Abstract
The role of estrogen receptor alpha (ERα) in breast cancer has been studied extensively, and its protein expression is prognostic and a primary determinant of endocrine sensitivity. However, much less is known about the role of ERβ and its relevance remains unclear due to the publication of conflicting reports. Here, we provide evidence that much of this controversy may be explained by variability in antibody sensitivity and specificity and describe the development, characterization, and potential applications of a novel monoclonal antibody targeting full-length human ERβ and its splice variant forms. Specifically, we demonstrate that a number of commercially available ERβ antibodies are insensitive for ERβ and exhibit significant cross-reaction with ERα. However, our newly developed MC10 ERβ antibody is shown to be highly specific and sensitive for detection of full-length ERβ and its variant forms. Strong and variable staining patterns for endogenous levels of ERβ protein were detected in normal human tissues and breast tumors using the MC10 antibody. Importantly, ERβ was shown to be expressed in a limited cohort of both ERα positive and ERα negative breast tumors. Taken together, these data demonstrate that the use of poorly validated ERβ antibodies is likely to explain much of the controversy in the field with regard to the biological relevance of ERβ in breast cancer. The use of the MC10 antibody, in combination with highly specific antibodies targeting only full-length ERα, is likely to provide additional discriminatory features in breast cancers that may be useful in predicting response to therapy.
Keywords: ESTROGEN RECEPTOR, ESTROGEN RECEPTOR BETA, BREAST CANCER, ANTIBODY
It is estimated that in 2011 over 230,000 women will be diagnosed with breast cancer in the United States alone [Siegel et al., 2011] with approximately 70% of these cases being classified as estrogen receptor (ER) positive breast tumors as defined by the expression of ER alpha (ERα) protein. For three decades, tamoxifen has been the most important therapeutic agent in the treatment of women with endocrine sensitive breast cancer since it effectively inhibits the proliferation inducing effects of 17α-estradiol (estrogen) in tumor cells. However, the use of ERα alone as an indicator of responsiveness to anti-estrogens is far from perfect as about 30% of ERα positive tumors do not respond to tamoxifen therapy [Osborne, 1998]. These observations have suggested that other estrogen receptors may be involved in mediating the responsiveness of endocrine sensitive tumors to hormonal agents. Following the discovery of a second estrogen receptor, ERα, in 1996 [Mosselman et al., 1996] many investigators began to explore the possible roles of this protein in mediating breast cancer development, progression, and response to therapy.
Like ERα, ERβ is a member of the nuclear receptor superfamily of proteins which functions as a ligand-mediated transcription factor [Mosselman et al., 1996]. The human gene for ERβ (ESR2) is comprised of eight exons which encode a 530-amino acid protein that is similar in structure to its closely related family member, ERα, as well as that of other nuclear hormone receptors. As with ERα, it contains five distinct protein domains designated as A/B, C, D, E, and F (Fig. 1). The A/B domain, located at the N-terminal end of the protein, contains an activation function (AF1) which has been shown to exhibit ligand independent activity [Tora et al., 1989]. The C domain contains a highly conserved DNA binding domain and is also involved with receptor dimerization. The D domain functions as a hinge region and is thought to contain a nuclear localization signal [Picard et al., 1990]. The ligand-binding domain lies within the E domain and contains another activation function referred to as AF2 [Tora et al., 1989]. At present, the functions of the F domain, located at the C-terminus, are not known.
Fig. 1.
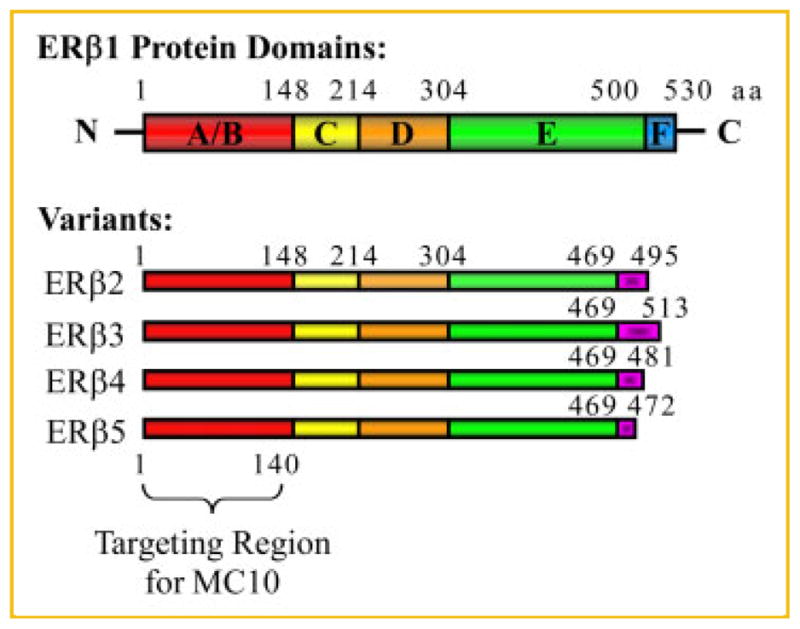
Diagram depicting the domain structures of human full-length ERα1 and its variant forms (ERα2–5) as well as the targeting region for the MC10 monoclonal ERβ antibody.
In addition to this “full-length” receptor (ERα1), the ERβ gene also encodes an additional four variants designated as ERα2, ERα3, ERα4, and ERα5 (Fig. 1). These variants are identical to that of ERα1 from amino acids 1–469. Amino acids 470–530, encoding the C-terminal portion of the E domain and the entire F domain of ERα1, are deleted in ERα2–5. However, each variant contains a unique C-terminal amino acid sequence which varies in length and results from alternative splicing of exon 8 [Moore et al., 1998; Lewandowski et al., 2002; Poola et al., 2005a] (Fig. 1).
Since the discovery of ERβ [Mosselman et al., 1996], its role in the development, progression, and treatment of breast cancer has been hotly debated, and to date, no real consensus regarding its clinical utility has been established. Potential explanations include the lack of standardized methodologies for detecting expression of ERα, the use of poorly validated antibodies, the presence of highly conserved variants whose functions remain unresolved and/or the inconsistent interpretation of what constitutes ERβ positivity. The first studies aimed at addressing the role of ERβ in breast cancer were conducted using mRNA-based assays [Leygue et al., 1998; Leygue et al., 1999; Speirs et al., 1999a; Speirs et al., 1999b; Iwao et al., 2000; Shaw et al., 2002; Park et al., 2003; Zhao et al., 2003]. However, it has been reported that mRNA levels of ERβ do not correlate with its protein levels in human tumors [Shaw et al., 2002; Balfe et al., 2004; O’Neill et al., 2004]. More recently, a significant number of immunohistochemical based studies have been performed using paraffin embedded breast tumor samples [Jarvinen et al., 2000; Jensen et al., 2001; Mann et al., 2001; Miyoshi et al., 2001; Omoto et al., 2001; Roger et al., 2001; Skliris et al., 2001, 2003, 2006; Murphy et al., 2002; Saunders et al., 2002; Fuqua et al., 2003; Iwase et al., 2003; Shaaban et al., 2003, 2008; Esslimani-Sahla et al., 2004; Fleming et al., 2004; Hopp et al., 2004; Myers et al., 2004; Nakopoulou et al., 2004; O’Neill et al., 2004; Poola et al., 2005b; Miller et al., 2006; Umekita et al., 2006; Gruvberger-Saal et al., 2007; Sugiura et al., 2007; Honma et al., 2008; Novelli et al., 2008; Motomura et al., 2010]. Unfortunately, the techniques for tissue preparation and processing, the antibodies employed, and the scoring systems used to determine ERβ positivity are highly variable making it extremely difficult to compare the results and draw specific conclusions as to the relevance of this protein in breast cancer. Complicating the matter even more is the fact that these studies differ significantly with regard to their patient populations, the number of samples utilized, menopausal status, ethnicity, types of therapies, and consideration of other important biomarkers such as ERα, progesterone receptor, and Her2 as well as patient follow-up times.
