Abstract
Endothelial dysfunction is an early manifestation of atherosclerosis caused in part by oxidized LDL (oxLDL). Since vitamin C, or ascorbic acid, prevents several aspects of endothelial dysfunction, the effects of oxLDL on oxidative stress and regulation of the ascorbate transporter, SVCT2, were studied in cultured EA.hy926 endothelial cells. Cells cultured for 18 h with 0.2 mg/ml oxLDL showed increased lipid peroxidation that was prevented by a single addition of 0.25 mM ascorbate at the beginning of the incubation. This protection caused a decrease in intracellular ascorbate, but no change in the cell content of GSH. In the absence of ascorbate, oxLDL increased SVCT2 protein and function during 18 h in culture. Although culture of the cells with ascorbate did not affect SVCT2 protein expression, the oxLDL-induced increase in SVCT2 protein expression was prevented by ascorbate. These results suggest that up-regulation of endothelial cell SVCT2 expression and function may help to maintain intracellular ascorbate during oxLDL-induced oxidative stress, and that ascorbate in turn can prevent this effect.
Keywords: oxidative stress, oxidized low density lipoprotein, ascorbic acid transport, SVCT2, endothelial cells
Introduction
Endothelial dysfunction is an early stage of atherosclerosis that is caused in part by damage from oxidized low density lipoprotein (oxLDL) [1, 2]. Treatment of endothelial cells in culture with OxLDL increases cellular reactive oxygen species and lipid peroxides [2–4], with corresponding decreases in low molecular weight antioxidants, such as ascorbate, GSH and α-tocopherol [4–6]. Of the latter group, ascorbate may be especially important, since endothelial cells in culture with physiologic plasma concentrations of ascorbate (50–100μM) can take up ascorbate to intracellular concentrations of 1–3 mM [5, 7]. These are similar to concentrations of GSH, which is considered the major intracellular low molecular weight antioxidant [8]. The sharp gradient of ascorbate across the plasma membrane is generated in most non-epithelial cells by the Sodium-dependent Vitamin C Transporter-2 (SVCT2) [9]. This transporter has a high affinity for ascorbate (apparent Km = 27–84 μM [7, 10]) and its messenger RNA [10] and protein [11] are expressed by endothelial cells.
Despite its importance in supplying intracellular ascorbate to endothelial cells, whether the SVCT2 is regulated by oxidative stress in these cells is unknown. To address this issue, we used pre-oxidized LDL to generate an oxidative stress in EA.hy926 endothelial cells and followed its effects on SVCT2 expression and function. These cells are a hybridoma line derived from human umbilical vein endothelial cells that retain endothelial features, including a cobblestone appearance with formation of capillary-like tubes in culture [12], expression of von Willebrand factor [13], oxidative modification of human LDL [14], and calcium-dependent eNOS activation [14, 15]. Overnight culture of EA.hy926 cells with oxLDL increased lipid peroxidation and decreased intracellular ascorbate, whereas it increased both protein expression and function of the SVCT2. Increases in intracellular ascorbate prevented oxLDL-induced increases in SVCT2 protein expression.
Methods and Materials
Materials
Analytical reagents were purchased from Sigma/Aldrich Chemical Co (St. Louis, MO).
Preparation of LDL-enriched lipoproteins
LDL-enriched lipoproteins were obtained from a human volunteer with familial hypercholesterolemia who was undergoing plasma apheresis every other week using dextran sulfate-cellulose adsorbent column (Liposorber, Kaneka Corp., Osaka, Japan). This resin binds apoprotein B-containing lipoproteins and removes them from plasma, which is then returned to the subject. The apoprotein B-enriched lipoproteins were eluted from the column resin with normal saline. The lipoprotein solution was oxidized immediately by incubation with 5 μM copper sulfate at 23 °C for 24 h. The reaction was terminated with the addition of EDTA to 5 mM. To remove the copper and EDTA, this preparation was dialyzed for 24 hours at 3 °C against 3 changes of phosphate buffered saline (PBS, 12.5 mM sodium phosphate, 140 mM sodium chloride, pH 7.4). It was stored up to 3 weeks before use in an opaque plastic tube at 3 °C. Previous gel electrophoresis showed that this preparation consisted largely of lipoproteins migrating in the beta range, corresponding to LDL [16]. Oxidation with copper as described did not affect electrophoretic migration, but doubled the malondialdehyde content of the preparation [16]. This suggests that although lipids underwent peroxidation, apolipoproteins were not damaged significantly, indicating that this LDL preparation, termed oxLDL, corresponds to minimally oxidized LDL.
