scholar]
- 33.Garcia SM, et al. Conservation. Reconsidering the consequences of selective fisheries. Science. 2012;335(6072):1045–1047. doi: 10.1126/science.1214594. [DOI] [PubMed] [Google Scholar]
- 34. Beverton RJH, Holt SJ (1957) On the dynamics of exploited fish populations. Fish Investment Series 2 (U.K. Ministry of Agriculture, Fisheries and Food, London), Vol 19.
- 35. Food and Agricultural Organization of the United Nations (FAO) (1998) Introduction to tropical fish stock assessment. Part 1: Manual. FAO Fisheries Technical Paper (FAO, Rome)
- 36.Jørgensen C, Ernande B, Fiksen O. Size-selective fishing gear and life history evolution in the Northeast Arctic cod. Evolutionary Applications. 2009;2(3):356–370. doi: 10.1111/j.1752-4571.2009.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huse G, Johansen GO, Bogstad L, Gjosaeter H. Studying spatial and trophic interactions between capelin and cod using individual-based modelling. ICES J Mar Sci. 2004;61(7):1201–1213. [Google Scholar]
- 38.Scheffer M, Baveco JM, Deangelis DL, Rose KA, Vannes EH. Super-individuals a simple solution for modeling large populations on an individual basis. Ecol Modell. 1995;80(2-3):161–170. [Google Scholar]
- 39. Richter AP, Eikeset AM, Van Soest DP, Stenseth NC (2011) Towards the optimal management of the Northeast Arctic cod fishery. Fondazione Eni Enrico Mattei Working Paper 591. Available at www.feem.it/userfiles/attach/20115201140194NDL2011-040.pdf.
- 40. International Council for the Exploration of the Sea (ICES) (2009) Report of the Arctic Fisheries Working Group (ICES, Copenhagen)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.