These realities highlight the need to develop more reliable and consistent strategies to detect ERβ expression which will ultimately enable scientists to further define the relevance of this protein in breast cancer progression and treatment. As a start, we have utilized multiple highly controlled cell model systems and technical approaches to confirm that a number of the commercially available ERβ antibodies are non-specific and insensitive for detection of this protein. Even more troubling is the fact that some of them actually cross-react with ERα and therefore lead to erroneous conclusions during data analysis. To address these issues, we have now developed and characterized a novel, highly specific and sensitive, monoclonal ERβ antibody (MC10) which detects all forms of ERα. We have also identified a highly specific commercially available antibody which recognizes only ERα1 (PPG5/10). We hypothesize that the use of both of these antibodies in parallel will allow for a more accurate and complete characterization of the expression of ERβ in human breast cancer biopsies and will further our ability to elucidate the potential roles of this protein, and its variants, in breast cancer. Identification of highly specific ERβ antibodies, and thorough characterization of such antibodies, is an essential first step in our quest to eventually use ERβ as a predictive and/or prognostic biomarker in breast cancer.
MATERIALS AND METHODS
CELL CULTURE
293T cells and parental U2OS osteosarcoma cells were purchased from American Type Culture Collection and grown in phenol red-free Dulbecco’s modified Eagle’s medium/F12 medium (DMEM/F12) (Sigma-Aldrich, St. Louis, MO) containing 10% Fetal Bovine Serum (FBS) (ISC Bioexpress, Kaysville, UT) and 1% antibiotic/antimycotic (AA) (Invitrogen, Carlsbad, CA). Doxycycline inducible U2OS-ERα, and -ERβ cell lines were originally developed in our laboratory as described previously [Monroe et al., 2003]. U2OS-ERα and ERβ cells were routinely maintained in phenol red-free DMEM/F12 containing 10% FBS, 1% AA, 5 mg/L blasticidin S (Roche Applied Science, Indianapolis, IN), and 500 mg/L zeocin (Invitrogen).
EXPRESSION AND PURIFICATION OF THE ERβ FUSION PROTEIN FOR ANTIBODY GENERATION
The 5′-region of the human ERβ gene encoding amino acids 1–140 was PCR amplified and cloned into the pGEX-5X-3 vector (GE Healthcare, Piscataway, NJ) using BamH1 and XhoI restriction enzyme sites. The ERα-GST construct was sequenced to ensure nucleotide integrity and the fusion protein was expressed in DH5α Escherichia coli cells and purified using a glutathione affinity column (Qiagen, Valencia, CA) as specified by the manufacturer. Purified ERα-GST fusion protein was dialyzed three times in 1×PBS and subsequently used for antibody development.
DEVELOPMENT OF ERβ MONOCLONAL ANTIBODIES
Balb/c mice were immunized with the purified ERβ-GST fusion protein (1–140 aa) and spleen cells were subsequently isolated and fused to HAT sensitive myeloma F/O cells to form hybridomas. Immunizations, cell fusions, and production of individual hybridoma clones were performed by the Mayo Clinic Antibody Core Facility. Fused hybridoma cells were plated as a single cell suspension into 96 well plates for further propagation and characterization. Individual clones were first screened for GST or ERβ antibody production by Enzyme-Linked Immunosorbent Assay (ELISA) analysis using both GST and ERβ protein as antigen. ERβ positive and GST negative clones were further screened by western blotting for detection of ERβ protein. All ELISA- and western-positive clones were finally confirmed for ERβ antibody production via immunofluorescence staining using U2OS-ERβ expressing cells. ERβ positive clones were then again sub-cloned and rescreened as above. This screening method led to the identification of two highly positive clones which were designated as Mayo Clinic (MC) 9 and 10. For large scale production of the ERβ monoclonal antibody, MC10 hybridoma cells were sent to Cocalico Biologicals Inc (Reamstown, PA), injected into the peritoneal cavity of Balb/C mice and allowed to proliferate at which time ascites fluid was collected. Following collection, the ascites fluid was centrifuged and the supernatant was used as the source of the MC10 ERβ monoclonal antibody described in this manuscript.
WESTERN BLOT AND IMMUNOPRECIPITATION ANALYSIS
293T cells were transfected with Flag-tagged ERα or ERβ pcDNA4/TO expression constructs (Invitrogen). Cells were harvested following two days of transfection and lysed in NETN buffer (150 mM NaCl, 1 mM EDTA, 20 mM Tris [pH 8.0], 0.5% Nonidet P-40). Cell lysates were centrifuged and supernatants were immunoprecipitated using 1 μg of either Flag (M2) (Sigma-Aldrich), ERα (H-20) (Santa Cruz, Santa Cruz, CA), ERβ (MC9 and MC10), or mouse IgG (Beckman Coulter, Brea, CA) antibodies. Whole cell lysates and immunocomplexes were separated by SDS-PAGE, transferred to PVDF membranes, probed with primary [Flag, ERα, and ERβ (MC9 and MC10)] and secondary antibodies and visualized using enhanced chemiluminescence (GE Healthcare).
PREPARATION OF CELL PELLETS FOR IMMUNOHISTOCHEMISTRY AND IMMUNOFLUORESCENCE
U2OS cells stably expressing Flag-tagged ERα or ERβ, under the control of a doxycycline promoter, were expanded in a total of 10 T150 tissue culture flasks. All U2OS cell lines were cultured in both the presence and absence of doxycycline to generate ER+ and ER− preparations respectively. At approximately 80% confluence, growth medium was removed, cell monolayers were washed twice with 1×PBS and fixed in 10% neutral buffered formalin for 5 min. Following fixation, cells were scraped from each flask, pooled and incubated in formalin at 4°C for a minimum of 12 h. Pooled cells were pelleted, processed, and paraffin embedded prior to use for immunohistochemistry and immunofluorescent staining. The resulting paraffin blocks were sectioned directly and also incorporated into tissue microarrays (TMAs) for use as positive and negative controls on a single slide for testing the specificity of antibodies for immunofluorescence and immunohistochemistry.
IMMUNOHISTOCHEMICAL AND IMMUNOFLUORESCENCE STAINING
Numerous staining protocols were tested using the following ERβ antibodies on sections of the formalin-fixed, paraffin embedded, cell pellets described above: Calbiochem GR40, Santa Cruz sc-6820, Chemicon AB1410, Thermo MA1-81281 (PPG5/10), and Mayo Clinic MC10. EDTA pH8.1 and Borg pH 9.5 antigen retrievals were tested on all antibodies. In addition, a citrate-based retrieval solution, low-pH Target Retrieval Solution (Dako, Carpentaria, CA) was used for the Chemicon, MC10, and PPG5/10 antibodies. Antibodies were tested in dilutions ranging from 1:50 to 1:400, with most commercial antibodies providing the best staining results at 1:100. For those antibodies with weak staining at the standard incubation time of 1 h, incubation times were increased to overnight. Envision Dual+ Link HRP (Dako) was the secondary label used for all antibodies in the initial evaluations. Staining was visualized by both immunofluorescence and immunohistochemistry for all antibodies. For immunohistochemistry, secondary antibodies were used as described in more detail below. Slides were digitized using an Olympus NDP brightfield system. For immunofluorescence staining, Alexa Fluor 568 secondary antibodies (Invitrogen) were used to visualize the primary antibody staining. Hoechst 33342 was used to stain nuclear DNA. Slides were viewed and images captured using a Zeiss 510 confocal microscope. In addition to immunofluorescence and immunohistochemistry for ERβ, sections were also stained with antibodies against Flag (M2, Sigma-Aldrich) and ER-alpha (clone ID5, M7047, Dako) as controls.