Cell Culture
EA.hy926 cells were generously provided by Dr. Cora Edgell (University of North Carolina, Chapel Hill, NC, USA) and cultured in Dulbecco’s minimal essential medium that contained 20 mM D-glucose, 10% (v/v) fetal bovine serum, and HAT media supplement (Sigma/Aldrich Chemical Co., St. Louis, MO). Cells were cultured to confluence over 24–48 h at 37 °C in humidified air containing 5% CO2.
Assays of ascorbate transport, ascorbate, GSH and lipid peroxidation
Ascorbate transport was measured as previously described using cells cultured in 12 well plates [17]. For assay of intracellular ascorbate and GSH, after incubations as indicated, the medium was removed and the 6-well culture plate was cooled on ice. The cells were rinsed twice with 2 ml of ice-cold Krebs-Ringer Hepes buffer (KRH). The latter consisted of 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4). For assay of ascorbate and GSH, cell layer was treated with 0.1 ml of 25% metaphosphoric acid (w/v), followed by 0.35 ml of a buffer containing 0.1 M Na2HPO4 and 0.05 mM EDTA, pH 8.0. Adherent cells were scraped from the plate and the lysate was centrifuged at 3 °C for 1 min at 13,000 ×g. Duplicate aliquots of supernatant were taken for assay of ascorbate [18] and GSH [19]. Intracellular GSH and ascorbate concentrations were calculated using the previously measured intracellular water space of EA.hy926 cells, which was 3.6 ± 1.2 μl/mg protein [15].
Lipid peroxidation in oxLDL and cells was measured as malondialdehyde by HPLC. Adherent cell monolayers in 6-well plates were rinsed 3 times in KRH. After the last rinse, KRH was aspirated and the cells were lysed with 0.5 ml of deionized water, and then treated with 20 μl of 10 mg/ml butylated hydroxytoluene in ethanol. The lysate was dissolved in an equal volume of 10 mg/ml sodium dodecyl sulfate in deionized water and assayed for malondialdehyde by HPLC according to the method of Tebbe, et al. [20], as previously detailed [21].
Detection of SVCT2 protein
SVCT2 protein was quantified by immunoblotting as previously described [16] using a goat anti-rat primary antibody SC-9926 (Santa Cruz Biochemicals, Santa Cruz, CA) at a 1:200 dilution. This antibody also detects the human SVCT2. The secondary antibody was rabbit anti-goat (A8919, Sigma/Aldrich Chemical Co, St. Louis, Mo) used at a 1:5000 dilution.
Data Analysis
The results were calculated as mean ± standard error, except where indicated. Statistical significance was determined by analysis of variance with post-hoc testing using the software program Sigma Stat 2.0 (Jandel Scientific, San Rafael, CA). Significance was based on a p value of < 0.05.
Results
Culture of EA.hy926 cells with 0.2 mg/ml oxLDL for 18 h increased the cell content of malondialdehyde by 80% (Fig. 1A, compare square with circle at zero ascorbate added). This increase in cellular malondialdehyde due to the oxLDL in the cells was completely prevented by a single addition of ascorbate at concentrations of 0.25 mM and higher (Fig. 1A, circles). Malondialdehyde in the cell culture medium (i.e., in the oxLDL) was also decreased almost to baseline when cells were initially incubated with ascorbate concentrations of about 0.25 mM and higher (Fig. 1B). Although increased malondialdehyde measured in both cells and medium is mostly pre-existing malondialdehyde in the oxLDL, the finding that ascorbate decreased malondialdehyde in both cells and medium shows that at least part of the increase in lipid peroxidation was due to further cell-mediated LDL oxidation. Despite the increase in lipid peroxidation, no changes in cellular GSH content were observed by culture with oxLDL, ascorbate, or both (Fig. 1C).