Antibodies which showed specificity for ERβ, i.e., exhibited staining in ERβ expressing cells with no staining in ERα expressing cells or in cells expressing neither ERα or ERβ, were selected for further refinement of staining protocols. The protocols developed in these studies were designed specifically for detection of ERβ in formalin-fixed, paraffin embedded human tissues used for standard clinical diagnostic purposes. Ultimately, protocols were finalized for only the PPG5/10 and MC10 ERβ antibodies since these were the only two ERβ antibodies which were highly specific and sensitive for detection of this protein. Sections obtained from formalin-fixed, paraffin embedded human tissue were deparaffinized in xylene, washed in decreasing concentrations of ethanol, and rehydrated in distilled water. Antigen retrieval was performed by placing slides in a preheated citrate-based solution (low-pH Target Retrieval Solution (Dako)) in a streamer at 98°C for 40 min. The staining procedure was carried out in a Dako Autostainer Plus as follows. Tissue sections were treated with Peroxidase Blocking Reagent (Dako) for 5 min, washed with 1×Wash Buffer (Dako), and treated with Background Sniper (Biocare Medical, Concord, CA) for 10 min. The primary antibodies for ERβ were diluted in Background Reducing Antibody Dilutent (Dako) and incubated for 60 min at room temperature. The PPG5/10 antibody was used at 1:100 and MC10 was used at 1:300. After washing once in Wash Buffer, sections were incubated in a mouse MACH3-HRP two-step system (Biocare Medical) for 20 min each. Slides were washed twice with 1×Wash Buffer between steps and before applying Betazoid DAB (Biocare Medical) for 5 min for colorimetric visualization. Counterstaining with hematoxylin and eosin, followed by dehydration in increasing concentrations of ethyl alcohol and xylene, was performed prior to cover slipping. These studies were approved by the Mayo Clinic Institutional Review Board and were conducted under the following protocol: IRB #08-008233.
SCORING OF ERβ PROTEIN EXPRESSION IN HUMAN TISSUES
Expression of ERβ was scored manually by a dedicated breast pathologist (CR) for proportion and intensity of nuclear staining and the intensity of cytoplasmic staining. The proportion score represents the estimated percentage of positive cells with nuclear staining. No staining, or less than 1%, was considered negative. Intensity staining was assigned none, weak, moderate, or strong for both nuclear and cytoplasmic staining.
CONSTRUCTION OF ERβ VARIANT EXPRESSION VECTORS
Our laboratory previously cloned Flag-tagged ERβ1 and ERβ2 into the pcDNA4/TO expression vector (Invitrogen) as described [Monroe et al., 2003; Secreto et al., 2007]. Flag-tagged ERβ variants 3–5 were generated using a PCR based approach and primers containing the unique C-terminal sequences for each variant. PCR amplified fragments were sub-cloned into the pcDNA4/TO expression vector. DNA sequencing was performed to ensure proper orientation and sequence integrity.
TRANSIENT TRANSFECTION AND CONFOCAL MICROSCOPY
For detection of ERβ1 and its variants by western blotting, 293T cells were plated in 10 cm dishes, grown to approximately 70% confluence and transiently transfected with 10 μg of individual ERβ expression constructs using FuGENE®6 (Roche Diagnostics, Indianapolis, IN). Twenty-four hours post-transfection, cells were lysed in NETN buffer and western blot analysis was performed as described above. For determination of the sub-cellular localization of ERβ variants, parental U2OS cells were grown on cover slips to approximately 30% confluence and subsequently transfected with 250 ng of individual ERβ expression constructs using FuGENE®6 (Roche Diagnostics) for 24 h. Cells were subsequently fixed with methanol for 1 h and permeabilized with 0.1% Triton X-100 for 5 min on ice. Slides were pre-incubated in 5% goat serum for 1 h to block non-specific binding sites and subsequently incubated with a 1:50 dilution of either Flag, MC10, or PPG5/10 antibodies for another hour. Slides were washed with 1×PBS and incubated with a FITC-conjugated anti-mouse secondary antibody (Jackson ImmunoResearch, United Kingdom) for 30 min at room temperature. DAPI was used as a counter-stain for nuclei (Invitrogen). Immunofluorescent detection was conducted using a Zeiss Laser Scanning Microscope 510.
RESULTS
CHARACTERIZATION OF NEWLY DEVELOPED MONOCLONAL ERβ ANTIBODIES
In order to determine the specificity of our newly developed monoclonal antibodies for ERβ, 293T cells were transiently transfected with human Flag-tagged ERα or ERβ expression constructs. Twenty-four hours post-transfection, cells were lysed and immunoprecipitations were performed using an ERα specific antibody, our MC9 and MC10 ERβ antibodies, a Flag antibody or an IgG control. Western blotting was then performed using these same antibodies. As shown in Figure 2A, the MC9 and MC10 antibodies did not immunoprecipitate ERα protein, nor did they detect ERα expression in whole cell extracts. However, these two antibodies were able to specifically immunoprecipitate and detect significant levels of ERβ protein in whole cell extracts of cells expressing ERβ similar to that of the Flag antibody (Fig. 2B). While both MC9 and MC10 were equally sensitive for detection of ERβ via immunoprecipitation and western blotting, the MC10 antibody was chosen for use in all of the experiments described below. These data demonstrate that the newly developed ERβ monoclonal antibodies are highly specific for detection of ERβ without cross-reaction to ERα or other non-specific proteins.
Fig. 2.
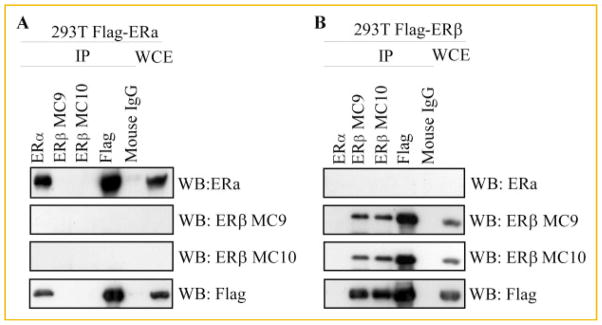
Specificity of MC9 and MC10 monoclonal antibodies for ERβ protein as determined by immunoprecipitation and western blotting. 293T cells were transfected with either a Flag-tagged ERα (A) or Flag-tagged ERβ (B) expression vector for 24 h and equal amounts of cell lysates were immunoprecipitated with indicated antibodies. Immunoprecipitated proteins (IP) were separated by SDS-PAGE and western blotting (WB) was performed using indicated antibodies. Detection of non-immunoprecipitated ERα or ERβ protein levels were also determined by western blotting in whole cell extracts (WCE).
COMPARISON OF MC10 TO COMMERCIALLY AVAILABLE ERβ ANTIBODIES VIA IMMUNOHISTOCHEMISTRY
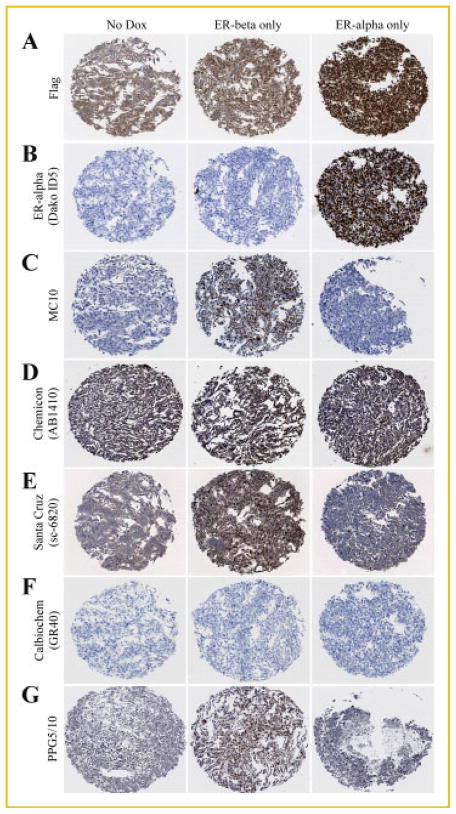
We hypothesized that much of the controversy with regard to the role of ERβ in mediating breast cancer progression and therapeutic responses was largely due to the lack of highly specific antibodies to detect the protein levels of this receptor in human tissues. Therefore, we proceeded to compare the ability of the MC10 antibody to specifically detect ERβ protein via immunohistochemistry, with that of other commercially available antibodies targeting ERβ. In order to accomplish this goal, it was essential to first utilize highly controlled cell model systems which were known to be completely ER negative or to specifically express ERα or ERβ. Therefore, we utilized our U2OS cell models which express Flag-tagged versions of these receptors under the control of a doxycycline inducible promoter allowing us to turn on or turn off their expression [Monroe et al., 2003]. U2OS cells were cultured in the presence or absence of doxycycline, collected, paraffin embedded, and processed for immunohistochemistry. As a positive control, sections of these cell pellets were first stained with a Flag-specific antibody. As shown in Figure 3A, some non-nuclear background staining was observed in non-doxycycline induced U2OS cells. However, intense nuclear staining was observed in the doxycycline induced U2OS-ERα and -ERβ cell lines confirming the expression of the ERs in these model systems (Fig. 3A). We next examined the staining patterns in sections of these cell pellets using the ERα specific antibody purchased from Dako (ID5) which is utilized at the Mayo Clinic for determination of hormone sensitivity of breast cancer patients. As expected, this antibody was highly specific for detection of ERα with no cross-reaction to ERβ (Fig. 3B). Our newly developed MC10 monoclonal antibody exhibited strong nuclear staining only in cells expressing ERβ with no cross-reaction to ERα and no background staining in the absence of doxycycline (Fig. 3C). In contrast, the ERβ specific antibody purchased from Chemicon (AB1410) exhibited extremely high levels of nuclear and cytoplasmic staining in all cell pellets (Fig. 3D). The ERβ antibody purchased from Santa Cruz (sc-6820) revealed strong reactivity in the ERβ expressing cells, however, appreciable levels of background staining, both cytoplasmic and nuclear, were also observed in ERα expressing cells and non-doxycycline induced U2OS cells (Fig. 3E). The antibody purchased from Calbiochem (GR40) was unable to detect expression of ERβ (Fig. 3F). In contrast, the ERβ specific monoclonal antibody (PPG5/10), which can be purchased from Thermo Scientific, Serotec, GeneTex and Dako, exhibited strong nuclear staining in ERβ expressing cells with slight background staining observed in the non-doxycycline induced and ERα expressing cell pellets (Fig. 3G). These studies further demonstrate that our newly developed MC10 antibody, unlike that of many of the commercially available antibodies, is able to specifically detect ERβ protein via immunohistochemistry without cross-reaction to ERα or other non-specific proteins.
Fig. 3.
Specificity and comparison of the MC10 monoclonal antibody for ERβ protein to that of other commercial antibodies as determined by immunohistochemistry. U2OS cells expressing either Flag-tagged ERβ or ERα under the control of a doxycycline inducible promoter were pelleted, paraffin embedded, sectioned, and processed for immunohistochemistry. Sections of un-induced cells, ERβ expressing cells and ERα expressing cells were stained with a monoclonal Flag antibody (1:100) (A), an ERα antibody (Dako ID5, 1: 50) (B), or the following ERβ antibodies: MC10 (1:300) (C), Chemicon (AB1410, 1:400) (D), Santa Cruz (sc-6820, 1:100) (E), Calbiochem (GR40, 1:100) (F), and PPG5/10 (1:100) (G).
COMPARISON OF MC10 TO COMMERCIALLY AVAILABLE ERβ ANTIBODIES VIA IMMUNOFLUORESCENCE
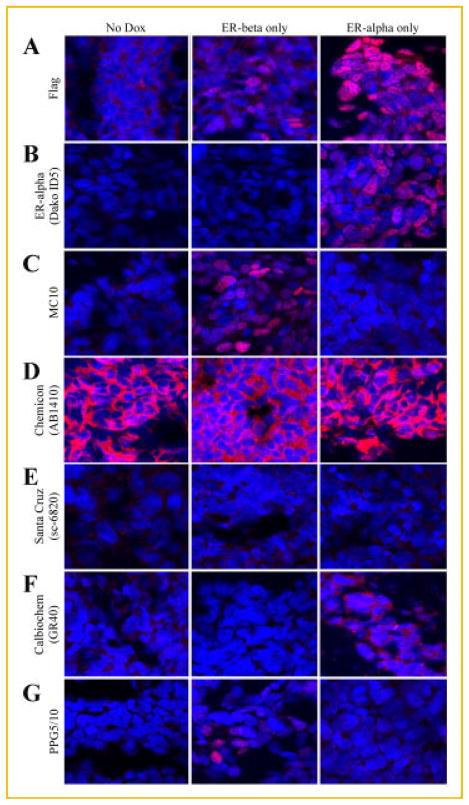
The specificity of the MC10 and commercially available antibodies for their targeted proteins were further examined using immunofluorescence detection in the U2OS cell models. As expected, nuclear staining was observed in ERα and ERβ expressing U2OS cells using the Flag antibody with little to no background in the absence of doxycycline (Fig. 4A). As with the results observed in Figure 3B, the ERα specific antibody (Dako-ID5) was highly selective for expression of this protein (Fig. 4B). The MC10 antibody exhibited strong nuclear staining only in ERβ expressing cells and exhibited no cross-reactivity with ERα or other non-specific proteins (Fig. 4C). The antibody purchased from Chemicon (AB1410) exhibited strong cytoplasmic staining in ERα and ERβ expressing cells as well as in the non-doxycycline induced U2OS cells (Fig. 4D). Unlike that observed using immunohistochemistry in Figure 3E, the Santa Cruz antibody (sc-6820) did not result in any appreciable levels of staining as detected by immunofluorescence (Fig. 4E). The antibody purchased from Calbiochem (GR40) was unable to detect ERβ expression, but did result in weak cytoplasmic staining in ERα expressing cells (Fig. 4F). The PPG5/10 antibody again detected ERβ protein expression with no cross-reaction to ERα (Fig. 4G). These studies further confirm the sensitivity and selectivity of the MC10 antibody for the detection of ERβ using immunofluorescence.
Fig. 4.
Specificity and comparison of the MC10 monoclonal antibody for ERβ protein to that of other commercial antibodies as determined by immunofluorescence. U2OS cells expressing either Flag-tagged ERβ or ERα under the control of a doxycycline inducible promoter were pelleted, paraffin embedded, sectioned, and processed for immunofluorescence. Sections of un-induced cells, ERβ expressing cells and ERα expressing cells were stained with a monoclonal Flag antibody (1:100) (A), an ERα antibody (Dako ID5, 1: 50) (B), or the following ERβ antibodies: MC10 (1:300) (C), Chemicon (AB1410, 1:400) (D), Santa Cruz (sc-6820, 1:100) (E), Calbiochem (GR40, 1:100) (F), and PPG5/10 (1:100) (G).
DETECTION OF ERβ PROTEIN IN HUMAN TISSUE SAMPLES USING MC10 AND PPG5/10 ANTIBODIES
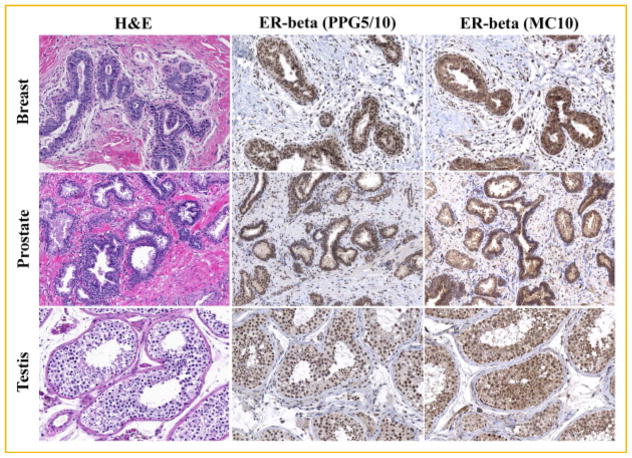
The above studies revealed that the MC10 and PPG5/10 ERβ antibodies were specific for detection of ERβ protein. However, the cell model systems utilized in these experiments over-expressed ERβ1 and were therefore not necessarily representative of endogenous receptor levels in human tissues. Additionally, these cell model systems do not account for the potential presence of other ERβ variants. To address this issue, we next examined the ability of these two antibodies to detect ERβ via immunohistochemistry in human tissues known to express this protein. As shown in Figure 5, substantial staining was observed using both antibodies in normal human breast, prostate, and testis tissue. However, the staining patterns were not identical between these two antibodies. More specifically, substantial cytoplasmic staining was observed using the MC10 antibody while the large majority of staining using the PPG5/10 antibody was localized to the nuclei (Fig. 5). The pathological reports regarding the staining of these tissues for both the PPG5/10 and MC10 antibodies are summarized in Table I.
Fig. 5.
Detection of ERβ protein in normal human tissues using the MC10 and PPG5/10 monoclonal antibodies. Serial sections of normal human breast, prostate, and testis tissue were processed for immunohistochemistry and stained with the PPG5/10 (1:100) and MC10 (1:300) antibodies. One section was also stained with hematoxylin and eosin (H&E) for histological purposes.