Figure 1.
Oxidative stress induced by oxLDL and protection by ascorbate. EA.hy926 cells were cultured in the presence (circles) or absence (square) of 0.2 mg/ml oxLDL and the indicated ascorbate concentration. After 18 h, aliquots of the medium were removed for assay of malondialdehyde (Panel B) and the cells were rinsed twice in KRH and taken for assay of malondialdehyde (Panel A) and GSH (Panel C). Results are shown from 4 or more experiments, with an “*” indicating p < 0.05 compared to time zero.
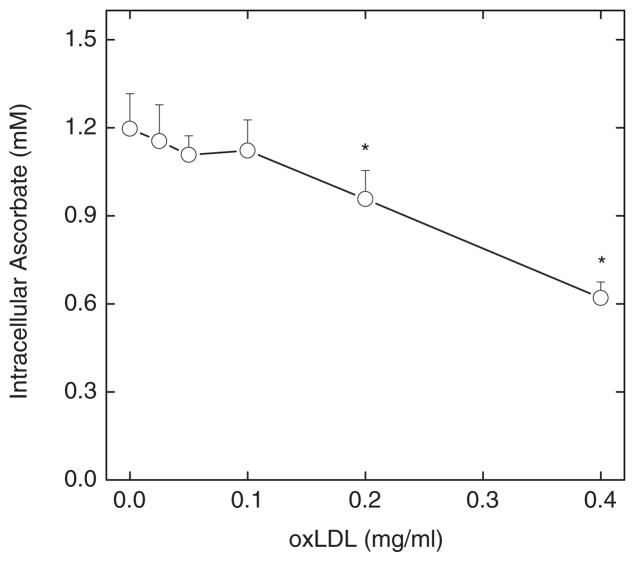
However, culture with oxLDL decreased intracellular ascorbate concentrations, as shown in Fig. 2. EA.hy926 cells that were treated with a single addition of 0.25 mM ascorbate and cultured for 18 h in the absence of oxLDL contained about 1.2 mM ascorbate (Fig. 2, at zero oxLDL). Intracellular ascorbate was decreased by culture with increasing concentrations of oxLDL, although the effect became significant only at oxLDL concentrations of 0.2 mg/ml oxLDL and higher (Fig. 2). These results show that the protection from lipid peroxidation in cells and medium observed in Fig. 1 comes at the expense of decreased ascorbate in the cells, presumably due to its oxidation. It should be noted that extracellular ascorbate after 18 of culture under these conditions was below the level of assay sensitivity (< 0.4 μM).
Figure 2.
OxLDL decreases intracellular ascorbate. Cells were cultured with 0.25 mM ascorbate and the indicated concentration of oxLDL. After 18 h, the cells were rinsed 3 times in KRH and taken for assay of intracellular ascorbate. Results are shown from 5 experiments, with “*” indicating p < 0.05 compared to cells not treated with oxLDL.
The preceding results show that cells cultured for 18 h in the presence of oxLDL are under oxidative stress. To determine whether this might affect ascorbate transport, SVCT2 expression and transport were assessed. SVCT2 protein was evident as a band of about 70 kilodaltons (Fig. 3A) that showed variable baseline expression in that in some assays it was very faint. The intensity of this band was increased after 18 h of culture with increasing concentrations of oxLDL. When the results of 5 experiments were analyzed by densitometry and expressed relative to β-actin in each blot, SVCT2 protein expression was progressively increased, becoming significant at 0.05 mg/ml oxLDL (Fig. 3B, circles). Non-oxidized LDL did not affect SVCT2 protein expression (Fig. 3B, square). This several-fold increase in SVCT2 protein was associated with a doubling in rates of ascorbate transport under the same incubation conditions (Fig. 3C).
Figure 3.
OxLDL increases SVCT2 message and protein. EA.hy926 cells were cultured for 18 h with the indicated concentrations of oxLDL then taken for assay of SVCT2 protein by immunoblotting. Panel A shows a representative immunoblot of the SVCT2, using β-actin as a control for gel loading. Panel B shows the densitometry from 5 assays of SVCT2 protein expression, normalized to β-actin and expressed as mean + SD in standardized units of density (circles). The single square shows data from 3 experiments for the indicated concentration of non-oxidized LDL. Panel C shows oxLDL effects on radiolabeled ascorbate transport. An asterisk (*) indicates p < 0.05 compared to the sample not treated with oxLDL.