TABLE I.
Pathological Scoring for ERβ in Normal Human Breast, Prostate, and Testis Tissue Sections as Detected by the PPG5/10 and MC10 ERβ Monoclonal Antibodies.
| Tissue type | PPG5/10
|
MC10
|
||
|---|---|---|---|---|
| Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | |
| Breast | >95%; Strong | Weak | <1% | Moderate |
| Prostate | 60–75%; Strong | Weak | <1% | Weak to Moderate |
| Testis | 80–90%; Strong | Weak | 50–60%; Strong | Moderate |
DETECTION OF ERβ VARIANTS USING THE MC10 ANTIBODY
In order to confirm that the MC10 antibody cross-reacts with all variant forms of ERβ, pcDNA4/TO expression vectors for ERβ1-5 were transiently expressed individually in 293T cells. Twenty-four hours post transfection, whole cell lysates were prepared and western blotting was performed using the MC10 and PPG5/10 antibodies. As expected, the MC10 antibody was shown to cross-react with all ERβ variants while the PPG5/10 antibody was shown to be specific for detection of only ERβ1 (Fig. 6).
Fig. 6.
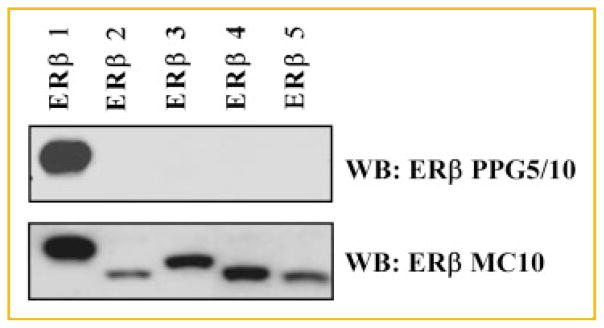
Detection of full-length ERβ1 and its variants (ERβ2-5) by the MC10 and PPG5/10 monoclonal antibodies. 293T cells were transiently transfected with expression vectors for ERβ1–5 for 24 h. Equal amounts of whole cell lysates were separated by SDS-PAGE and western blotting (WB) was performed using the PPG5/10 and MC10 antibodies. PPG5/10 was shown to only detect ERβ1 while the MC10 antibody was shown to cross-react with ERβ1-5.
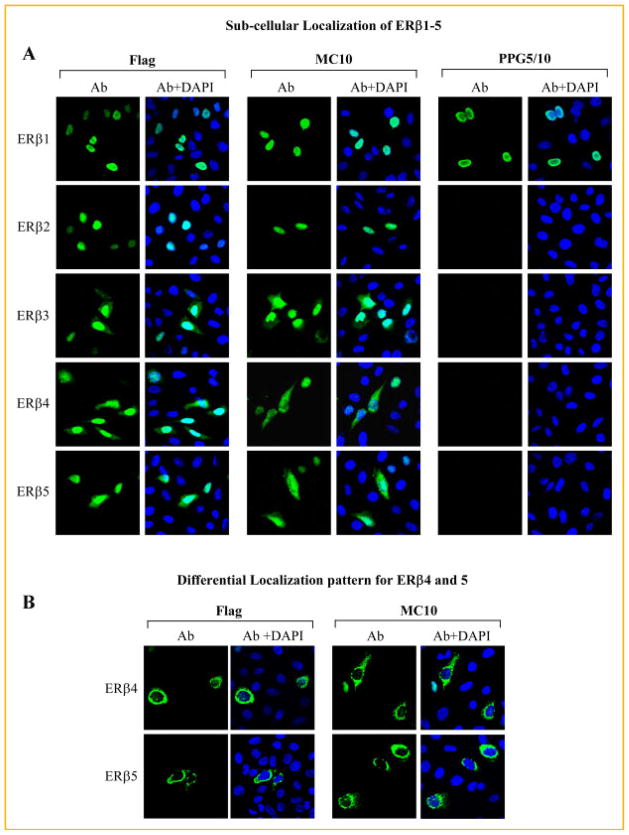
SUB-CELLULAR LOCALIZATION OF ERβ VARIANTS
Since appreciable levels of cytoplasmic staining were observed in human tissue samples stained with the MC10 antibody (Fig. 5), and since this antibody cross-reacts with all ERβ variants, we proceeded to determine and compare the sub-cellular localization of ERβ1–5. Along these lines, parental (ER negative) U2OS cells were plated on cover slips and transiently transfected with Flag-tagged ERβ1–5. Localization of these proteins was first analyzed by immunofluorescent confocal microscopy using a Flag-specific antibody. As shown in Figure 7A, ERβ1 was completely localized to the nucleus. Similar staining patterns were also observed for ERβ2 (Fig. 7A). However, in addition to nuclear staining, appreciable levels of ERβ3–5 were detected in the cytoplasm (Fig. 7A). Identical staining patterns were detected with the MC10 antibody (Fig. 7A). As with the western blotting results (Fig. 6), the PPG5/10 antibody was only able to detect full-length ERβ1 (Fig. 7A). Interestingly, a significant number of cells transfected with either ERβ4 or ERβ5 exhibited a completely different staining pattern characterized as primarily peri-nuclear with some cytoplasmic staining but no nuclear staining (Fig. 7B). This pattern was identical when using the both the Flag and MC10 antibodies (Fig. 7B). As with Figure 7A, no such staining patterns were detected by the PPG5/10 antibody (data not shown). To our knowledge, these studies are the first to have comprehensively characterized the sub-cellular localization of all ERβ variants and suggest that the cytoplasmic and/or peri-nuclear staining observed in human tissues with the MC10 antibody (Fig. 5) are likely explained by expression of ERβ3, ERβ4, or ERβ5, or any combination of the three.
Fig. 7.
Sub-cellular localization of ERβ1–5. U2OS cells were plated on cover slips and transiently transfected with Flag-tagged expression vectors for ERβ1–5 for 24 h. Cells were subsequently fixed, permeabilized, and incubated with either the monoclonal Flag (1:50), MC10 (1:50), or PPG5/10 (1:50) antibodies. Localization of ERβ1–5 was determined using a FITC-labeled anti-mouse secondary antibody and confocal microscopy. A: The PPG5/10 antibody was only able to detect ERβ1 expression while the MC10 antibody was able to detect all forms of ERβ. ERβ1 and ERβ2 were completely localized within the cell nucleus while appreciable levels of cytoplasmic localization were also observed for ERβ3–5 using both the Flag and MC10 antibodies. B: A significant number of cells transfected with ERβ4 and ERβ5 expression vectors also exhibited a differential sub-cellular localization pattern characterized by strong peri-nuclear and punctate cytoplasmic staining in the absence of nuclear staining with both the Flag and MC10 antibodies.