Since ascorbate prevented lipid peroxidation due to oxLDL, its effects on SVCT protein expression were evaluated with the results shown in Fig. 4A as a representative immunoblot and in Fig. 4B after quantification by densitometry of the data from 5 experiments. Again, culture for 18 h with 0.2 mg/ml oxLDL increased the intensity of the 70 kilodalton band compared to control. Ascorbate alone had no effect on the baseline SVCT2 protein, but progressively decreased the intensity of the SVCT2 band compared to oxLDL alone (compare 2nd bar to last 3 bars in Fig. 4B). A significant effect was observed at loading ascorbate concentration of 80 μM.
Figure 4.

Ascorbate effects on oxLDL-induced increases SVCT2. EA.hy926 cells were cultured for 18 h with the indicated concentrations of oxLDL and ascorbate, and then taken for assay of SVCT2 expression by immunoblotting. Panel A. shows a representative immunoblot of the SVCT2, with β-actin as a control for gel loading. Panel B shows the densitometry from 5 assays of SVCT2 protein expression, normalized to β-actin and expressed as mean + SD in arbitrary density units. Bars not sharing the same letters are different from one another at p < 0.05.
Discussion
EA.hy926 cells cultured for 18 h with minimally oxidized LDL showed increased lipid peroxidation that was prevented by loading the cells with ascorbate to a concentration of about 1.2 mM, which is likely to be in the physiologic range in vivo [22]. Similar results were observed in previous studies using different types of endothelial cells, different durations of treatment, and different extents of LDL oxidation [5, 21, 23, 24]. Although oxLDL increased oxidative stress in the endothelial cells used in the present studies, this increase was not severe, since intracellular GSH was unaffected. On the other hand, intracellular ascorbate was decreased by oxLDL, showing that it was more sensitive than GSH to oxLDL-induced oxidative stress, an effect we previously observed following 30 min of treatment of bovine artery endothelial cells with ferricyanide [8]. Intracellular GSH is maintained both by synthesis and robust recycling from GSSG by glutathione reductase [25]. GSH in turn can recycle ascorbate from dehydroascorbate, either directly [26] or as an electron donor to thiol transferase enzymes [27]. The oxLDL-induced decrease in ascorbate in the absence of a change in GSH suggests that ascorbate recycling, rather than GSH synthesis and recycling, failed to keep up with the oxidative stress induced by oxLDL in culture. Thus although GSH has been considered the major intracellular redox buffer [28], when the ascorbate concentration is high, it appears to protect GSH from oxidation.
The major new findings of this work are that 1) treatment of EA.hy926 cells with oxLDL in culture up-regulated expression and function of the SVCT2 and that 2) intracellular ascorbate prevented this effect of oxLDL. This extends our previous findings in RAW264.7 macrophages that an oxidative stress due to oxLDL enhances SVCT2 expression and function [16]. SVCT2 mRNA expression is also increased in viable peri-infarct area of brain in rats undergoing to ischemia-reperfusion injury [29], a treatment known to cause increased oxidative stress. An increase in intracellular ascorbate mediated by increased SVCT2 expression may have special implications for ascorbate function in endothelial cells, since in addition to scavenging radical species, ascorbate enhances several important endothelial cell functions. These include serving as a co-factor for dioxygenase enzymes that mediate hydroxylation of collagen and HIF-1α [30], preserving tetrahydrobiopterin to maintain endothelial nitric oxide synthesis [31], preventing apoptosis [32–34], and enhancing endothelial barrier function [35].