DETECTION OF ERβ PROTEIN IN HUMAN BREAST TUMORS
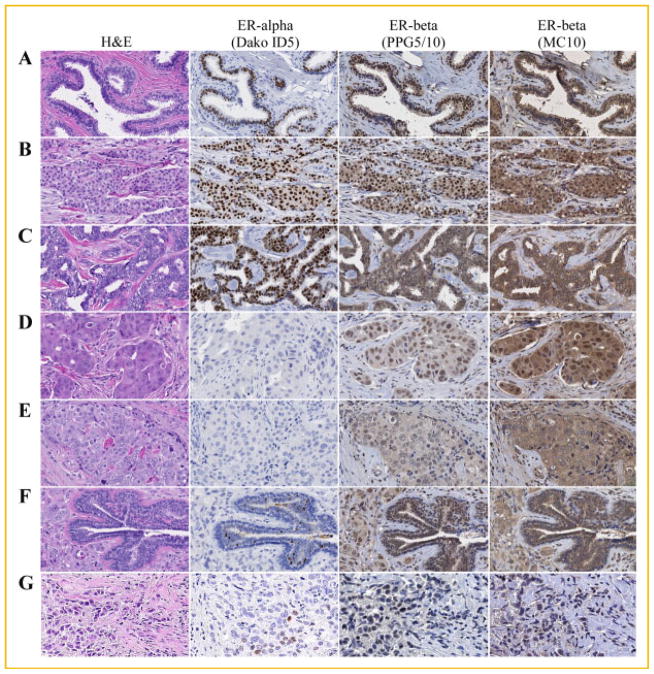
The original goals of this study were to produce and/or identify an antibody that was specific for the detection of ERβ protein, without cross-reaction to ERα or other unrelated proteins, which would ultimately enhance our ability to further characterize the role of ERβ in human breast cancer. Along these lines, we next screened normal breast tissue and a subset of human breast tumors for ERα and ERβ protein expression using an ERα specific antibody (Dako-ID5), as well as the MC10 and PPG5/10 ERβ specific antibodies. Normal breast ducts were shown to be highly positive for ERβ with primarily nuclear staining detected with the PPG5/10 antibody and strong cytoplasmic staining detected with the MC10 antibody (Fig. 8A). Interestingly, of the six breast tumors examined, five exhibited some degree of ERβ positivity with both the PPG5/10 and MC10 antibodies (Fig. 8B–F). Of these, one ERα+ tumor exhibited nuclear and cytoplasmic staining with both antibodies (Fig. 8B). However, there was significantly more cytoplasmic staining observed with the MC10 antibody (Fig. 8B). Another ERα+ tumor exhibited strong cytoplasmic staining using both antibodies with little to no nuclear staining for ERβ (Fig. 8C). Of the three ERα-tumors, all exhibited ERβ positivity with both antibodies to varying degrees (Fig. 8D–F). Finally, the weakly ERα+ breast tumor shown in Figure 8G was essentially negative for ERβ using both antibodies. The pathological reports regarding the staining of these specimens for ERβ as determined by both the PPG5/10 and MC10 antibodies are summarized in Table II.
Fig. 8.
Detection of ERβ protein in normal and cancerous human breast tissue using the MC10 and PPG5/10 monoclonal antibodies. Serial sections of a normal human breast, as well as six randomly selected breast tumors were processed for immunohistochemistry and stained with the ERα specific Dako ID5 antibody (1:50) and the ERβ specific PPG5/10 (1:100) and MC10 (1:300) antibodies as indicated. One section was also stained with hematoxylin and eosin (H&E) for histological purposes. A: Normal human breast tissue. B,C: ERα+ human breast tumors. D–F: ERα-human breast tumors. G: A weakly ERα+ and ERβ-human breast tumor. Note the different staining patterns for ERβ as detected by the PPG5/10 and MC10 antibodies for many of these specimens.
TABLE II.
Pathological Scoring for ERβ in Normal Human Breast and Breast Tumor Sections as Detected by the PPG5/10 and MC10 ERβ Monoclonal Antibodies.
| Tissue | PPG5/10
|
MC10
|
||
|---|---|---|---|---|
| Nuclear | Cytoplasmic | Nuclear | Cytoplasmic | |
| Normal Breast | >90%; Strong | Weak | <1% | Moderate |
| Tumor B | 80%; Strong | Weak | <1% | Strong |
| Tumor C | <1% | Moderate | <1% | Strong |
| Tumor D | 70%; Moderate | Weak | 30–40%; Moderate | Moderate to Strong |
| Tumor E | <1% | Weak | <1% | Moderate |
| Tumor F | 10–20%; Strong | Weak | <1% | Moderate |
| Tumor G | <1% | None | <1% | Weak |
DISCUSSION
In the present study, we describe the development and characterization of a novel monoclonal ERβ antibody, MC10, which is highly specific and sensitive for detection of full-length ERβ1 as well as all four of its variant forms (ERβ2-5). We have also analyzed the specificity and sensitivity of other commercially available ERβ antibodies and have shown that, with the exception of PPG5/10, they are either insensitive or non-specific for detection of this protein. This study is also the first to analyze and compare the sub-cellular localization of all of the ERβ variants and has demonstrated that some of these variants exhibit substantial cytoplasmic and peri-nuclear localization patterns. Immunohistochemical staining of normal human tissues and breast cancer biopsies using the PPG5/10 and MC10 antibodies have revealed distinct staining patterns including tumors which exhibit primarily nuclear staining, and others which exhibit primarily cytoplasmic staining, both in the presence and absence of ERα. While significant conclusions regarding the expression of ERβ in breast cancer cannot be drawn from this limited cohort of breast tumors, it is apparent that ERβ is expressed in a proportion of ERα-tumors and that the sub-cellular localization of ERβ as detected by the PPG5/10 and MC10 antibodies is variable.
The differences in nuclear staining between the two antibodies could be explained by the fact that the PPG5/10 antibody recognizes the C-terminal end of ERβ1 while the MC10 antibody was designed against the N-terminal region. It is possible, and potentially likely, that the N-terminal region of ERβ is much less accessible than the C-terminal domain due to tertiary protein structure and/or interaction with co-factors in the nucleus which are not disrupted by the antigen retrieval methods. While little is known about the functional role of the N-terminal domain(s) of ERβ, we have previously demonstrated that this region, particularly the AF1 domain, confers specificity for at least two known ERβ target genes, TIEG1 and RBBP1, and is essential for their regulation in response to estrogen [Monroe et al., 2006; Hawse et al., 2008]. This domain was also shown to be necessary for interaction with the classic co-regulators, SRC1 and SRC2, as they pertain to regulation of these two target genes [Monroe et al., 2006; Hawse et al., 2008]. Interaction with, and epitope masking by, such co-factors in the nucleus could lead to less nuclear staining for ERβ when using the MC10 antibody compared to the PPG5/10 antibody. Increased levels of cytoplasmic staining as detected by the MC10 antibody are likely explained by the presence of ERβ variants 3–5 since the MC10 antibody was produced using a N-terminal domain which is 100% conserved between all ERβ variants while the PPG5/10 antibody was designed to recognize the C-terminal region of only ERβ1. Furthermore, we have demonstrated that ERβ3–5 at least partially localize to cytoplasmic regions. Since these antibodies result in distinct staining patterns in human tissues, it is our opinion that their combined use in large cohorts of well-annotated samples is likely to provide a more comprehensive and accurate analysis regarding the biological significance of total ERβ protein expression in breast cancer. Additionally, the use of both antibodies will allow for further stratification of ERβ+ tumors on the basis of nuclear versus cytoplasmic staining and as a function of ERβ1 positivity in the presence or absence of ERβ variants.
A number of past in vitro studies have shown that re-expression of ERβ1 in ER negative breast cancer cells results in the reduction of both basal and estrogen induced proliferation rates [Lazennec et al., 2001; Secreto et al., 2007; Thomas et al., 2011]. Expression of ERβ1 in ERα positive breast cancer cells also results in suppression of proliferation following estrogen exposure [Paruthiyil et al., 2004; Strom et al., 2004; Sotoca et al., 2008]. Furthermore, ERβ expression has been shown to increase the effectiveness of selective estrogen receptor modulators (SERMs) such as endoxifen, 4-hydroxytamox-ifen, raloxifene, and fulvestrant in cell culture model systems [Strom et al., 2004; Hodges-Gallagher et al., 2008; Wu et al., 2011]. The summation of these studies suggests that ERβ could serve as a prognostic and/or predictive marker in breast tumors.