Despite the increase in SVCT2 induced by oxLDL, intracellular ascorbate decreased in cells cultured with oxLDL. Oxidation of intracellular ascorbate would have caused a concomitant increase in DHA, which might have contributed to SVCT2 up-regulation [36]. The decrease in cellular ascorbate could have also contributed to up-regulation of the SVCT2 in the presence of oxLDL, as has been observed in liver following in vivo ascorbate depletion in mice unable to synthesize ascorbate [37]. This agrees with our finding that increased SVCT2 expression due to oxLDL was prevented in a dose-dependent manner by intracellular ascorbate. Such ascorbate-induced down-regulation of SVCT2 expression could therefore have contributed to the decrease in intracellular ascorbate observed after culture with oxLDL. Previous studies showed that cell loading with ascorbate alone decreased ascorbate transport in osteoblasts [38] and for SVCT2 expression in cultured macrophages [16]. A similar effect was not observed in the present studies in part because baseline SVCT2 expression was low and variable in EA.hy926 endothelial cells.
In conclusion, endothelial cells cultured in the presence of oxLDL underwent oxidative stress that was decreased by ascorbate and associated with increased expression of the ascorbate transporter SVCT2. Although the molecular mechanism of oxLDL-induced SVCT2 expression remains to be determined, this finding supports the notion that increased ascorbate provided by the SVCT2 is important for endothelial cell survival or function. It also places the SVCT2 in a group of antioxidant proteins including thioredoxin reductase, heme oxygenase-1, cystine transporters and GSH synthetic enzymes that are up-regulated by oxidative stress [24, 39].
Acknowledgments
This work was supported by NIH grant DK050435. Media for cell culture was prepared by the Cell Culture Core of the Vanderbilt Diabetes Research and Training Center (DK20593).
References
- 1.Steinberg D, Parthasarathy S, Carew TE, et al. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrecher UP, Parthasarathy S, Leake DS, et al. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci USA. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinbrecher UP. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988;959:20–30. doi: 10.1016/0005-2760(88)90145-2. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt A, Salvayre R, Delchambre J, et al. Prevention by α-tocopherol and rutin of glutathione and ATP depletion induced by oxidized LDL in cultured endothelial cells. Br J Pharmacol. 1995;116:1985–1990. doi: 10.1111/j.1476-5381.1995.tb16402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A, Frei B. Both intracellular and extracellular vitamin C inhibit atherogenic modification of LDL by human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1583–1590. doi: 10.1161/01.atv.17.8.1583. [DOI] [PubMed] [Google Scholar]
- 6.Diaz MN, Frei B, Vita JA, et al. Mechanisms of disease - Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 7.May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch Biochem Biophys. 2005;434:178–186. doi: 10.1016/j.abb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 8.May JM, Qu ZC, Li X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem Pharmacol. 2001;62:873–881. doi: 10.1016/s0006-2952(01)00736-5. [DOI] [PubMed] [Google Scholar]
- 9.Tsukaguchi H, Tokui T, Mackenzie B, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 10.Best KA, Holmes ME, Samson SE, et al. Ascorbate uptake in pig coronary artery endothelial cells. Mol Cell Biochem. 2005;271:43–49. doi: 10.1007/s11010-005-3442-0. [DOI] [PubMed] [Google Scholar]
- 11.Qiao H, Li L, Qu ZC, et al. Cobalt-induced oxidant stress in cultured endothelial cells: Prevention by ascorbate in relation to HIF-1alpha. Biofactors. 2009;35:306–313. doi: 10.1002/biof.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer J, Margolis M, Schreiner C, et al. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992;153:437–449. doi: 10.1002/jcp.1041530302. [DOI] [PubMed] [Google Scholar]
- 13.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pech-Amsellem MA, Myara I, Pico I, et al. Oxidative modifications of low-density lipoproteins (LDL) by the human endothelial cell line EA.hy 926. Experientia. 1996;52:234–238. doi: 10.1007/BF01920713. [DOI] [PubMed] [Google Scholar]
- 15.Jones W, Li X, Perriott LM, et al. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 16.Chi X, May JM. Oxidized lipoprotein induces the macrophage ascorbate transporter (SVCT2): protection by intracellular ascorbate against oxidant stress and apoptosis. Arch Biochem Biophys. 2009;485:174–182. doi: 10.1016/j.abb.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May JM, Qu ZC. Redox regulation of ascorbic acid transport: Role of transporter and intracellular sulfhydryls. Biofactors. 2004;20:199–211. [Google Scholar]
- 18.Mendiratta S, Qu Z-C, May JM. Erythrocyte ascorbate recycling: Antioxidant effects in blood. Free Radic Biol Med. 1998;24:789–797. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- 19.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 20.Tebbe B, Wu SL, Geilen CC, et al. L-ascorbic acid inhibits UVA-induced lipid peroxidation and secretion of IL-1α and IL-6 in cultured human keratinocytes in vitro. J Invest Dermatol. 1997;108:302–306. doi: 10.1111/1523-1747.ep12286468. [DOI] [PubMed] [Google Scholar]
- 21.Sabharwal AK, May JM. alpha-Lipoic acid and ascorbate prevent LDL oxidation and oxidant stress in endothelial cells. Mol Cell Biochem. 2008;309:125–132. doi: 10.1007/s11010-007-9650-z. [DOI] [PubMed] [Google Scholar]
- 22.May JM, Qu ZC. Ascorbic acid efflux and re-uptake in endothelial cells: maintenance of intracellular ascorbate. Mol Cell Biochem. 2009;325:79–88. doi: 10.1007/s11010-008-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negre-Salvayre A, Mabile L, Delchambre J, et al. α-Tocopherol, ascorbic acid, and rutin inhibit synergistically the copper-promoted LDL oxidation and the cytotoxicity of oxidized LDL to cultured endothelial cells. Biol Trace Elem Res. 1995;47:81–91. doi: 10.1007/BF02790104. [DOI] [PubMed] [Google Scholar]
- 24.Siow RC, Sato H, Leake DS, et al. Induction of antioxidant stress proteins in vascular endothelial and smooth muscle cells: protective action of vitamin C against atherogenic lipoproteins. Free Radic Res. 1999;31:309–318. doi: 10.1080/10715769900300871. [DOI] [PubMed] [Google Scholar]
- 25.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 26.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 27.Wells WW, Xu DP, Washburn MP. Glutathione: Dehydroascorbate oxidoreductases. Methods Enzymol. 1995;252:30–38. doi: 10.1016/0076-6879(95)52006-6. [DOI] [PubMed] [Google Scholar]
- 28.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 29.Berger UV, Lu XC, Liu W, et al. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem. 2003;86:896–906. doi: 10.1046/j.1471-4159.2003.01891.x. [DOI] [PubMed] [Google Scholar]
- 30.De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci. 2004;61:209–219. doi: 10.1007/s00018-003-3203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heller R, Unbehaun A, Schellenberg B, et al. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 32.Dhar-Mascareño M, Cárcamo JM, Golde DW. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic Biol Med. 2005;38:1311–1322. doi: 10.1016/j.freeradbiomed.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Haendeler J, Zeiher AM, Dimmeler S. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur J Pharmacol. 1996;317:407–411. doi: 10.1016/s0014-2999(96)00759-5. [DOI] [PubMed] [Google Scholar]
- 34.Rössig L, Hoffmann J, Hugel B, et al. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation. 2001;104:2182–2187. doi: 10.1161/hc4301.098284. [DOI] [PubMed] [Google Scholar]
- 35.May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol. 2009;297:C169–C178. doi: 10.1152/ajpcell.00674.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stait SE, Leake DS. The effects of ascorbate and dehydroascorbate on the oxidation of low-density lipoprotein. Biochem J. 1996;320:373–381. doi: 10.1042/bj3200373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano A, Aigaki T, Maruyama N, et al. Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch Biochem Biophys. 2010;496:38–44. doi: 10.1016/j.abb.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Dixon SJ, Wilson JX. Adaptive regulation of ascorbate transport in osteoblastic cells. J Bone Miner Res. 1992;7:675–681. doi: 10.1002/jbmr.5650070612. [DOI] [PubMed] [Google Scholar]
- 39.Mostert V, Hill KE, Burk RF. Loss of activity of the selenoenzyme thioredoxin reductase causes induction of hepatic heme oxygenase-1. FEBS Lett. 2003;541:85–88. doi: 10.1016/s0014-5793(03)00309-0. [DOI] [PubMed] [Google Scholar]