Unfortunately, the value of ERβ protein expression in the clinical setting has not been realized. Indeed, significant discrepancies have been reported for both the rate of ERβ expression in breast cancer, as well as its predictive and prognostic value with regard to patient outcomes and responses to targeted therapies. Our findings strongly suggest that some of these discrepancies are related to the use of highly variable and non-specific antibodies. Many studies, including the present report, have demonstrated that ERβ is expressed in normal breast epithelial cells [Jarvinen et al., 2000; Roger et al., 2001; Shaaban et al., 2003; Skliris et al., 2001, 2003] and several studies have indicated that ERβ expression levels are suppressed or lost in many breast cancers [Leygue et al., 1998, 1999; Iwao et al., 2000; Miyoshi et al., 2001; Roger et al., 2001; Shaw et al., 2002; Park et al., 2003; Skliris et al., 2003; Zhao et al., 2003; Bardin et al., 2004]. However, other studies analyzing the frequency of ERβ expression in breast tumors have reported extremely variable results which range from 17–100% [Jarvinen et al., 2000; Jensen et al., 2001; Mann et al., 2001; Miyoshi et al., 2001; Omoto et al., 2001; Skliris et al., 2001, 2006; Saunders et al., 2002; Fuqua et al., 2003; Myers et al., 2004; Nakopoulou et al., 2004; O’Neill et al., 2004; Poola et al., 2005b; Miller et al., 2006; Umekita et al., 2006; Sugiura et al., 2007]. This wide range of ERβ positivity is in stark contrast to the well accepted and reported rate of ERα expression which is observed in approximately 70% of all breast cancer cases. Additionally, the frequency of ERβ expression in ERα negative tumors which has been reported in the literature ranges from 13–83% [Jensen et al., 2001; Mann et al., 2001; Saunders et al., 2002; Shaaban et al., 2003; O’Neill et al., 2004; Honma et al., 2008]. With regard to the potential functions of ERβ in breast cancer, a number of more recent studies have suggested that the presence of this receptor correlates with improved rates of recurrence, disease-free survival, and overall survival [Mann et al., 2001; Omoto et al., 2001; Murphy et al., 2002; Iwase et al., 2003; Esslimani-Sahla et al., 2004; Fleming et al., 2004; Hopp et al., 2004; Myers et al., 2004; Nakopoulou et al., 2004; Sugiura et al., 2007]. However, others indicate little to no correlation [O’Neill et al., 2004; Miller et al., 2006] or even worse prognosis [Speirs et al., 1999a; Speirs et al., 1999b; Jensen et al., 2001]. Lastly, several studies have reported that the presence of ERβ in breast tumors increases the effectiveness of tamoxifen therapy [Poola et al., 2005b; Honma et al., 2008; Novelli et al., 2008; Shaaban et al., 2008] or aromatase inhibitor therapy [Motomura et al., 2010].
These matters become further complicated when considering the fact that the majority of studies have utilized a single ERβ antibody, which either detects only ERβ1 or cross-reacts with all forms of ERβ, making it impossible to analyze the individual contributions of the full-length receptor or its variants. While the functions of ERβ2–5 remain poorly understood, a few publications have suggested that their presence in breast tumors is associated with increased overall survival and increased sensitivity to tamoxifen therapy [Mann et al., 2001; Murphy et al., 2002; Esslimani-Sahla et al., 2004; Hopp et al., 2004; Honma et al., 2008]. It should be of note that these studies have employed antibodies which were designed to cross react with all forms of ERβ (ERβ1–5) and therefore make it impossible to attribute these favorable responses to either the full-length receptor or one or more of its variants. A couple of reports using an ERβ2 specific antibody have shown that its presence in breast tumors correlates with increased overall survival [Sugiura et al., 2007; Shaaban et al., 2008] and one report stated the same for ERβ5 [Shaaban et al., 2008]. Another factor to be considered is the sub-cellular localization of these receptors since we have now shown that ERβ3–5 can localize to cytoplasmic and peri-nuclear regions of the cell in addition to the nucleus. A number of past studies have noted the appearance of cytoplasmic staining for ERβ, but the majority have ignored the potential significance of this observation with the exception of one manuscript which reported that cytoplasmic ERβ2 positivity was correlated with decreased overall survival [Shaaban et al., 2008]. Of note, our studies suggest that ERβ2 is localized within the nuclei of cells and again call into question the specificity of this particular antibody.
In summary, we describe the development and characterization of a novel ERβ monoclonal antibody, MC10, which is highly specific for the detection of ERβ1 and its four variant forms via western blotting, immunoprecipitation, immunohistochemistry, and immunofluorescence. We also provide evidence that some commercially available ERβ antibodies are either non-specific or insensitive for the detection of ERβ with the exception of the ERβ1 specific PPG5/10 antibody. The use of these, and other, non-specific ERβ antibodies in clinical samples has likely created much of the controversy regarding the functions of this protein in breast cancer. Additionally, our results raise significant concerns relating to the interpretation of data presented in previous manuscripts which utilized either commercially obtained, or independently developed, ERβ antibodies in the absence of thorough characterization with regard to their sensitivity and specificity. The present data also reveal that ERβ variants 3–5 exhibit some extent of cytoplasmic and peri-nuclear sub-cellular localization and further research is needed to determine whether such staining patterns in breast tumors could be of predictive and/or prognostic value. The summation of our studies highlight the need to further analyze the role of ERβ in breast cancer using highly specific and validated antibodies. Additionally, the use of ERβ antibodies which do not detect all ERβ variants, or the use of a single ERβ antibody which does not discriminate between ERβ1 and its variants, is unlikely to reveal the complete biological significance of total ERβ expression in breast cancer and may in part explain the conflicting studies which have been reported for ERβ in the literature. The use of the newly developed MC10 antibody, in combination with other highly specific ERβ antibodies such as PPG5/10, may provide additional discriminatory features of value in predicting the response of breast cancer patients to endocrine therapies and/or their association with other clinicopathological factors. Lastly, the ability to accurately identify ERβ expression in ERα negative breast tumors could provide a basis to treat a large number of women with safe and effective hormonal based therapies which at present are not considered as an option.
Acknowledgments
The authors would like to thank Sarah Withers, Kenneth Peters, Kevin Pitel, and Elizabeth Bruinsma for their excellent technical support. Additionally, they would like to thank the Mayo Clinic Antibody Core Facility for their assistance with the development and generation of the monoclonal ERβ antibody described here. This work was supported by Susan G. Komen for the Cure KG100142 (JRH), the Breast Cancer Research Foundation (JNI and TCS), a generous gift from Bruce and Martha Atwater (TCS, JRH, JNI, MPG), a Mayo Clinic Breast Cancer SPORE Career Development Award (P50CA116201) (JRH), a Paul Calabresi K12 award NCI CA90628 (JRH), and the Mayo Foundation.
References
- Balfe PJ, McCann AH, Welch HM, Kerin MJ. Estrogen receptor beta and breast cancer. Eur J Surg Oncol. 2004;30:1043–1050. doi: 10.1016/j.ejso.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, Gustafsson JA, Rochefort H. Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res. 2004;10:5769–5776. doi: 10.1158/1078-0432.CCR-04-0389. [DOI] [PubMed] [Google Scholar]
- Fleming FJ, Hill AD, McDermott EW, O’Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydro-xytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–383. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
- Fuqua SA, Schiff R, Parra I, Moore JT, Mohsin SK, Osborne CK, Clark GM, Allred DC. Estrogen receptor beta protein in human breast cancer: Correlation with clinical tumor parameters. Cancer Res. 2003;63:2434–2439. [PMC free article] [PubMed] [Google Scholar]
- Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A, Ferno M. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res. 2007;13:1987–1994. doi: 10.1158/1078-0432.CCR-06-1823. [DOI] [PubMed] [Google Scholar]
- Hawse JR, Subramaniam M, Monroe DG, Hemmingsen AH, Ingle JN, Khosla S, Oursler MJ, Spelsberg TC. Estrogen receptor beta isoform-specific induction of transforming growth factor beta-inducible early gene-1 in human osteoblast cells: an essential role for the activation function 1 domain. Mol Endocrinol. 2008;22:1579–1595. doi: 10.1210/me.2007-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges-Gallagher L, Valentine CD, El Bader S, Kushner PJ. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat. 2008;109:241–250. doi: 10.1007/s10549-007-9640-6. [DOI] [PubMed] [Google Scholar]
- Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA. Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res. 2004;10:7490–7499. doi: 10.1158/1078-0432.CCR-04-1114. [DOI] [PubMed] [Google Scholar]
- Iwao K, Miyoshi Y, Egawa C, Ikeda N, Noguchi S. Quantitative analysis of estrogen receptor-beta mRNA and its variants in human breast cancers. Int J Cancer. 2000;88:733–736. doi: 10.1002/1097-0215(20001201)88:5<733::aid-ijc8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Iwase H, Zhang Z, Omoto Y, Sugiura H, Yamashita H, Toyama T, Iwata H, Kobayashi S. Clinical significance of the expression of estrogen receptors alpha and beta for endocrine therapy of breast cancer. Cancer Chemother Pharmacol. 2003;52(Suppl 1):S34–S38. doi: 10.1007/s00280-003-0592-1. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Pelto-Huikko M, Holli K, Isola J. Estrogen receptor beta is coexpressed with ERalpha and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol. 2000;156:29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, Wahlstrom T, Warner M, Coombes RC, Gustafsson JA. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci USA. 2001;98:15197–15202. doi: 10.1073/pnas.211556298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142:4120–4130. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski S, Kalita K, Kaczmarek L. Estrogen receptor beta. Potential functional significance of a variety of mRNA isoforms. FEBS Lett. 2002;524:1–5. doi: 10.1016/s0014-5793(02)03015-6. [DOI] [PubMed] [Google Scholar]
- Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–3201. [PubMed] [Google Scholar]
- Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of estrogen receptor beta1, beta2, and beta5 messenger RNAs in human breast tissue. Cancer Res. 1999;59:1175–1179. [PubMed] [Google Scholar]
- Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, Li Y, Younes M. Estrogen receptor beta expression in invasive breast cancer. Hum Pathol. 2001;32:113–118. doi: 10.1053/hupa.2001.21506. [DOI] [PubMed] [Google Scholar]
- Miller WR, Anderson TJ, Dixon JM, Saunders PT. Oestrogen receptor beta and neoadjuvant therapy with tamoxifen: Prediction of response and effects of treatment. Br J Cancer. 2006;94:1333–1338. doi: 10.1038/sj.bjc.6603082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y, Taguchi T, Gustafsson JA, Noguchi S. Clinicopathological characteristics of estrogen receptor-beta-positive human breast cancers. Jpn J Cancer Res. 2001;92:1057–1061. doi: 10.1111/j.1349-7006.2001.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem. 2003;90:315–326. doi: 10.1002/jcb.10633. [DOI] [PubMed] [Google Scholar]
- Monroe DG, Secreto FJ, Hawse JR, Subramaniam M, Khosla S, Spelsberg TC. Estrogen receptor isoform-specific regulation of the retinoblastoma-binding protein 1 (RBBP1) gene: Roles of AF1 and enhancer elements. J Biol Chem. 2006;281:28596–28604. doi: 10.1074/jbc.M605226200. [DOI] [PubMed] [Google Scholar]
- Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Motomura K, Ishitobi M, Komoike Y, Koyama H, Nagase H, Inaji H, Noguchi S. Expression of estrogen receptor beta and phosphorylation of estrogen receptor alpha serine 167 correlate with progression-free survival in patients with metastatic breast cancer treated with aromatase inhibitors. Oncology. 2010;79:55–61. doi: 10.1159/000319540. [DOI] [PubMed] [Google Scholar]
- Murphy LC, Leygue E, Niu Y, Snell L, Ho SM, Watson PH. Relationship of coregulator and oestrogen receptor isoform expression to de novo tamoxifen resistance in human breast cancer. Br J Cancer. 2002;87:1411–1416. doi: 10.1038/sj.bjc.6600654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O’Higgins NJ, Hill AD, Young LS. Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer. 2004;91:1687–1693. doi: 10.1038/sj.bjc.6602156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S, Keramopoulos A. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol. 2004;57:523–528. doi: 10.1136/jcp.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli F, Milella M, Melucci E, Di Benedetto A, Sperduti I, Perrone-Donnorso R, Perracchio L, Venturo I, Nistico C, Fabi A, Buglioni S, Natali PG, Mottolese M. A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: An observational prospective study. Breast Cancer Res. 2008;10:R74. doi: 10.1186/bcr2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill PA, Davies MP, Shaaban AM, Innes H, Torevell A, Sibson DR, Foster CS. Wild-type oestrogen receptor beta (ERbeta1) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. Br J Cancer. 2004;91:1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto Y, Inoue S, Ogawa S, Toyama T, Yamashita H, Muramatsu M, Kobayashi S, Iwase H. Clinical value of the wild-type estrogen receptor beta expression in breast cancer. Cancer Lett. 2001;163:207–212. doi: 10.1016/s0304-3835(00)00680-7. [DOI] [PubMed] [Google Scholar]
- Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- Park BW, Kim KS, Heo MK, Ko SS, Hong SW, Yang WI, Kim JH, Kim GE, Lee KS. Expression of estrogen receptor-beta in normal mammary and tumor tissues: Is it protective in breast carcinogenesis? Breast Cancer Res Treat. 2003;80:79–85. doi: 10.1023/A:1024406223619. [DOI] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- Picard D, Kumar V, Chambon P, Yamamoto KR. Signal transduction by steroid hormones: Nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1990;1:291–299. doi: 10.1091/mbc.1.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poola I, Abraham J, Baldwin K, Saunders A, Bhatnagar R. Estrogen receptors beta4 and beta5 are full length functionally distinct ERbeta isoforms: Cloning from human ovary and functional characterization. Endocrine. 2005a;27:227–238. doi: 10.1385/ENDO:27:3:227. [DOI] [PubMed] [Google Scholar]
- Poola I, Fuqua SA, De Witty RL, Abraham J, Marshallack JJ, Liu A. Estrogen receptor alpha-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERbeta1 and ERbeta5: potential molecular targets for chemoprevention. Clin Cancer Res. 2005b;11:7579–7585. doi: 10.1158/1078-0432.CCR-05-0728. [DOI] [PubMed] [Google Scholar]
- Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- Saunders PT, Millar MR, Williams K, Macpherson S, Bayne C, O’Sullivan C, Anderson TJ, Groome NP, Miller WR. Expression of oestrogen receptor beta (ERbeta1) protein in human breast cancer biopsies. Br J Cancer. 2002;86:250–256. doi: 10.1038/sj.bjc.6600035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secreto FJ, Monroe DG, Dutta S, Ingle JN, Spelsberg TC. Estrogen receptor alpha/beta isoforms, but not betacx, modulate unique patterns of gene expression and cell proliferation in Hs578T cells. J Cell Biochem. 2007;101:1125–1147. doi: 10.1002/jcb.21205. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27:1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Udokang K, Mosquera JM, Chauhan H, Jones JL, Walker RA. Oestrogen receptors alpha and beta differ in normal human breast and breast carcinomas. J Pathol. 2002;198:450–457. doi: 10.1002/path.1230. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Skliris GP, Carder PJ, Lansdown MR, Speirs V. Immunohistochemical detection of ERbeta in breast cancer: Towards more detailed receptor profiling? Br J Cancer. 2001;84:1095–1098. doi: 10.1054/bjoc.2001.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95:616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skliris GP, Munot K, Bell SM, Carder PJ, Lane S, Horgan K, Lansdown MR, Parkes AT, Hanby AM, Markham AF, Speirs V. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201:213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- Sotoca AM, van den Berg H, Vervoort J, van der Saag P, Strom A, Gustafsson JA, Rietjens I, Murk AJ. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008;105:303–311. doi: 10.1093/toxsci/kfn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs V, Malone C, Walton DS, Kerin MJ, Atkin SL. Increased expression of estrogen receptor beta mRNA in tamoxifen-resistant breast cancer patients. Cancer Res. 1999a;59:5421–5424. [PubMed] [Google Scholar]
- Speirs V, Parkes AT, Kerin MJ, Walton DS, Carleton PJ, Fox JN, Atkin SL. Coexpression of estrogen receptor alpha and beta: Poor prognostic factors in human breast cancer? Cancer Res. 1999b;59:525–528. [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Toyama T, Hara Y, Zhang Z, Kobayashi S, Fujii Y, Iwase H, Yamashita H. Expression of estrogen receptor beta wild-type and its variant ERbetacx/beta2 is correlated with better prognosis in breast cancer. Jpn J Clin Oncol. 2007;37:820–828. doi: 10.1093/jjco/hym114. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Strom A, Lindberg K, Gustafsson JA. Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G(2)/M checkpoint signaling. Breast Cancer Res Treat. 2011;127:417–427. doi: 10.1007/s10549-010-1011-z. [DOI] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Umekita Y, Souda M, Ohi Y, Sagara Y, Rai Y, Takahama T, Yoshida H. Expression of wild-type estrogen receptor beta protein in human breast cancer: Specific correlation with HER2/neu overexpression. Pathol Int. 2006;56:423–427. doi: 10.1111/j.1440-1827.2006.01983.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13:R27. doi: 10.1186/bcr2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: Regulation by methylation. Oncogene. 2003;22:7600–7606. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]